Introduction
Invasive fungal infections are leading causes of
mortality and morbidity in patients with hematological malignancies
and prolonged neutropenia following chemotherapy (1,2). In
general, fluconazole is used as a prophylactic antifungal treatment
for patients with hematological malignancies who are predicted to
exhibit prolonged neutropenia following aggressive chemotherapy.
Thus, mold infections become more common in patients with
fluconazole prophylaxis and Fusarium is the second most
common cause of mold infection. The incidence of Fusarium
spp. infections in patients with acute leukemia in Europe is
0.06% (3) and was 1.2% among 750
allogeneic and 0.2% among 1,537 autologous marrow transplant
recipients in the United States (4). Disseminated fusariosis accounts for
70% of Fusarium infections in immunocompromised patients,
particularly in patients with acute leukemia with prolonged and
profound neutropenia, and patients undergoing hematopoietic stem
cell transplantation (5).
Case report
A 20-year-old male was diagnosed with acute
lymphoblastic leukemia, precursor B cell type and received
induction chemotherapy with TPOG-ALL-2002 VHR protocol (6) with partial remission in the induction
course. The patient did not finish the consolidation course due to
prolonged neutropenia. Later, the patient relapsed and received
chemotherapy with FLAG-IDA (idarubicin, fludarabine, cytarabine and
G-CSF) (7). The patient exhibited
prolonged febrile neutropenia for more than one month during the
initial course of chemotherapy, but exhibited rapid relapse again,
soon following neutropenia recovery. In the second course of
treatment, the patient received 400 mg oral fluconazole daily as an
antifungal prophylaxis. The febrile neutropenia was found two days
later. Antibiotic treatment with imipenem/cilastatin (500 mg, i.v.,
every 6 h), vancomycin (1,000 mg, i.v., every 12 h) and micafungin
(100 mg, i.v., daily) was then administrated. After four days,
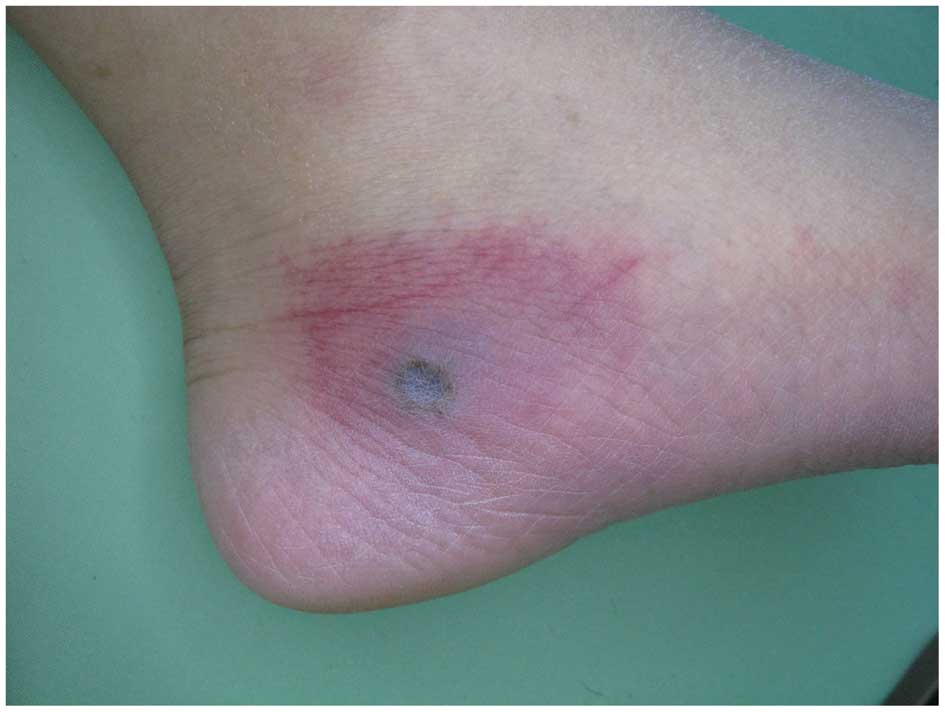
multiple skin lesions, starting from the legs and spreading to the
face and upper extremities were identified. The lesions exhibited
necrotic centers surrounded by spreading erythema (Fig. 1). The lesions worsened and the
antifungal treatment was replaced with caspofungin (100 mg, i.v.,
daily). A biopsy of the skin lesions showed the presence of hyphae
occupying the vascular space. The Gomori methanamine silver and
periodic acid-Schiff stains were positive. The histopathological
diagnosis was angioinvasive aspergillosis and treatment with
voriconazole (200 mg, i.v., every 12 h) was initiated in addition
to caspofungin with poor response. The amphotericin B was not
administered due to ethical issues (the patient and family refused
further aggressive treatment owing to refractory acute
lymphoblastic leukemia with poor therapeutic response). A
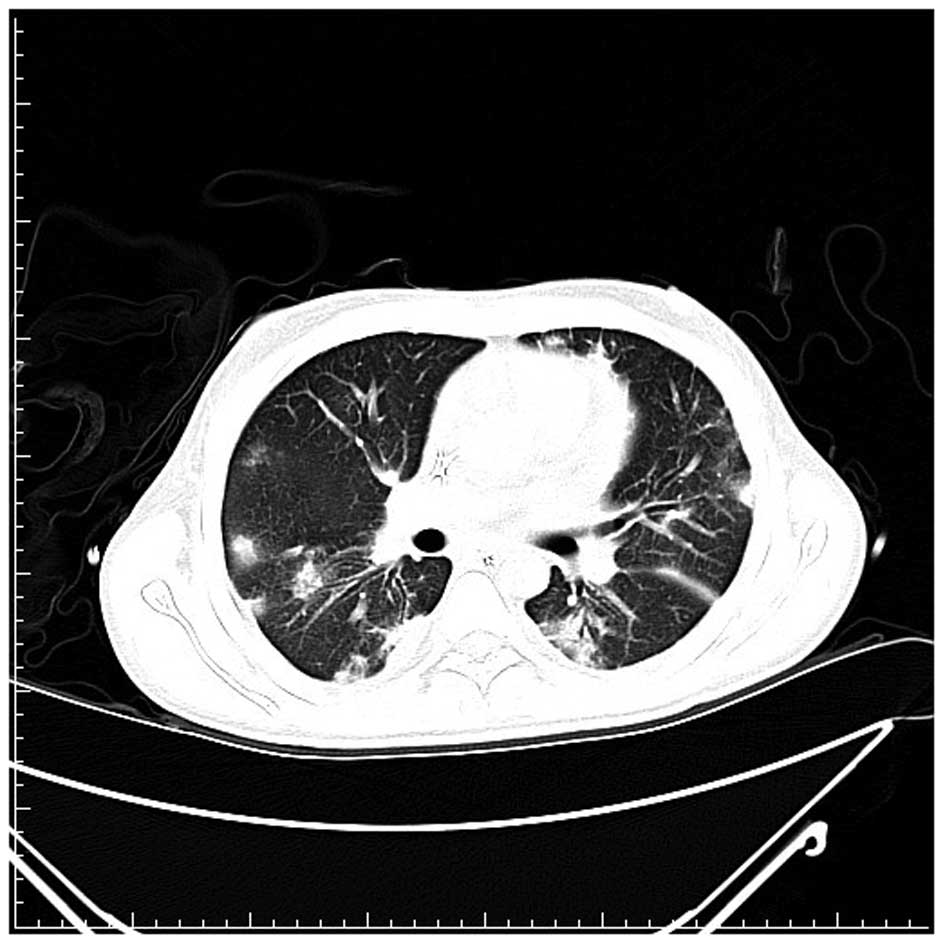
high-resolution computed tomography scan of the lungs found
multiple speculated and round consolidative densities in the lungs
(Fig. 2). After three weeks, the
sputum and skin tissue cultures finally demonstrated Fusarium
spp. colonization (Fig. 3). The
patient succumbed to the disease two months later. Written consent
was provided from the patient’s family.
Discussion
Fusarium is widely distributed in soil,
plants and air and is common in tropical and temperate regions.
This pathogen can cause a broad spectrum of human diseases, which
may be locally invasive or disseminated. In immunocompromised
patients, particularly in high-risk patients (those with prolonged
and profound neutropenia), Fusarium infection is typically
invasive and disseminated (5). In a
previous study of invasive fusariosis in 84 patients with
hematological malignancies, patients with acute leukemia occurred
most frequently (56%) and the majority of patients (83%) were
neutropenic at diagnosis (8). The
incidence of Fusarium spp. infection in patients with acute
leukemia in Europe is 0.06% (3).
Previously, in a 10-year follow-up study from a US institution, the
incidence of Fusarium infection was 1.2% among 750
allogeneic and 0.2% among 1,537 autologous marrow transplant
recipients (4).
Fusarium infection in immunocompromised
patients is airborne or inoculated through the breakdown of the
skin barrier. Skin lesions may involve any skin sites, with
predominance in the extremities. Fusarium infection presents
more frequently as metastatic skin lesions with initial
presentation of subcutaneous lesions, to erythematous indurations,
followed by target-like central necrotic lesions (4). The lesions are evoked rapidly within
one to five days, at various stages of evolution and occasionally
with myalgias (9). Skin biopsies
are extremely easy to perform and confirm clinical suspicion.
However, the histopathological images of skin lesions resemble
those of Aspergillus infections. The two infections exhibit
vascular invasion and branching septate hyphae (4); therefore, identification from
Aspergillus may be difficult.
As with the case of Aspergillus, the
radiological observations of pulmonary Fusarium infection
are non-specific, including non-specific infiltrates to nodular
and/or cavity formation, depending on the timing of the examination
(9). The prognosis is considerably
worse in patient pulmonary infiltrates (10).
The definite diagnosis of Fusarium infection
requires Fusarium spp. isolation from blood and tissue
culture (such as the skin, lungs and sinuses). However, the
Fusarium species may contaminate laboratory specimens and
pseudo-outbreaks of fusariosis may occur (11). Thus, the clinician must be cautious
of interpretation. Unlike Aspergillus, the Fusarium
species may be isolated from cultures of blood samples in 40% of
cases. With the presence of disseminated skin lesions, the presence
of the fungus in blood cultures may increase to 56% (8). In the majority of cases, fungemia is
likely to develop in a median of five days (range, one to 10 days)
following the appearance of skin lesions (9). However, in the present case, the blood
culture did not yield Fusarium species despite disseminated
skin lesions.
For patients with persistently febrile neutropenia,
empiric treatment with caspofungin is initially recommended and
liposomal amphotericin B is an alternative choice (12). There is no treatment guideline for
Fusarium infection. Nucci et al(8) suggested high-dose amphotericin B or
lipid formation of amphotericin B since specific Fusarium
species may be resistant to azoles. The greatest challenge to
clinicians is the early diagnosis of the Fusarium infection
and preemptive treatment, since the histopathology of
Fusarium may be confused with Aspergillus and the
galactomannan test may not be useful for distinguishing
Fusarium from Aspergillus(13). Despite aggressive treatment, the
survival rate of the Fusarium infection in patients with
persistent neutropenia is only 4% (8). Early granulocyte recovery is
significantly associated with favorable response and survival and
growth factors (G-CSF or GM-CSF) or granulocyte transfusions, which
may shorten the neutropenic period to aid immunity recovery
(15). Combination antifungal
therapy is well-tolerated with acceptable minor toxicity (14,15)
and, theoretically, may have benefits to stabilizing the infection
and preventing fatal progression. However, such data are limited in
hematological patients with Fusarium infection. In a
previous report of immunocompromised patients with disseminated
Fusarium infection, 70% of patients (14 cases) exhibited a
positive response to combination antifungal therapy (16). In one multicenter retrospective
study of 61 hematological patients with confirmed or predicted
invasive mold infections, comparing liposomal amphotericin B plus
caspofungin, liposomal amphotericin B plus a triazole and
voriconazole plus one echinocandin drug, no statistical differences
were identified among these groups at the end of treatment and
12-week survival (15). In the
current case, the patient was recommended amphotericin B plus
caspofungin or amphotericin B plus voriconazole treatment in
combination; however, the patient and family refused due to ethical
issues. The optimal treatment for patients with Fusarium
infection requires further investigation and well-designed clinical
trails to address this issue.
References
|
1
|
Pagano L, Caira M, Candoni A, et al: The
epidemiology of fungal infections in patients with hematologic
malignancies: the SEIFEM-2004 study. Haematologica. 91:1068–1075.
2006.PubMed/NCBI
|
|
2
|
Böhme A, Ruhnke M, Buchheidt D, et al:
Treatment of invasive fungal infections in cancer patients -
recommendations of the Infectious Diseases Working Party (AGIHO) of
the German Society of Hematology and Oncology (DGHO). Ann Hematol.
88:97–110. 2009.
|
|
3
|
Girmenia C, Pagano L, Corvatta L, et al:
The epidemiology of fusariosis in patients with haematological
diseases. Gimema Infection Programme. Br J Haematol. 111:272–276.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boutati EI and Anaissie EJ:
Fusarium, a significant emerging pathogen in patients with
hematologic malignancy: ten years’ experience at a cancer center
and implications for management. Blood. 90:999–1008.
1997.PubMed/NCBI
|
|
5
|
Nucci M and Anaissie E: Fusarium
infections in immunocompromised patients. Clin Microbiol Rev.
20:695–704. 2007. View Article : Google Scholar
|
|
6
|
Liang DC, Yang CP, Lin DT, et al:
Long-term results of Taiwan Pediatric Oncology Group studies 1997
and 2002 for childhood acute lymphoblastic leukemia. Leukemia.
24:397–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fleischhack G, Hasan C, Graf N, Mann G and
Bode U: IDA-FLAG (idarubicin, fludarabine, cytarabine, G-CSF), an
effective remission-induction therapy for poor-prognosis AML of
childhood prior to allogeneic or autologous bone marrow
transplantation: experiences of a phase II trial. Br J Haematol.
102:647–655. 1998. View Article : Google Scholar
|
|
8
|
Nucci M, Anaissie EJ, Queiroz-Telles F, et
al: Outcome predictors of 84 patients with hematologic malignancies
and Fusarium infection. Cancer. 98:315–319. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dignani MC and Anaissie EJ: Human
fusariosis. Clin Microbiol Infect. 10(Suppl 1): 67–75. 2004.
View Article : Google Scholar
|
|
10
|
Chaoui D, Legrand O, Roche N, et al:
Incidence and prognostic value of respiratory events in acute
leukemia. Leukemia. 18:670–675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grigis A, Farina C, Symoens F, Nolard N
and Goglio A: Nosocomial pseudo-outbreak of Fusarium
verticillioides associated with sterile plastic containers. Infect
Control Hosp Epidemiol. 21:50–52. 2000.
|
|
12
|
Rüping MJ, Vehreschild JJ and Cornely OA:
Antifungal treatment strategies in high risk patients. Mycoses.
51(Suppl 2): 46–51. 2008.
|
|
13
|
Tortorano AM, Esposto MC, Prigitano A,
Grancini A, Ossi C, Cavanna C and Cascio GL: Cross-reactivity of
Fusarium spp. in the Aspergillus Galactomannan
enzyme-linked immunosorbent assay. J Clin Microbiol. 50:1051–1053.
2012.
|
|
14
|
Cetkovsky P, Kouba M, Markova M, et al:
Combination of voriconazole and anidulafungin as primary therapy in
hematologic patients. Biol Blood Marrow Transplant. 16:S2642010.
View Article : Google Scholar
|
|
15
|
Rojas R, Molina JR, Jarque I, et al:
Outcome of antifungal combination therapy for invasive mold
infections in hematological patients is independent for chosen
combination. Mediterr J Hematol Infect Dis. 4:e20120112012.
View Article : Google Scholar
|
|
16
|
Liu JY, Chen WT, Ko BS, et al: Combination
antifungal therapy for disseminated fusariosis in immunocompromised
patients: a case report and literature review. Med Mycol.
49:872–878. 2011.PubMed/NCBI
|