Introduction
Metastasis is the predominant cause of death in
cancer patients, and the majority of patients with malignant tumors
will develop varying degrees of metastasis by the time they reach
the late stage of the disease (1–5). As
metastasis is the leading cause of treatment failure and tumor
relapse, the inhibition of tumor metastasis is a key element of
anticancer treatment. Numerous patients with malignant tumors are
in a hypercoagulable state, which can cause various types of
thromboembolism. The latest data show that 9% of cancer patients
die of thromboembolism. Venous thromboembolism (VTE) is one of the
major complications of malignant tumors, with an incidence of 4–6%
(6–9). VTE is second only to the malignancy
itself as a cause of mortality in cancer patients (10–11).
Studies have shown that a hypercoagulable state is closely
associated with the metastasis of malignant tumors (12). The present study aimed to
investigate whether Huisheng oral solution (HSOS) inhibits tumor
metastasis by improving the hypercoagulable state in patients with
malignancies.
HSOS was developed based on a traditional recipe,
known as Huazheng Huisheng Dan, which was recorded in a medical
book from the Qing Dynasty. After 17 years of clinical use, HSOS as
an adjuvant chemotherapy agent has been demonstrated to
significantly improve the quality of life and survival of cancer
patients (13–15). In view of the correlation between
the clinical efficacy of HSOS in malignant tumors and blood stasis
syndrome, and the correlation between blood stasis syndrome and the
hypercoagulable state and thromboembolism, we hypothesized that
promoting blood circulation and removing blood stasis may inhibit
the metastasis of malignant tumors based on traditional Chinese
medicine theory (16). By
developing a middle- to old-aged C57 mouse model of Lewis lung
carcinoma (LLC) with concurrent thromboembolism, we investigated
the effect of HSOS treatment on the hypercoagulable state, assessed
its role in preventing tumor metastasis through promoting blood
circulation and removing blood stasis, and explored the possible
mechanisms involved. The results obtained may provide convincing
experimental evidence to support the notion that Chinese medicine
plays an active role in the inhibition of tumor metastasis.
Materials and methods
Materials and equipment
Animals and tumor cell lines
Eight- to twelve-month-old female C57BL/6 mice of
specific pathogen-free grade, weighing between 27 and 30 g, and
male New Zealand rabbits, weighing between 2 and 2.5 kg, were
provided by the Huaxi Laboratory Animal Center of Sichuan
University [certificate nos. SCXK (chuan) 2008–09 and SCXK (chuan)
2008–10; Chengdu, China]. The animals were allowed to adapt to
their respective environment and food for six to seven days prior
to the start of the experiments. The LLC cell line was obtained
from the Laboratory of Tumor Biology at West China Hospital of
Sichuan University (Chengdu, China). The study was approved by the
Ethical Review Committee of the Society for Laboratory Automation
and Screening (SLAS, Chengdu, China), and all animal procedures
were approved by the Institutional Animal Care and Use Committee of
the Society for Laboratory Automation and Screening.
Drugs and reagents
HSOS (20110203; Chengdu Tianfu Pharmaceutical,
Chengdu, China), trypsin (Amresco, Solon, OH, USA), fetal bovine
serum (Gibco, Carlsbad, CA, USA), Dulbecco’s modified Eagle’s
medium (DMEM; Gibco), ADP (Chrono-log Corporation, Havertown, PA,
USA), collagen (Chrono-log Corporation) and thrombin (384–386;
Chrono-log Corporation) were obtained commercially. Platelet
washing solution and modified Tyrode’s solution have been described
previously (17,18). VEGF and D-dimer ELISA kits (201204;
Shanghai Fengxiang Biological Technology Co., Ltd., Shanghai,
China), anti-CD34 primary antibody (13012; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), biotinylated goat
anti-rabbit IgG (V0527; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China), the DAB chromogenic kit
(753223A; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.),
sodium citrate and EDTA-2Na were also obtained commercially.
Main equipment
An MLR-351 CO2 incubator (Sanyo, Osaka,
Japan), CKX41 inverted microscope (Olympus, Tokyo, Japan), 81m-25
optical microscope (Olympus), CELL-DYN1700 blood cell analyzer
(Abbott Diagnostics, Abbott Park, IL, USA), 560CA platelet
aggregation detector (Chrono-log Corporation), MK3 microplate
reader (Thermo Electron, Waltham, MA, USA), KEN-006 high-speed
centrifuge (Kendro Instruments, Newtown, CT, USA), clean bench
(Wujiang Huazhao Purifying Equipment Co., Ltd., Jiangsu, China) and
refrigerator (Revco Technologies, Asheville, NC, USA) were used in
this study.
Methods
Cell suspension preparation
LLC cells in logarithmic phase were used in this
study. After pouring off the supernatant, adherent cells were
digested with 0.25% trypsin, and harvested cells were suspended in
10% DMEM. The suspended cells were then centrifuged at 150 × g for
3 min, re-suspended with DMEM and counted to adjust the density of
cells to 1×107 cells/ml.
Tumor cell injection
Tumor cell suspension (0.2 ml per mouse) was
subcutaneously injected into the left armpit of female C57BL/6
mice.
Animal groups and treatments
The mice injected with tumor cell suspension were
divided into two groups: a model group (n=50) and a treatment group
(n=50). Fifty normal mice were used as normal controls. The
treatment group was intragastrically administered with HSOS at 0.25
ml·d−1 (~16.7-fold the human dose) for 25 consecutive
days, while the normal control group and model group were
administered equal volumes of normal saline. Twenty-four New
Zealand rabbits were divided into either a treatment group or a
normal control group (n=12 for each group), and both groups were
intragastrically administered HSOS at 5
ml·kg−1·d−1 (~10-fold the human dose). The
doses used in this study were determined based on a pilot
study.
Preparation of platelet-rich plasma
(PRP) and washed platelets
Blood samples collected in tubes containing 3.8%
sodium citrate [blood samples were mixed with sodium citrate at a
ratio of 1:9 (vol/vol)] were centrifuged at 100 × g for 5 min,
resulting in a PRP supernatant. The pellet was further centrifuged
at 1,200 × g for 10 min, resulting in a platelet-poor plasma (PPP)
supernatant. The number of platelets in the resulting PRP was
adjusted to 400–500×109/l with the resulting PPP for the
determination of ADP- and collagen-induced platelet aggregation.
The PRP was centrifuged at 400 × g for 8 min, and the supernatant
was decanted. The resulting platelet pellet was washed twice with
platelet washing solution and re-suspended in modified Tyrode’s
solution. The number of platelets was adjusted to
400–500×109/l for the determination of the rate of
thrombin-induced platelet aggregation.
Measurements
Blood cell counts
One hour after drug administration on day 25, blood
samples were drawn from the eyes of 25 mice in each group (~0.7 ml
per mouse) and placed in tubes containing 10% EDTA-2Na (yielding a
final concentration of 4%). Blood samples (0.25 ml) were used for
counting blood cells (mainly platelets, leukocytes and red blood
cells).
Plasma D-dimer and VEGF levels
The remaining blood samples were centrifuged at 900
× g for 20 min, and the supernatants were collected for the
measurement of plasma levels of D-dimer and VEGF levels using
commercial kits according to the manufacturers’ instructions.
Rate of platelet aggregation
One hour after drug administration on day 25, heart
blood samples were collected from the remaining mice in each group
(~1 ml per mouse) and used to prepare washed platelets. Modified
Tyrode’s solution was used as a blank control for the determination
of the rate of thrombin-induced platelet aggregation. Subsequently,
10 μl of thrombin (100 U·ml−1) was added to 500 μl of
washed platelets, and the maximum rate of platelet aggregation
after 5 min was measured.
One hour after drug administration on day 10, blood
samples were obtained from an ear artery of New Zealand rabbits
with a vacuum blood collection needle (~10 ml per rabbit) to
prepare the washed platelets. Following this, 2 μl thrombin (100
U·ml−1) was added to 500 μl of washed platelets, and the
maximum rate of platelet aggregation after 5 min was measured. In
addition, PRP was prepared to determine the rates of ADP- and
collagen-induced platelet aggregation. PPP was used a blank
control. ADP (10 μl) or collagen (1 μl) was added to 500 μl of the
PRP to obtain a final concentration of 5 μM or 2 μg·ml−1
to determine the maximum rate of platelet aggregation after 5
min.
Number of thrombi or metastatic
nodules
Tumor-bearing mice were sacrificed to collect the
brain, lungs, mesentery, femoral vein and external iliac vein
tissues. The tissues were then fixed in 10% formalin,
paraffin-embedded (two wax blocks for coronal sections of the
brain, two wax blocks for five lung lobes, and one wax block for
the femoral vein, external iliac vein or mesentery), sectioned and
subjected to HE staining. The number of thrombi or metastatic
nodules was counted under a light microscope. Metastatic nodules
were classified into four grades based on their diameter: I,
<0.5 mm; II, 0.5–1 mm; III, 1–2 mm; and IV, >2 mm. The total
number of metastatic nodules was calculated as follows: (Number of
grade I nodules × 1) + (number of grade II nodules × 2) + (number
of grade III nodules × 3) + (number of grade IV nodules × 4)
(19).
Tumor weight and reduced rate of tumor
growth
Tumors were removed from the animals and weighed,
and the reduced rate of tumor growth (%) was calculated as follows:
(Average tumor weight for the model group − average tumor weight
for the treatment group/average tumor weight for the model group) ×
100%.
Thymus and spleen coefficients
The thymus and spleen were removed from the animals
and weighed to calculate organ coefficients using the following
formula: Organ coefficient = organ weight/body weight excluding
tumors. In addition, sections of spleen tissue were prepared to
observe histopathological changes.
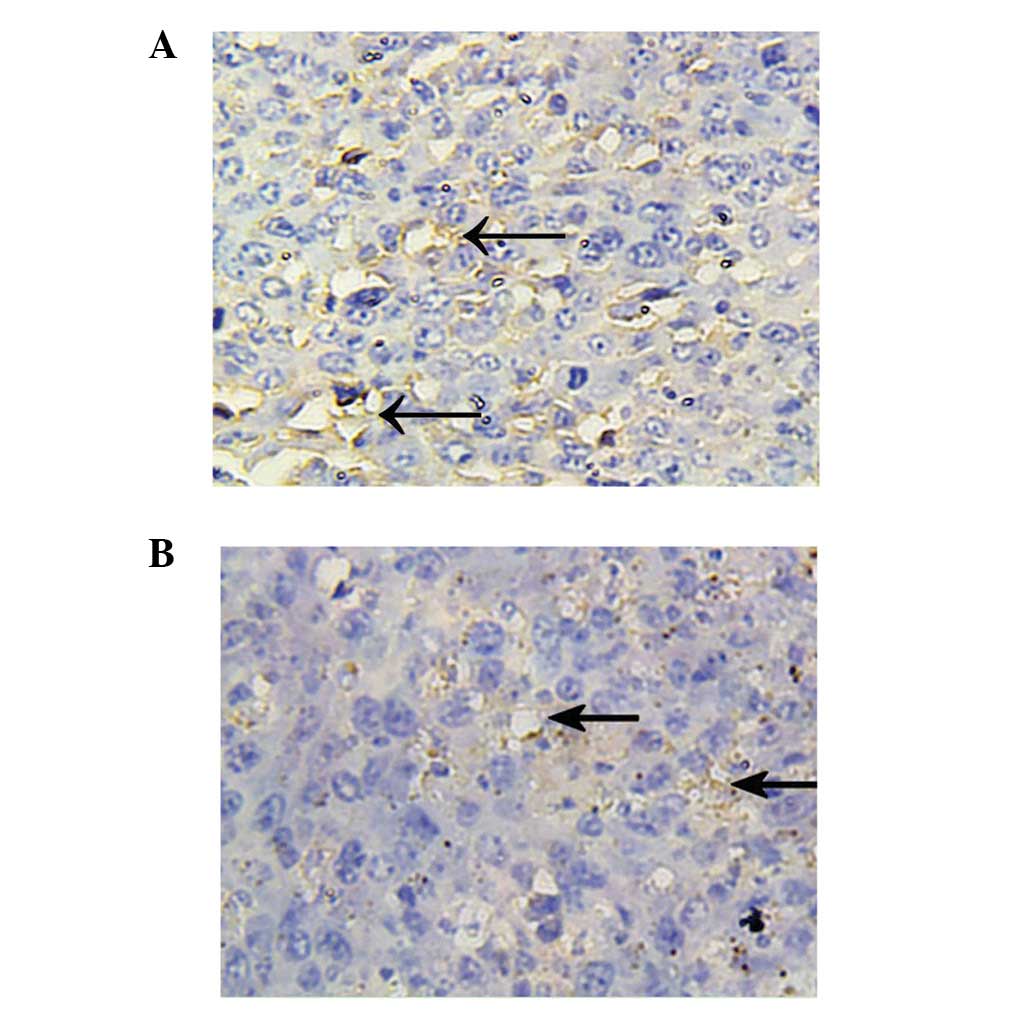
Tumor microvessel density (MVD)
Tumor tissue was fixed in 10% paraformaldehyde,
paraffin-embedded and sectioned. The expression of CD34 in tumor
tissue was detected using the immunohistochemical SP method with a
streptavidin-peroxidase kit (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) according to the manufacturer’s
instructions. Stained sections were observed under a microscope,
and positive cells were stained brown-yellow or yellow in the
cytoplasm. The average optical density was determined using the
Image-Pro Plus 6.0 image analysis system (Media Cybernetics, Inc.,
Rockville, MD, USA).
Statistical analysis
All statistical analyses were performed using SPSS
17.0 software (IBM, New York, NY, USA). Numerical data are
expressed as the means ± standard deviation. Blood cell counts,
plasma levels of D-dimer and VEGF and rates of platelet aggregation
were compared using one-way analysis of variance. Homogeneity of
variance was tested. The LSD test was used when P>0.05 and
Dunnett’s T3 test was used when P<0.05. Tumor weights, the
number of metastatic nodules and MVD in tumor tissue were compared
using the independent samples t-test. The incidence of thrombosis
or pulmonary metastasis was compared using the χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of HSOS treatment on the incidence
of pulmonary thrombosis in mice with LLC
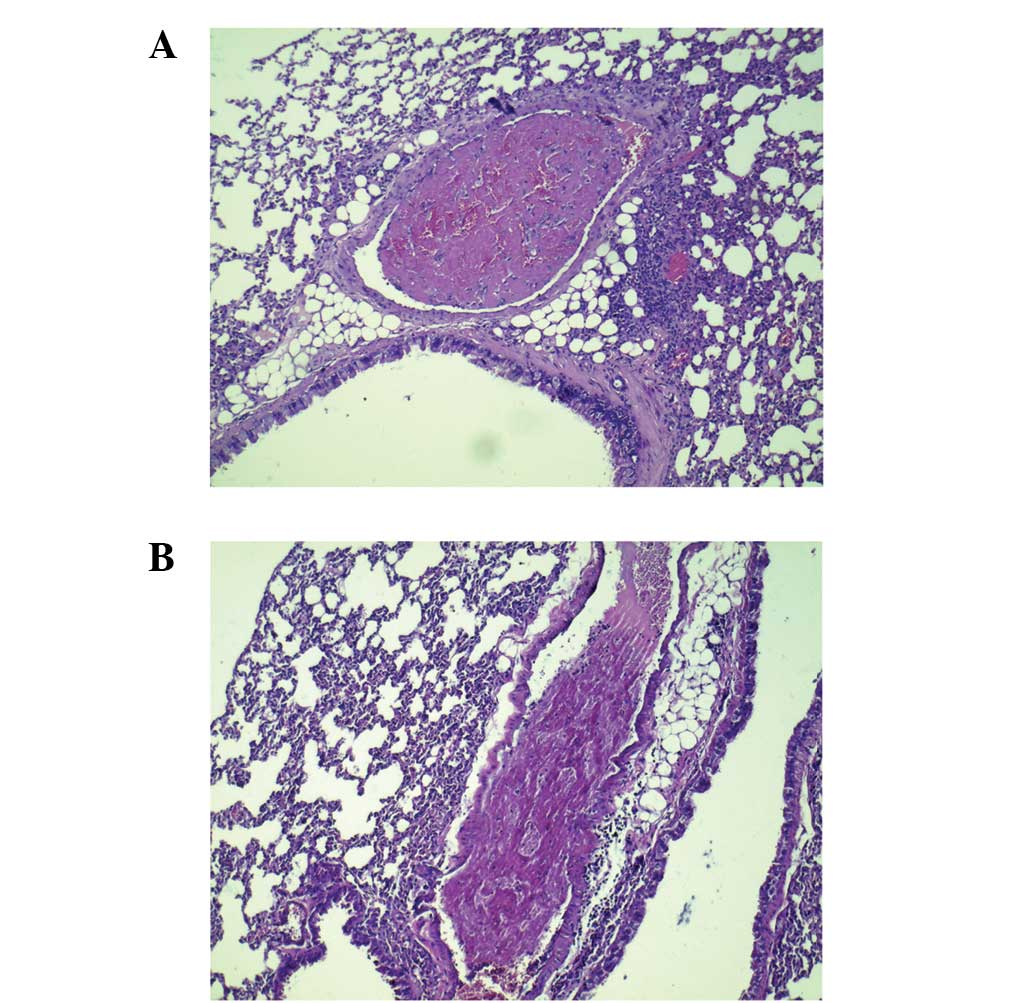
Thrombi were observed only in lung tissues. Compared
with the normal control mice, the incidence of pulmonary thrombosis
was significantly higher in model mice (0/50 vs. 9/50, P<0.01).
HSOS treatment significantly reduced the incidence of pulmonary
thrombosis compared with that in the model mice (2/50, P=0.055)
(Fig. 1).
Effect of HSOS treatment on the incidence
of pulmonary metastasis in mice with LLC
Metastatic nodules were observed only in lung
tissues. Under the optical microscope, tumor emboli were visible in
pulmonary blood vessels. Perivascular tumor nodules were noted and
tumor emboli could be observed in each nodule. Tumor nodules were
focal or multifocal, and varied in size. Tumor nodules increased in
size typically by expansion, with compression of surrounding
parenchyma of the lung, or focal invasion into adjacent parenchyma
of lung, which destroyed the entire lung lobe (Figs. 2–4).
In the model group, the number and size of tumor nodules were
large, and diffusion of tumor nodules in the entire lung lobe was
observed in certain animals. By contrast, no diffusion of tumor
nodules in the entire lung lobe was noted in the treatment group.
The average number of metastatic nodules (P=0.031) and the
incidence of pulmonary metastasis (44/50 vs. 36/50, P=0.046) were
significantly lower in the treatment group than those in the model
group.
Effect of HSOS treatment on tumor growth
in mice with LLC
As shown in Table
III, the mean tumor weight was marginally lower in the
treatment group than in the model group, and the reduced rate of
tumor growth was 9.7%.
 | Table IIIEffect of HSOS treatment on immune
organs, tumor growth and metastasis (means ± SD, n=50). |
Table III
Effect of HSOS treatment on immune
organs, tumor growth and metastasis (means ± SD, n=50).
| Group | Body weight (g) | Tumor weight (g) | Thymus
coefficient | Spleen
coefficient | Number of
metastatic nodules per mouse |
|---|
| Normal control | 27.62±3.43 | - |
0.00179±0.00043 | 0.0045±0.0021 | - |
| Model | 29.24±4.37 | 5.15±1.17 |
0.00106±0.0006a |
0.0129±0.0031a | 5±4.38 |
| Treatment | 28.04±2.9 | 4.65±0.75 |
0.0014±0.0008a,b |
0.0154±0.0041a,b | 2.61±4.55b |
Effect of HSOS treatment on blood cell
counts and plasma D-dimer levels in mice with LLC
Compared with the normal control group, the white
blood cell count and plasma D-dimer levels were significantly
higher, and the red blood cell and platelet counts were
significantly lower, in the model and treatment groups (P<0.01
for all). However, the white blood cell count (P=0.027) and plasma
D-dimer levels (P=0.048) were significantly lower, and the platelet
count (P=0.015) was significantly higher, in the treatment group
compared with that in the model group, although there was no
significant difference in the red blood cell count between the two
groups (P=0.77) (Table I).
 | Table IEffect of HSOS treatment on blood cell
counts and plasma levels of D-dimer and VEGF in mice with LLC
(means ± SD, n=25). |
Table I
Effect of HSOS treatment on blood cell
counts and plasma levels of D-dimer and VEGF in mice with LLC
(means ± SD, n=25).
| Blood cell count | Plasma |
|---|
|
|
|
|---|
| Group | WBC
(×109) | RBC
(×1012) | Platelets
(×109) | D-dimer (μg/l) | VEGF (ng/l) |
|---|
| Normal control | 5.62±1.76 | 10.5±0.36 | 907.3±106.55 | 46.02±11.81 | 77.46±48 |
| Model | 19.13±5.66a | 6.61±0.83a | 343.43±115.84a | 69.39±17.81a | 178.7±85.64a |
| Treatment | 16.74±3.26a,b | 6.56±0.6a | 435.87±178.71a,b | 60.72±16.67a,b | 112.26±66.72b |
Effect of HSOS treatment on the rate of
thrombin-, ADP- or collagen-induced platelet aggregation
The rate of thrombin-induced platelet aggregation
showed no significant difference among each group in either mice or
rabbits. The rate of ADP-induced platelet aggregation also showed
no significant difference among each group in rabbits. However, the
rate of collagen-induced platelet aggregation was significantly
lower in the treatment group than that in the normal control group
in rabbits (P<0.01) (Table
II).
 | Table IIEffect of HSOS treatment on the rates
of thrombin-, ADP- and collagen-induced platelet aggregation (means
± SD). |
Table II
Effect of HSOS treatment on the rates
of thrombin-, ADP- and collagen-induced platelet aggregation (means
± SD).
| Rabbits | Mice |
|---|
|
|
|
|---|
| Normal control | Treatment | Normal control | Model | Treatment |
|---|
| Sample size | 12 | 12 | 10 | 10 | 10 |
| Thrombin | 96.26±12.32 | 90±26.89 | 95.12±27.84 | 96.50±27.41 | 95.62±29.05 |
| ADP | 52±15 | 56.4±21.38 | | | |
| Collagen | 101.81±11.37 | 82.18±9.96a | | | |
Effect of HSOS treatment on plasma VEGF
levels and MVD in tumor tissue in mice with LLC
Plasma levels of VEGF were significantly higher in
the model and treatment groups than those in the normal control
group (P<0.01 for both). However, the plasma level of VEGF in
the treatment group was significantly lower than that in the model
group (P=0.013) (Table I). The
average optical density of MVD in tumor tissue was significantly
lower in the treatment group than that in the model group
(8061.85±3944.75 vs. 13169.88±9989.43, P<0.01) (Fig. 5).
Effect of HSOS treatment on immune organs
in mice with LLC
Compared with the normal control group, the thymus
coefficient was significantly lower in the model and treatment
groups (P<0.05 for both); however, the thymus coefficient was
significantly higher in the treatment group than that in the model
group (P=0.045). The spleen coefficient was significantly higher in
the model group and treatment group than that in the normal control
group (P<0.01 for both); however, the thymus coefficient was
significantly higher in the treatment group than that in the model
group (P=0.022) (Table III). In
both the model and treatment groups, diffuse hyperplasia of spleen
lymphocytes was visible, and the red pulp was compressed and
shrunken (Fig. 6).
Discussion
In this study, we selected multiple tissues prone to
thromboembolism, including the brain, lung, mesentery, femoral vein
and external iliac vein, to observe the occurrence of thrombosis in
mice with LLC. Thrombosis associated with LLC metastasis was
detected only in the lung. We speculate that this may be due to the
fact that LLC cells have a higher affinity for lung tissue. Tumor
cells migrate from the injection site with the blood flow to
pulmonary blood vessels and form tumor thrombi, which then invade
the surrounding lung tissue and result in the occurrence of
metastatic nodules (20). When
tumor thrombi invade pulmonary blood vessels, vascular endothelial
injury occurs. Tumor cells attach to the damaged endothelium and
gather into groups to form whirlpools (21). In addition, tumor cells may release
a large number of procoagulant substances (22). The presence of vascular endothelial
injury, coagulation-promoting substances and hemorheological
changes promotes the occurrence of pulmonary vascular thrombosis.
In addition, our preliminary results showed a low incidence of
pulmonary thromboembolism (1/22) in 6- to 8-week-old C57 mice,
which made statistical analysis difficult. For this reason, a
relatively large number of middle- to old-aged C57 mice were used
in this study in order that the incidence of pulmonary thrombosis
(9/50) met the requirements for statistical analysis.
Our observation that treatment with HSOS
significantly reduced the incidence of pulmonary thromboembolism
and pulmonary metastasis in mice with LLC indicates that HSOS
reduces the incidence of pulmonary metastasis by possibly
decreasing the incidence of pulmonary thromboembolism. As
thrombosis is one of the manifestations of the coagulation states
of the body, HSOS may inhibit tumor metastasis by potentially
improving the hypercoagulable state of the body.
The finding that treatment with HSOS significantly
decreased plasma D-dimer levels in mice with LLC and the rate of
collagen-induced platelet aggregation in rabbits indicates that
HSOS is capable of improving abnormal coagulation and fibrinolysis
caused by malignant tumors and thereby reducing the incidence of
pulmonary thromboembolism. This may be one of the mechanisms by
which HSOS treatment inhibits cancer metastasis. The increase in
D-dimer levels indicates that the generation of thrombin increased,
which often leads to thrombosis. The presence of thrombosis
enhances fibrinolysis (23),
increases blood viscosity and slows down blood flow. Thus, tumor
cells may easily attach to the vessel wall and migrate from the
blood vessels to form metastatic nodules (24). As HSOS is composed of a variety of
substances that may promote blood circulation and remove blood
stasis, it may improve blood flow state, decrease blood viscosity,
suppress thrombosis, reduce plasma D-dimer levels and indirectly
inhibit pulmonary metastasis. Conversely, individual tumor cells in
the blood are more likely to be killed by the immune system, while
tumor cells aggregated with platelets are able to form tumor
thrombi and evade the immune system, resulting in the development
of metastasis (25). HSOS treatment
may effectively inhibit collagen-induced platelet aggregation,
prevent the formation of tumor thrombi and thereby inhibit tumor
metastasis.
Neovascularization is essential for tumor
proliferation and metastasis. VEGF is the most important angiogenic
factor (26). Studies have shown
that a hypercoagulable state in patients with malignant tumors
promotes tumor angiogenesis. In hypercoagulable states, activated
platelets secrete tumor cell growth factors and angiogenic factors,
such as platelet-derived growth factor (27), VEGF (28) and angiopoietin-1 (29). VEGF not only promotes the
proliferation and division of endothelial cells, and angiogenesis,
but also increases vascular permeability, thereby promoting tumor
growth and metastasis. Our observation that treatment with HSOS
reduced plasma VEGF levels in mice with LLC indicates that HSOS may
inhibit tumor angiogenesis. This result is further confirmed by our
observation that HSOS reduced MVD in the tumor tissue. Overall,
these findings indicate that the improvement of hypercoagulability
and the inhibition of tumor angiogenesis is another important
mechanism by which HSOS inhibits tumor proliferation and
metastasis.
HSOS treatment improved leukocyte and platelet
counts in mice with LLC, indicating that HSOS is able to mitigate
abnormal peripheral blood cell counts caused by malignant tumors.
The increase in the number of leukocytes in mice with LLC may be
due to the fact that the tumor cells produced large amounts of
inflammatory cytokines to stimulate the release of bone marrow
myelocytes and the production of peripheral leukocytes (30). Abnormally increased numbers of
leukocytes may gather and adhere to microvessels, and block the
blood vessels. In addition, biologically active substances released
by leukocytes, such as leukotrienes and prostaglandins, may cause
vasoconstriction and platelet aggregation, worsen the
hypercoagulable the hyperviscosity state in tumor patients
(31), and thereby promote tumor
metastasis. Thus, HSOS treatment may reduce leukocyte-induced
inflammation and inhibit tumor metastasis. The decrease in red
blood cell count and platelet count in mice with LLC may be
associated with the consumption of platelets and red blood cells
during thrombosis, the destruction of bone marrow hematopoietic
function by tumor cells (32) and
the excessive consumption of vitamin B12 and folic acid caused by
malignant tumors. The observation that HSOS treatment elevated
platelet count in mice bearing LLC indicates that HSOS has a
protective effect on the body.
Treatment with HSOS may reduce tumor-induced damage
to the thymus, strengthen the tumor immune response in the spleen
and enhance immune function. Tumor cells secrete immunosuppressive
factors and cause thymic atrophy and functional impairment
(33). HSOS treatment may alleviate
the atrophy of the thymus and therefore exert a protective effect
on the thymus. The spleen is the largest peripheral immune organ.
Tumor-specific antigens stimulate the spleen to produce an immune
response, resulting in the active proliferation of spleen
lymphocytes (34). HSOS treatment
may further strengthen immune responses in the spleen, indicating
that in addition to the tumor immune response, HSOS is involved in
enhancing immune function. Enhanced immune function may improve the
recognition of tumor antigens and the capacity for killing tumor
cells, and reduce pulmonary thrombosis and metastasis. This is
another important mechanism underlying the antitumor effect of
HSOS.
In conclusion, HSOS is involved in inhibiting tumor
metastasis and growth. This inhibitory effect is associated with
promoting blood circulation, removing blood stasis and improving
hypercoagulability. In the present study, HSOS treatment
effectively improved the following hypercoagulability parameters in
mice with LLC: D-dimer, platelet aggregation, white blood cell
count and the number of pulmonary thrombi. In addition, HSOS
treatment inhibits tumor metastasis and growth, possibly by
inhibiting tumor angiogenesis and modulating immune function. Our
study correlates the anticancer effects of HSOS with the
improvement of hypercoagulability, providing a new avenue for the
treatment of malignant tumors with Chinese medicine.
In the present study, the reduced rate of tumor
growth in aging mice treated with HSOS was 9.7%. We have previously
investigated the effect of HSOS treatment on thromboembolism in 6-
to 8-week-old mice with LLC and found that the reduced rate of
tumor growth was ~40%, which is similar to that reported by others
(35). It remains to be investigated whether the lower reduced rate
of tumor growth observed in the present study is associated with
diminished immune function in aging mice.
References
|
1
|
Gao J and Zhang JB: Cancer invasion and
metastasis: basic and clinical aspects. Beijing Science Press;
Beijing: pp. 254–263. 2003
|
|
2
|
Wang X, Zhu Y, Ma Y, et al: The role of
cancer stem cells in cancer metastasis: New perspective and
progress. Cancer Epidemiol. 37:60–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Umemura S, Tsubouchi K, Yoshioka H, et al:
Clinical outcome in patients with leptomeningeal metastasis from
non-small cell lung cancer: Okayama Lung Cancer Study Group. Lung
Cancer. 77:134–139. 2012. View Article : Google Scholar
|
|
4
|
Xu Z: New concept and new way of treatment
of cancer metastasis. People’s Military Medical Press; Beijing: pp.
26–29. 2006
|
|
5
|
Gerl R and Vaux DL: Apoptosis in the
development and treatment of cancer. Carcinogenesis. 26:263–270.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang LX, Zhang X and Li J: Diagnosis and
treatment of prothrombotic state in patients with malignant tumors.
J Pract Oncol. 26:440–442. 2011.
|
|
7
|
Li XY, Xu L and Tan H: Anticoagulant and
thrombolytic treatment. Science and Technology Literature
Publishing House; Beijing: pp. 3102011
|
|
8
|
Wang CY, Yang F, Li GF, et al: Incidence
and clinical features of malignant venous thromboembolism: an
analysis of 862 cases. Acta Academiae Medicinae Militaris Tertiae.
32:2256–2257. 2010.
|
|
9
|
Khorana AA, Francis CW, Culakova E, et al:
Thromboembolism in hospitalized neutropenic cancer patients. J Clin
Oncol. 24:484–490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heit JA: Cancer and venous
thromboembolism: scope of the problem. Cancer Control. 12(Suppl 1):
S5–S10. 2005.
|
|
11
|
Zhang ZG, Han L and Zhao LB: Malignancies
and thromboembolic disease. J Pract Oncol. 25:346–347. 2010.
|
|
12
|
Hu L, Yang H, Su L, et al: Clinical
research of the hypercoagulable state in 180 patients with
malignant tumor. Med Res Ed. 27:30–32. 2010.
|
|
13
|
Cheng LZ and Ge HL: Clinical efficacy of
Huisheng Oral Solution as an auxiliary treatment for lung cancer.
Chin J Prim Med Pharm. 12:1580–1582. 2005.
|
|
14
|
Li HT, Fu GQ and Wang CQ: Clinical
observation of treating PHC by combining TACE with Huisheng oral
liquid. West China Med J. 18:516–517. 2003.
|
|
15
|
Xiong G, Zhou J, Hu YQ, et al: Huisheng
Oral Solution improves quality of life of patients with advanced
malignant tumors during chemotherapy. Shandong Med J. 51:57–58.
2011.
|
|
16
|
Fu J: Huisheng Oral Solution: pharmacology
and clinical applications. Shandong Med J. 51:110–111. 2011.
|
|
17
|
Johnson LN, Winter KM, Reid S, et al:
Cryopreservation of buffy-coat-derived platelet concentrates in
dimethyl sulfoxide and platelet additive solution. Cryobiology.
62:100–106. 2011.
Mathew M, Schilling T and Oettel M:
Connectivity percolation in suspensions of hard platelets. Phys Rev
E Stat Nonlin Soft Matter Phys. 85:0614072012.
|
|
18
|
Gao J: Invadation and metastases of
cancer. Union Press of Beijing Medical University and Peking Union
Medical College; Beijing: pp. 621996
|
|
19
|
Das UN: Essential fatty acids and their
metabolites as modulators of stem cell biology with reference to
inflammation, cancer, and metastasis. Cancer Metastasis Rev.
30:311–324. 2011. View Article : Google Scholar
|
|
20
|
Debaugnies F, Azerad MA, Noubouossié D, et
al: Evaluation of the procoagulant activity in the plasma of cancer
patients using a thrombin generation assay. Thromb Res.
126:531–535. 2010. View Article : Google Scholar
|
|
21
|
Kröger K, Weiland D, Ose C, et al: Risk
factors for venous thromboembolic events in cancer patients. Ann
Oncol. 17:297–303. 2006.PubMed/NCBI
|
|
22
|
Anderson JA and Weitz JI: Hypercoagulable
states. Clin Chest Med. 31:659–673. 2010. View Article : Google Scholar
|
|
23
|
De Cicco M: The prothrombotic state in
cancer: pathogenic mechanisms. Crit Rev Oncol Hematol. 50:187–196.
2004.PubMed/NCBI
|
|
24
|
Xiao B, Ma LL, Zhang SD, et al:
Correlation between coagulation function, tumor stage and
metastasis in patients with renal cell carcinoma: a retrospective
study. Chin Med J (Engl). 124:1205–1208. 2011.
|
|
25
|
Xue Y, Religa P, Cao RH, et al: Anti-VEGF
agents confer survival advantages to tumor-bearing mice by
improving cancer-associated systemic syndrome. Proc Natl Acad Sci
USA. 105:18513–18518. 2008. View Article : Google Scholar
|
|
26
|
Kepner N and Lipton A: A mitogenic factor
for transformed fibroblasts from human platelets. Cancer Res.
41:430–432. 1981.PubMed/NCBI
|
|
27
|
Möhle R, Green D, Moore MA, et al:
Constitutive production and thrombin-induced release of vascular
endothelial growth factor by human megakaryocytes and platelets.
Proc Natl Acad Sci USA. 94:663–668. 1997.PubMed/NCBI
|
|
28
|
Ahmad SA, Liu W, Jung YD, et al: The
effects of angiopoietin-1 and -2 on tumor growth and angiogenesis
in human colon cancer. Cancer Res. 61:1255–1259. 2001.
|
|
29
|
Zhu XY, Zhang XZ and Zhong XY: Effect of
shenqi fuzheng injection for hemopoietic and immune function
reconstruction in patients with hematologic malignancies undergoing
chemotherapy. Zhongguo Zhong Xi Yi Jie He Za Zhi. 30:205–206.
2010.(In Chinese).
|
|
30
|
Quan D, Gallinger S, Nhan C, et al: The
role of liver resection for colorectal cancer metastases in an era
of multimodality treatment: a systematic review. Surgery.
151:860–870. 2012. View Article : Google Scholar
|
|
31
|
Iwasaki A, Hamanaka W, Harnada T, et al:
Significance of platelet counts in patients who underwent surgical
treatment for lung metastasis. Int Surg. 92:103–109.
2007.PubMed/NCBI
|
|
32
|
Qu YZ, Guan JZ, Pan H and Song Y: Effects
of the Aidi Dripping Pills on immune functions of the tumor-bearing
mouse. J Tradit Chin Med. 30:122–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li JH, Chen CF, Li WW, et al: Effects of
Ethanol Extract of POLLEN TYPHAE on Immune Function in C57BL/6
Tumor Bearing Mice. Medicinal Plant. 2:12–14. 2011.
|
|
34
|
Huang GJ, Wang XM, Mao XY and Du ZT: An
experimental study of antitumor effects of Huisheng Oral Solution.
Chin Trad Patent Med. 20:37–39. 1998.
|