Introduction
Bladder carcinoma is considered to be the most
common malignant tumor of the urothelium and 94% of baldder
carcinomas are composed of transitional cells (1,2). Its
distinct symptoms are microscopic or macroscopic hematuria and less
frequent symptoms include difficulty urinating, frequent urination
and therapy-resistant urinary tract infection. Therefore, it is
important to detect bladder carcinoma from voided urine specimens
at the earliest possible stage.
At present, urine cytology, radiographic imaging and
cystoscopy are the most common methods for diagnosis and follow-up
of patients with bladder cancer. Cytology is noninvasive and has a
high specificity. However, its sensitivity in urinary specimens is
limited as the majority of noninvasive cancers (stage pTa) are
missed (3–5). Therefore, cytology alone is not
reliable enough to serve as a basis for therapeutic decisions.
Although full bladder ultrasound examination may show an
intravesicular mass or consequential dilatation of the bladder, its
sensitivity depends mainly on the quality of the apparatus and the
experience of the clinician. Furthermore, it is extremely difficult
for ultrasound examination to detect a tumor small in size or
situated at the posterior bladder wall (6,7). In
addition to discomfort and risk of patient morbidity (urinary tract
infection, pain and contrast reaction), cystoscopy fails to detect
flat tumors and carcinomas in situ in the bladder (8). Thus, a reliable and noninvasive method
must be developed to detect bladder cancer in its earliest
stages.
A number of previous studies have used single
fluorescence in situ hybridization (FISH) probes to detect
gene abnormalities in malignant cells; however, this may have
limited sensitivity and specificity (9,10).
With the progression of tumor studies, increasing numbers of new
chromosomal instabilities and aneuploidies have been found in
bladder cancer, particularly those involving chromosomes 1, 3, 7,
9, 11 and 17 (11–13). Therefore, hybridizing various probes
for different pericentromeric and other chromosomal regions in a
single step may facilitate the accurate diagnosis of bladder cancer
clinically. The present study aimed to evaluate the diagnostic
usefulness of multicolor FISH assay, and its sensitivity and
specificity as a non-invasive method, for the diagnosis of bladder
cancer.
Materials and methods
Patient population and samples
A total of 83 voided urine specimens (54 from males
and 29 from females), obtained between June 2010 and May 2012, were
collected. These included 16 samples from patients with superficial
tumors (mean age, 71 years; two cases could not be evaluated by
FISH evaluation) and 37 samples from patients with muscle-invasive
tumors (mean age, 75 years; one case could not be evaluated by FISH
evaluation). In addition, 30 patients with bladder inflammation
were included in the control group (one case could not be evaluated
by FISH evaluation). Voided urine specimens from all bladder cancer
patients with biopsy-diagnosed bladder cancer were used as the gold
standard for evidence of disease. This study was approved by the
Medical Ethics Committee of Sun Yat-sen University (Guangzhou,
Guangdong, China).
FISH processing
The urine samples were collected in the morning (the
first urination of the day) for FISH analysis. First, voided urine
samples were centrifuged at 2,582 × g for 10 min and incubated in a
hypotonic solution of potassium chloride (0.075 M). Next, the cell
pellets were fixed with methanol acetic acid (2:1). Slides produced
from the resuspended cells were used for the subsequent FISH
assays.
Specific probe kits used in this study included
CEP17, CEP3, CEP7 and 9p21 labeled with various fluorescent dyes
(Beijing GP Medical Technologies, Ltd., Beijing, China). Following
the guidelines of the kit, slides were developed at room
temperature and then dehydrated in a series of ethanol washes (70,
85, and 100% for 2 min each). Next, a 10 μl probe mixture was added
to each slide and sealed under a small glass coverslip. The target
DNA and probe were codenatured at 7°C for 5 min, followed by
hybridization at 42°C.
Post-hybridization washes were performed with 2X SSC
for 10 min and 2X SSC/0.1% NP-40 for 5 min at 47°C. Counterstaining
was performed with DAPI. Signal analysis was performed using a
computer applied imaging system.
Means and three standard deviations of the
percentages of nuclei with abnormal signal patterns were calculated
as the cutoff values. Voided urine samples from 20 normal
individuals were used to establish the cutoff values (Table I).
 | Table IDefining the optimal cutoff values for
FISH-positive voided urine specimens (average percentage of cells
with 0, 1, 3 or more signals in 100 consecutive cells from 20 urine
specimens from normal donors). |
Table I
Defining the optimal cutoff values for
FISH-positive voided urine specimens (average percentage of cells
with 0, 1, 3 or more signals in 100 consecutive cells from 20 urine
specimens from normal donors).
| CSP 3 | CSP 7 | CSP 17 | 9p21 |
|---|
|
|
|
|
|
|---|
| Number of
signals | 1 | 3 or more | 1 | 3 or more | 1 | 3 or more | 0 or 1 | 3 or more |
|---|
| Mean | 2.18 | 0.65 | 1.85 | 0.50 | 1.53 | 0.83 | 2.15 | 2.03 |
| SD | 1.38 | 0.61 | 1.28 | 0.50 | 1.34 | 0.63 | 1.25 | 1.32 |
| Cutoff, % | 6.32 | 2.48 | 5.69 | 2.00 | 5.55 | 2.72 | 5.90 | 5.99 |
By definition, abnormalities, including aneusomy of
locus-specific probes, chromosome monosomy and polysomy, were
diagnosed only when the percentage of cells with one or three or
more FISH signals exceeded the cutoff values. For each probe, 100
nuclei were studied.
Statistical analysis
A χ2 test was used to analyze the
correlation between FISH results and tumor stages and grades.
Severity of the genetic alterations was defined as the percentage
of cells with cytogenetic abnormalities according to the positive
criteria of FISH. Statistical analysis of the severity of the
genetic alterations among various tumor stages and grades was
performed by Mann-Whitney (MW) and Kruskal-Wallis (K-W) tests,
using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Genetic alterations in bladder
cancer
Based on the evaluation criteria of pathological
T-stage and tumor grade cancers, the 50 resected tumors with FISH
examination were classified as non-muscle-invasive (pTa, pT1) in 34
cases and muscle-invasive (pT2) in 16 cases. The tumor grade was
low in 37 cases and high in 13 cases.
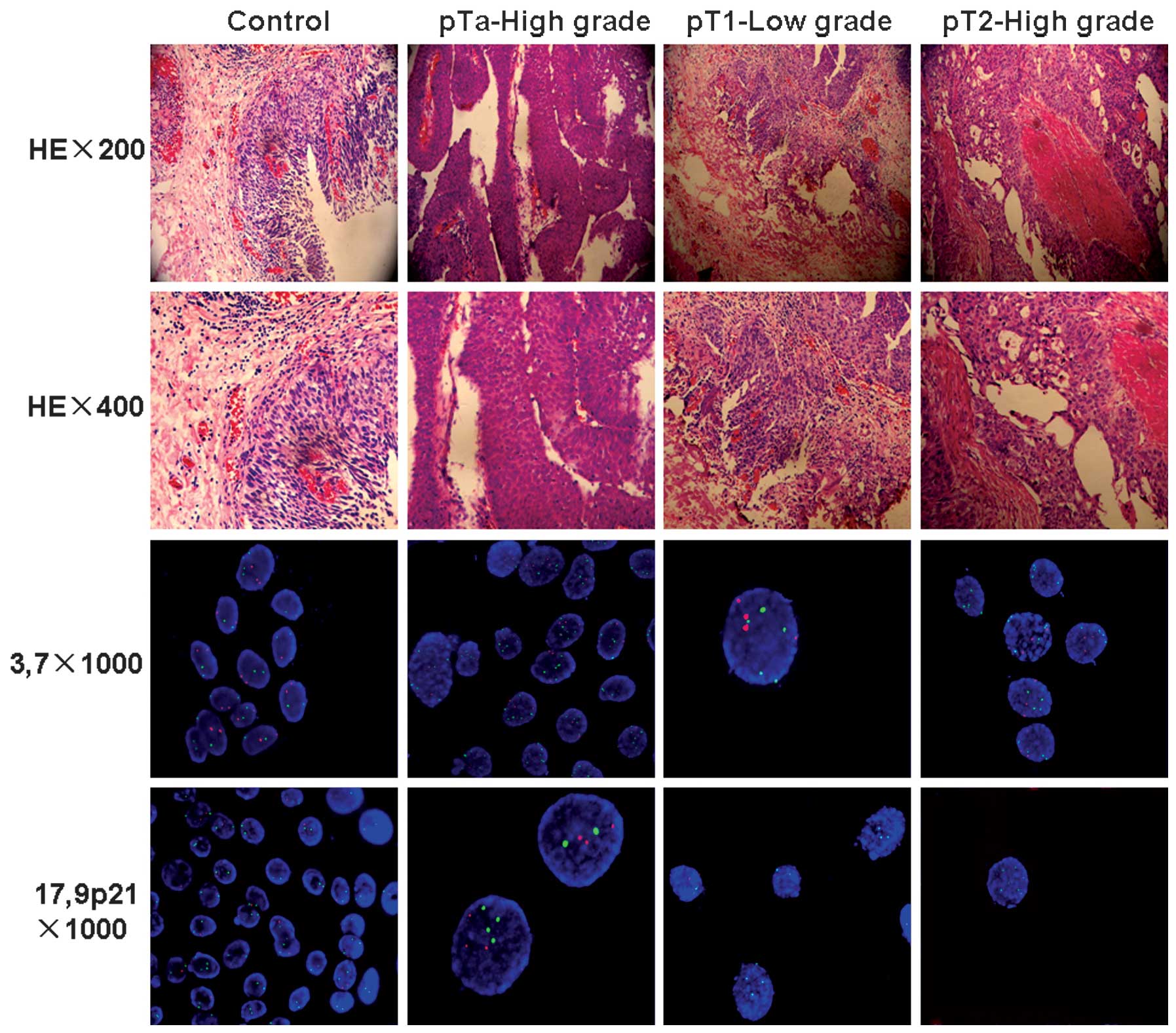
The positive rates of FISH in the urine samples were
compared with the histological findings of the transurethral
resection specimens. In total, 44 patients with FISH-positive
results were verified histologically as having bladder cancer
(Fig. 1). Negative results were
obtained in six cases, from which five were proven to be carcinomas
of superficial stage Ta and one was stage T2, based on the later
histological findings. In the non-malignant group, 29 cases were
FISH-negative and one case was not able to be detected by FISH. The
detailed results are shown in Tables
II and III. Altogether, based
on the histological findings, the specificity and sensitivity of
the FISH analysis were rated as 100 and 88%, respectively (Tables II and III).
 | Table IIFISH results in voided bladder cancer
urine specimens. |
Table II
FISH results in voided bladder cancer
urine specimens.
| FISH results | |
|---|
|
| |
|---|
| Diagnosis type | Positive | Negative | Could not be
evaluated | Total |
|---|
| Malignant |
| Bladder cancer | 44 | 6 | 3 | 53 |
| Non-malignant |
| Inflammation | 0 | 29 | 1 | 30 |
 | Table IIIMultiprobe FISH results in voided
bladder cancer urine specimens of various stages and grades. |
Table III
Multiprobe FISH results in voided
bladder cancer urine specimens of various stages and grades.
| FISH results | |
|---|
|
| |
|---|
| Bladder cancer | n/N | % | χ2 test
P-value |
|---|
| Stage | | | >0.05 |
| Non-muscle-invasive
(pTa, pT1) | 29/34 | 85.3 | |
| Muscle-invasive
(pT2) | 15/16 | 93.8 | |
| Grade | | | >0.05 |
| Low | 32/37 | 86.5 | |
| High | 12/13 | 92.3 | |
| Overall
sensitivity | 44/50 | 88.0 | |
The FISH assay showed that all cases with bladder
inflammation were negative, with only chromosomes 3, 7 and 17, and
the 9p21 locus, with abnormalities. Of the 44 FISH-positive
transitional carcinoma cases, 8 stage Ta, 19 stage T1 and 15 stage
T2 cases exhibited polysomy of chromosome 3. Polysomy of
chromosomes 7 and 17 was mainly distributed in 4 and 10 stage Ta
cases, 10 and 14 stage T1 cases and 10 and 15 stage T2 cases,
respectively. However, in all positive samples, there was no loss
of chromosomes 3, 7 and 17 detected. For 9p21, any copy number
change in >6 cells was recorded as positive, which included gain
of chromosomal material and heterozygous or homozygous deletions.
Ten stage Ta, 19 T1 and 11 T2 cases exhibited 9p21 abnormalities
(Table IV).
 | Table IVComparison between individual FISH
probes and the combined probe in 79 voided urine specimens. |
Table IV
Comparison between individual FISH
probes and the combined probe in 79 voided urine specimens.
| Combined | Centromeric probe
(polysomy) | Homozygous deletion
of 9p21 | Deletion of 9p21 |
|---|
|
|---|
| 3 | 7 | 17 |
|---|
| Inflammation
(n=29) | 2 (6.0) | 1 (3.5) | 3 (10.4) | 1 (3.5) | 0 (0.0) |
| pTa (n=14) | 8 (57.1) | 4 (28.6) | 10 (71.4) | 10 (71.4) | 10 (71.4) |
| pT1 (n=20) | 19 (95.0) | 10 (50.0) | 14 (70.0) | 19 (95.0) | 19 (95.0) |
| pT2 (n=16) | 15 (63.4) | 10 (62.5) | 15 (93.8) | 11 (68.8) | 15 (93.8) |
Relationship between the number of
abnormal cells and tumor progression
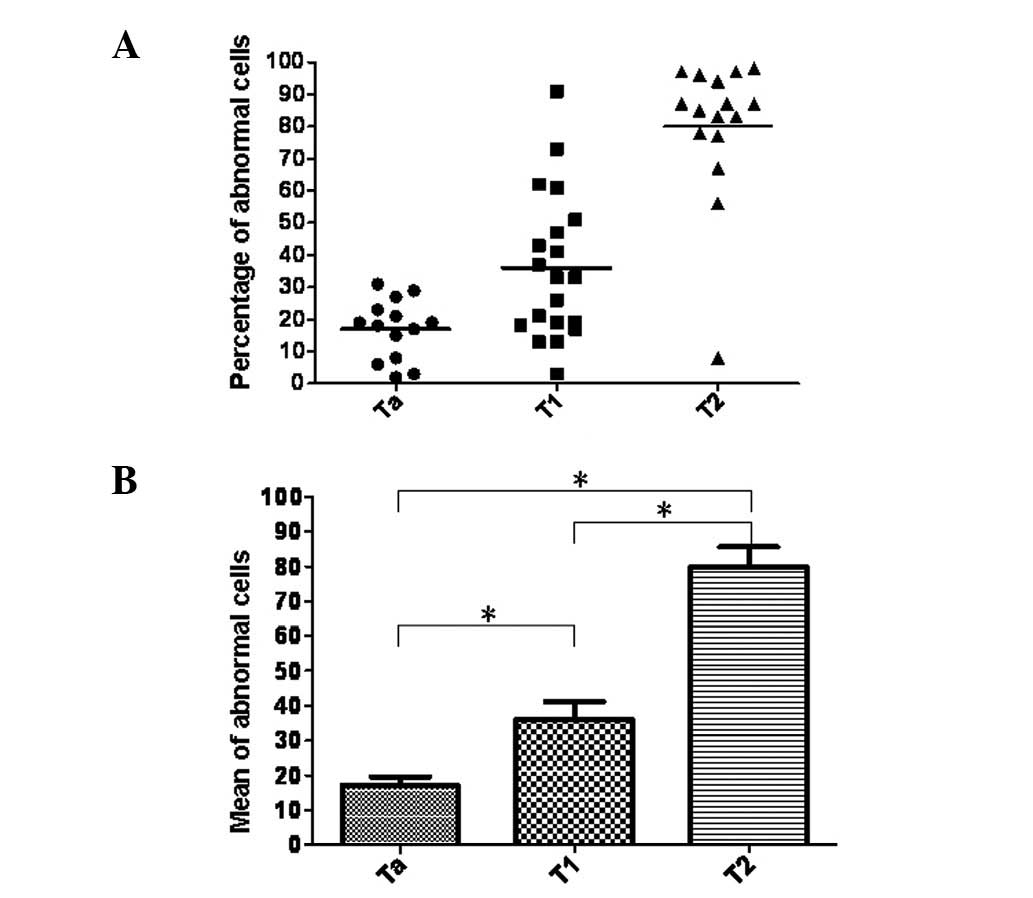
MW and K-W tests showed a significant correlation
between tumor progression and the number of abnormal cells with
cytogenetic alterations. Namely, the number of abnormal cells
defined by FISH testing was significantly higher in T1 and T2
(invasive) bladder carcinoma cases than in the superficial,
noninvasive Ta stage bladder carcinomas (P<0.05). In addition, a
significant difference was found between the number of abnormal
cells in muscle-invasive and non-muscle-invasive bladder cancer
(P<0.01) (Fig. 2). Furthermore,
there was a positive correlation between the frequency of abnormal
cells and the tumor grade and a significant difference was
indicated by the MW test comparing low grade and high grade tumors
(P<0.01) (Fig. 3).
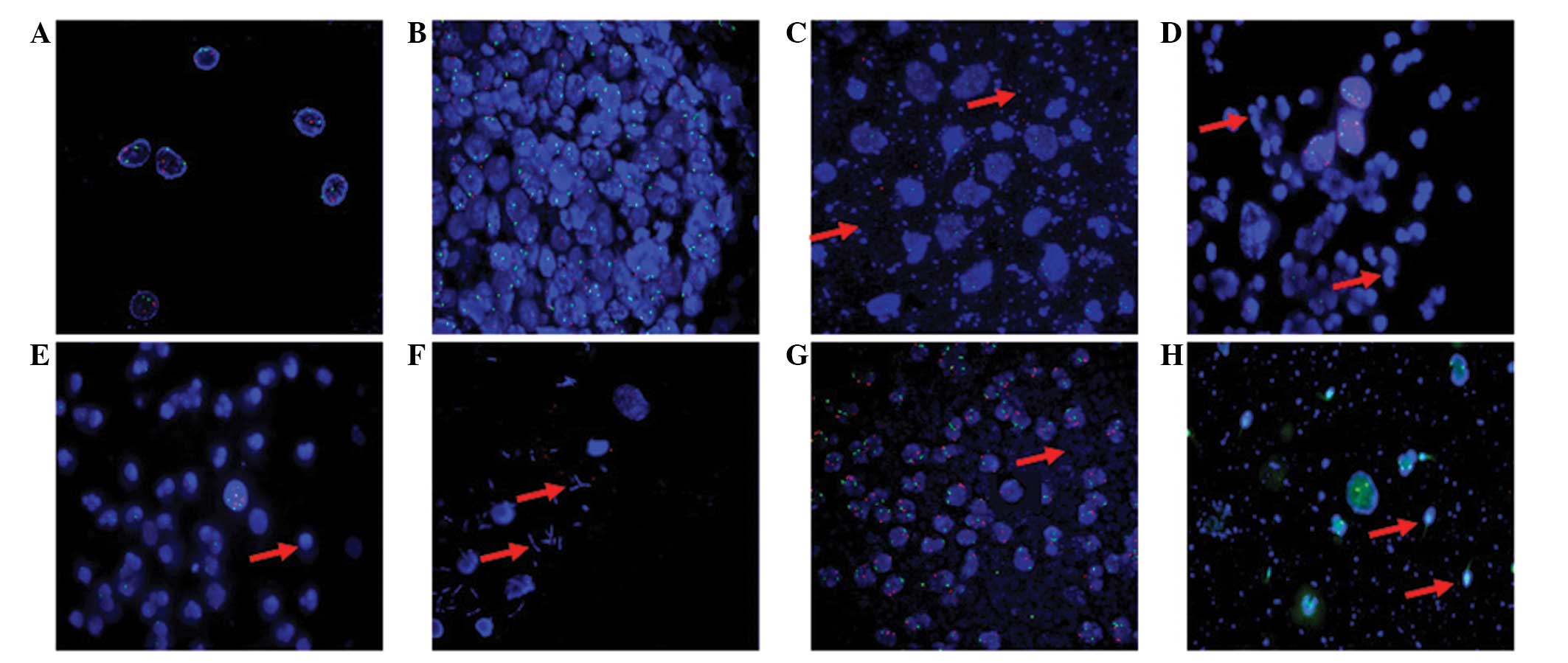
Various factors affecting FISH
evaluation
FISH evaluation was affected by various factors,
particularly by specific characteristics of the urine sample.
Fig. 4A shows an optimal cytospin
specimen in which the cells are evenly spread and the contours of
the cell nucleus are extremely clear. However, in Fig. 4B, the high cell density makes it
difficult to discern cell contours, which affect the positive rate
of FISH. Occasionally, a high proportion of mucus also leads to the
failure of pepsin function so that the probes for chromosomes 3, 7
17 and 9p21 fail to hybridize with the corresponding complementary
target sequences (Fig 4C). In
patients with severe inflammation, numerous lymphocytes and
neutrophils may cover the epithelial cells, resulting in errors in
signal dot counting (Fig. 4D and
E). Similarly, bacilli, cocci and sperm may make the background
too concentrated, which can affect the appearance of the green
signal (Figs. 4F–H).
Discussion
Bladder cancer is one of the most common
malignancies and represents the 13th most common cause of all
cancer mortalities worldwide (14).
The majority of patients with bladder cancer produce hematuria
whilst the other 10% exhibit asymptomatic microscopic hematuria,
which is positive for the morphological abnormality of urine
cytology (15). Thus, the main
method for diagnosis of bladder cancer is a combination of
cystoscopy, biopsy and voided urine cytology. Although urinary
cytology is non-invasive and highly sensitive for cases of
differentiated tumors, it also has disadvantages, for example being
examination-dependent (16).
Furthermore, cystoscopy is an invasive diagnostic intervention
accompanied by discomfort and potential complications. Therefore,
urine-based marker systems are important for the diagnosis and
treatment of bladder cancer.
The FISH technique was approved by the FDA for the
detection of urothelial carcinomas in July 2001 (17). Following this, a number of studies
have shown promising results for FISH technology (18,19).
In a comparative study of FISH with the diagnostic markers, BTA
Stat, hemoglobin strips and telomerase, FISH exhibited the highest
sensitivity (81% compared with 78, 74 and 46% for BTA Stat,
hemoglobin strips and telomerase, respectively) and the highest
specificity (96%) (17). The
present study found the specificity of the method to be 100% and
the sensitivity to be 87%. It was noted that 93.8% of the invasive
bladder tumors were detected (excluding the cases with FISH results
that could not be evaluated). However, FISH results misidentified
five cases with non-muscle-invasive (pTa, pT1) bladder cancer and
one case with muscle-invasive (pT2) bladder cancer. Possible
reasons for these false-negative FISH results include a low tumor
burden or tumor cells that were not readily excreted into the
urine, particularly in non-muscle-invasive stages (20). In addition, these results may be
associated with combination pattern of FISH probes, including
chromosomes 3, 7, 9 and 17, which are the most common abnormalities
found in urothelial carcinoma (21). These results showed that the FISH
assay significantly increases the sensitivity and specificity of
the diagnosis of bladder cancer, as described in previous studies
(22,23).
Waldman et al have shown that the severity of
chromosomal aberrations is closely associated with high-grade and
high-stage bladder carcinoma, aggressive behavior and a significant
reduction in the progression-free interval (24). Results of the present study indicate
that the severity of genetic alterations is significantly higher in
high-grade and high-stage carcinomas, since the frequency of
abnormal cells matching the positive criteria of FISH analysis is
positively correlated with tumor invasiveness (stages pTa-pT1 and
pT2) and histological grade (low-high). It has been suggested that
a tetraploidization process may lead to the majority of the
chromosomal aberrations detected by FISH (25). By contrast, aneusomy of chromosomes
7, 9, and 17 was hypothesized to be predictive of tumor recurrence
in a separate study (26). The
present study also showed that the positive rate for polyploidy of
chromosomes 3, 7 and 17 in invasive stages (pT1 and pT2) was
significantly higher than that in the superficial stage (pTa). This
indicated that the existence of multiple cytogenetic changes was
correspondingly associated with the aggression of bladder
cancer.
During FISH analysis in the present study, the final
diagnostic evaluation was influenced by various factors,
particularly characteristics of the urine sample. These included
the chemical features of the urine, neutrophil or lymphocyte
infiltration, bacteriuria, sperm and the time point of urination.
If excessive grume is found in the urine, the assimilation time of
pepsin can be extended. In addition, increasing the washing
frequency with 0.2X SSC may clear away adhesive neutrophils and
lymphocytes which make evaluation extremely difficult through their
association with epithelial cells. In general, instructing patients
with bacteriuria to take antibacterials three days before FISH is
important as bacteria may adhere to the epithelial cells and make
correct detection impossible, particularly in 9p21 signals.
Furthermore, the experience of the doctor may also be critical. It
is important not to regard the multiple signals of overlapping cell
as a chromosome multiplication. To avoid this, it is imperative
that the channel of the nuclear staining is always carefully
examined using DAPI.
At present, there is no effective method to replace
cystoscopy, however, FISH may represent an additional diagnostic
tool providing help in unclear cases. Furthermore, FISH has
advantages over urine cytology in detecting bladder cancer, for
example a higher specificity and sensitivity, which may play a
vital role in the initial diagnosis and treatment of patients with
bladder cancer.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 30900650/H1615,
81172232/H1615 and 81172564/H1625), Fund for the Precep-torial
Program of Higher Education (no. 20090171120070), Guangdong
Province Technology Project Foundation (nos. S2012010008378 and
2011B031800104) and National High-tech R&D Program of China
(863 Program; no. 2012AA02A603).
References
|
1
|
Chamie K and Litwin MS: Quality of bladder
cancer care in the USA. Expert Rev Pharmacoecon Outcomes Res.
11:619–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bellmunt J, Orsola A, Wiegel T, Guix M, De
Santis M, Kataja V, et al; ESMO Guidelines Working Group. Bladder
cancer: ESMO clinical practice guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 22(Suppl 6): vi45–vi49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alameddine M and Nassir A: The influence
of urine cytology on our practice. Urol Ann. 4:80–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mian C, Lodde M, Comploj E, Negri G,
Egarter-Vigl E, Lusuardi L, et al: Liquid-based cytology as a tool
for the performance of uCyt+ and Urovysion
Multicolour-FISH in the detection of urothelial carcinoma.
Cytopathology. 14:338–342. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dahmoush L and Cohen MB: FISHing and
beyond in urinary cytology. Diagn Cytopathol. 31:2012004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lapray JF, Costa P, Delmas V and Haab F:
The role of ultrasound in the exploration of pelvic floor
disorders. Prog Urol. 19:947–952. 2009.(In French).
|
|
7
|
Mazo EB, Gazhonova VE and Chepurov DA:
Three-dimentional echography in diagnosis and staging of urinary
bladder cancer. Urologiia. May–Jun;6–12. 2005.(In Russian).
|
|
8
|
Hermann GG, Mogensen K, Toft BG, Glenthøj
A and Pedersen HM: Outpatient diagnostic of bladder tumours in
flexible cystoscopes: evaluation of fluorescence-guided flexible
cystoscopy and bladder biopsies. Scand J Urol Nephrol. 46:31–36.
2012. View Article : Google Scholar
|
|
9
|
Panani AD, Kozirakis D, Anastasiou J,
Babanaraki A, Malovrouvas D and Roussos C: Is aneusomy of
chromosome 9 alone a valid biomarker for urinary bladder cancer
screening? Anticancer Res. 26:1161–1165. 2006.
|
|
10
|
Leonardo C, Merola R, Orlandi G, Leonardo
F, Rondoni M and De Nunzio C: C-erb-2 gene amplification and
chromosomal anomalies in bladder cancer: preliminary results. J Exp
Clin Cancer Res. 24:633–638. 2005.PubMed/NCBI
|
|
11
|
Caraway NP, Khanna A, Fernandez RL, Payne
L, Bassett RL Jr, Zhang HZ, et al: Fluorescence in situ
hybridization for detecting urothelial carcinoma: a
clinicopathologic study. Cancer Cytopathol. 118:259–268. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chuang KL, Chuang HC, Ng KF, Chang YH, Wu
CT, Chuang CK, et al: Urinary fluorescence in situ hybridization
assay for detecting urothelial carcinoma in Taiwanese patients. BJU
Int. 105:1413–1416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reid-Nicholson MD, Ramalingam P, Adeagbo
B, Cheng N, Peiper SC and Terris MK: The use of Urovysion
fluorescence in situ hybridization in the diagnosis and
surveillance of non-urothelial carcinoma of the bladder. Mod
Pathol. 22:119–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Madeb R, Golijanin D, Knopf J, Davis M,
Feng C, Fender A, et al: Long-term outcome of patients with a
negative work-up for asymptomatic microhematuria. Urology.
75:20–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Glas AS, Roos D, Deutekom M, Zwinderman
AH, Bossuyt PM and Kurth KH: Tumor markers in the diagnosis of
primary bladder cancer. A systematic review. J Urol. 169:1975–1982.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Halling KC, King W, Sokolova IA, Karnes
RJ, Meyer RG, Powell EL, et al: A comparison of BTA stat,
hemoglobin dipstick, telomerase and Vysis UroVysion assays for the
detection of urothelial carcinoma in urine. J Urol. 167:2001–2006.
2002. View Article : Google Scholar
|
|
18
|
Galván AB, Salido M, Espinet B, Placer J,
Pijuan L, Juanpere N, et al: A multicolor fluorescence in
situ hybridization assay: a monitoring tool in the surveillance
of patients with a history of non-muscle-invasive urothelial cell
carcinoma: A prospective study. Cancer Cytopathol. 119:395–403.
2011.
|
|
19
|
Fritsche HM, Burger M, Dietmaier W,
Denzinger S, Bach E, Otto W, et al: Multicolor FISH (UroVysion)
facilitates follow-up of patients with high-grade urothelial
carcinoma of the bladder. Am J Clin Pathol. 134:597–603. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laudadio J, Keane TE, Reeves HM, Savage
SJ, Hoda RS, Lage JM and Wolff DJ: Fluorescence in situ
hybridization for detecting transitional cell carcinoma:
implications for clinical practice. BJU Int. 96:1280–1285.
2005.
|
|
21
|
Dumache R, David D, Kaycsa A, Minciu R,
Negru S and Puiu M: Genetic and epigenetic biomarkers for early
detection, therapeutic effectiveness and relapse monitoring in
bladder cancer. Rev Med Chir Soc Med Nat Iasi. 115:163–167.
2011.
|
|
22
|
Song MJ, Lee HM and Kim SH: Clinical
usefulness of fluorescence in situ hybridization for diagnosis and
surveillance of bladder cancer. Cancer Genet Cytogenet.
198:144–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwak KW, Kim SH and Lee HM: The utility of
fluorescence in situ hybridization for detection of bladder
urothelial carcinoma in routine clinical practice. J Korean Med
Sci. 24:1139–1144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Waldman FM, Carroll PR, Kerschmann R,
Cohen MB, Field FG and Mayall BH: Centromeric copy number of
chromosome 7 is strongly correlated with tumor grade and labeling
index in human bladder cancer. Cancer Res. 51:3807–3813. 1991.
|
|
25
|
Sauter G, Simon R, Bubendorf L and
Mihatsch M: Molecular genetics of urinary bladder cancer
progression. Verh Dtsch Ges Pathol. 86:49–56. 2002.(In German).
|
|
26
|
Horstmann M, Patschan O, Hennenlotter J,
Senger E, Feil G and Stenzl A: Combinations of urine-based tumour
markers in bladder cancer surveillance. Scand J Urol Nephrol.
43:461–466. 2009. View Article : Google Scholar : PubMed/NCBI
|