Introduction
Ovarian cancer is a heterogeneous and rapidly
progressive disease of low prevalence and poor survival. It is one
of the major causes of cancer-related mortality in women. In 2011,
there were 4,705 deaths per 100,000 women from ovarian cancer in
Japan (1). Poor nutritional status
is common in ovarian cancer, and is a well-known variable that
affects cancer treatment and outcomes (2). In advanced stages, it is associated
with cachexia and ascites from malnutrition. The nutritional status
of a patient with malignant disease is known to be associated with
survival, and can be assessed by serum protein levels (2). The assessment of nutritional status is
essential for a diagnosis of nutritional compromise, and
measurements of serum concentrations of rapid turnover proteins
(RTPs) such as retinol binding protein (RBP), prealbumin (PA) and
transferrin (TF) have been reported to be more accurate for
assessment than albumin (3–7).
There is increasing evidence that a systemic
inflammatory response is of prognostic value in patients with
various types of cancer. An elevated serum C-reactive protein (CRP)
concentration is associated with a poor prognosis in colorectal,
breast and ovarian cancer. Hypoalbuminemia, which is often
associated with elevated CRP levels, has been reported to be a good
predictor of poor prognosis in many types of cancer (8–10).
Systemic inflammation occurs by various mechanisms involving
numerous pro-inflammatory cytokines and other soluble protein
mediators. Among them, soluble interleukin (IL)-2 receptor (sIL-2R)
is part of a membrane receptor for IL-2, which can be localized on
the surface of various lymphoid cells, including activated T cells,
natural killer cells, monocytes, eosinophils and certain tumor
cells. We have previously reported that increased production of
sIL-2R is correlated with an inhibition of cell-mediated immunity,
as well as with systemic inflammation and nutritional impairment,
and it may be involved in immunological mechanisms that induce
cancer cachexia (11,12). IL-17 is believed to stimulate
various cell types to produce proinflammatory mediators that
amplify intestinal inflammation, such as in inflammatory bowel
diseases, or rheumatoid arthritis (13–16).
The production of IL-17 has recently been reported to be associated
with systemic inflammation, immune suppression and hypoalbuminemia
in patients with gastrointestinal cancer (17). In ovarian cancer, we have reported
that serum levels of vascular endothelial growth factor (VEGF), a
glycosylated angiogenesis mediator, are elevated and correlated
with malnutrition and inflammation (18).
Tumor progression has been reported to be frequently
associated with systemic inflammation, and decreased serum albumin
levels can be observed in this clinical scenario. In the present
study, the correlation between decreased levels of RTP (including
RBP, PA and TF, and CRP) and other inflammation-related proteins
(such as sIL-2R, IL-17 and VEGF) was examined.
Materials and methods
Sample collection
Blood samples were collected from 41 patients with
ovarian cancer. The patient group included four patients with stage
I disease, two with stage II disease, 13 with stage III disease and
22 with stage IV disease. The enrolled patients had undergone
surgery or chemotherapy at the Department of Obstetrics and
Gynecology at Fukushima Medical University Hospital between May
2011 and February 2013. The patients were between 38 and 84 years
old (median, 59.2 years) and newly diagnosed, with histological
confirmation of the diagnosis. Blood samples were collected prior
to initiation of any treatment. Peripheral blood mononuclear cells
(PBMCs) were separated over Ficoll-Hypaque (Pharmacia-Biotech,
Uppsala, Sweden). The isolated PBMCs were washed twice with
RPMI-1640 (Wako Pure Chemical Industries Ltd., Osaka, Japan). This
study was approved by the ethics committee of Fukushima Medical
University (No. 1095), and written informed consent was obtained
from all patients and healthy donors.
Cytokine production assay
To measure the production of IL-17 by PBMCs, 20 ml
of heparinized blood was drawn, and PBMCs were separated by a
Ficoll-density gradient centrifugation procedure. A total of
106 PBMCs were cultured in 1 ml of RPMI-1640 medium
containing 10% heat-inactivated fetal calf serum (Gibco-BRL, St.
Louis, MO, USA) and 100 μg/ml phytohemagglutinin (Sigma, Rockville,
MD, USA) for 24 h under 5% CO2 at 37°C. After
cultivation, the aliquots of the supernatant were frozen and stored
at −80°C until use. The samples were then thawed and used to
measure the concentrations of IL-17 by enzyme-linked immunosorbent
assay (ELISA; Quantikine test kit; R&D Systems, Minneapolis,
MN, USA). Test samples were used only once after thawing.
Markers for nutritional status and
chronic inflammation
In order to evaluate the nutritional condition of
the patients, serum concentrations of RBP (latex agglutination
immunoassay), PA (turbidimetric immunoassay) and TF (turbidimetric
immunoassay) were measured. Routine hematologic investigation
included a hemogram and measurement of CRP levels. Counts of
neutrophils and lymphocytes, and their ratio (neutrophil/lymphocyte
ratio, NLR), in the peripheral blood of patients were used as
inflammation-related markers.
Measurements of sIL-2R and VEGF
Serum concentrations of sIL-2R and VEGF were
measured by ELISA (R&D Systems) according to the manufacturer’s
instructions.
Statistical analysis
Differences between the groups were evaluated using
Student’s t-test. Correlations between two variables were
quantified by Spearman’s rank correlation coefficient. P<0.05
was considered to indicate a statistically significant
difference.
Results
Serum concentrations of RTPs
A total of 41 samples from patients with ovarian
cancer were tested. The serum RBP concentrations in patients with
stage I, II, III and IV disease were 2.86±0.67, 3.35±0.75,
1.745±0.35 and 1.57±0.14 mg/dl, respectively, and were
significantly lower in patients with stage IV disease than in those
with stage I (P<0.01) or stage II (P<0.005) disease (Fig. 1). The serum concentrations of PA in
patients with stage I, II, III and IV disease were 24.24±4.58,
25.70±2.9, 13.49±2.57 and 11.79±1.20 mg/dl, respectively. Serum PA
concentrations were significantly lower in patients with stage IV
disease than in those with stage I or stage II (both P<0.005,
Fig. 2) disease, and patients with
stage III disease had lower concentrations than those with stage II
(P<0.05) and stage I (P<0.10) disease. The serum
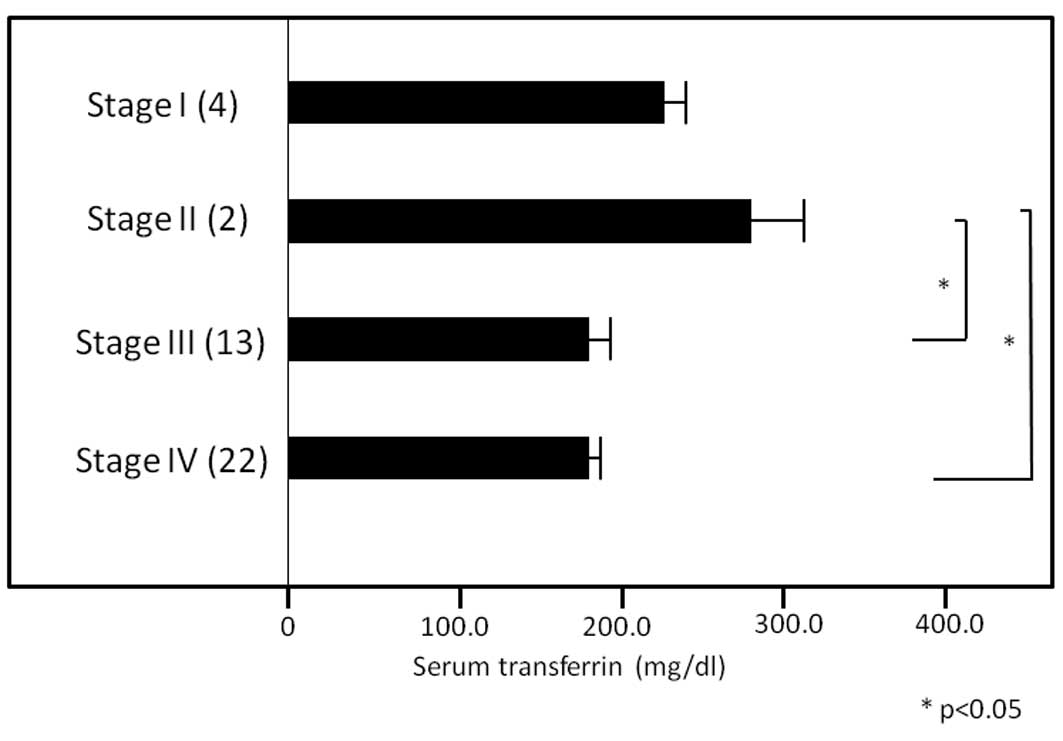
concentrations of TF in patients with stage I, II, III and IV
disease were 227.2±31.3, 286.2±27.0, 179.7±20.1 and 185.8±12.5
mg/dl, respectively, and those with stage III or stage IV disease
had lower concentrations than those with stage II disease (both
P<0.05, Fig. 3).
Correlations of CRP levels with RTP
levels and inflammation-related proteins
Table I shows the
correlations between CRP levels and serum levels of RTPs, including
RBP, PA and TF, NLR as an inflammatory marker, and
inflammation-related proteins, including VEGF, IL-17 and sIL-2R.
Significant inverse correlations were found between CRP levels and
RTP levels. NLR, VEGF levels and sIL-2R levels were significantly
correlated with CRP levels.
 | Table ICorrelations of serum levels of CRP
with rapid turnover proteins and inflammation-related proteins. |
Table I
Correlations of serum levels of CRP
with rapid turnover proteins and inflammation-related proteins.
| Coefficient
number | P-value |
|---|
| Retinol binding
protein (mg/dl) | −0.659 | 2.76E-06a |
| Prealbumin
(mg/dl) | −0.662 | 2.43E-06a |
| Transferrin
(mg/dl) | −0.758 | 9.69E-09a |
| Neutrophil/lymphocyte
ratio | 0.348 | 0.025a |
| Serum VEGF
(pg/ml) | 0.522 | 0.00047a |
| Production of
interleukin-17 (pg/ml) | −0.225 | 0.155 |
| Serum soluble
interleukin-2 receptor (pg/ml) | 0.764 | 5.97E-09a |
Correlations of serum levels of RTPs with
inflammation-related factors
Table II shows the
correlations between RBP, PA and TF levels, and NLR, IL-17
production, and serum VEGF and sIL-2R levels. The serum RBP
concentrations showed significant inverse correlations with NLR and
sIL-2R levels. PA levels were significantly and inversely
correlated with NLR and sIL-2R levels, and tended to be similarly
correlated with serum VEGF concentrations and IL-17 production. TF
levels were significantly and inversely correlated with all factors
tested in this study, including NLR, IL-17 production, and VEGF and
sIL-2R concentrations.
 | Table IICorrelation of rapid turnover protein
with the neutrophil/lymphocyte ratio and inflammation-related
proteins. |
Table II
Correlation of rapid turnover protein
with the neutrophil/lymphocyte ratio and inflammation-related
proteins.
| Retinol binding
protein (mg/dl) | Prealbumin
(mg/dl) | Transferrin
(mg/dl) |
|---|
| Neutrophil/lymphocyte
ratio |
−0.4340/0.0030a |
−0.4490/0.0019a |
−0.4700/0.0011a |
| Serum VEGF
(pg/ml) | −0.1130/0.4570 | −0.2500/0.0930 |
−0.3080/0.0370a |
| Production of
interleukin-17 (pg/ml) | −0.0800/0.5630 | −0.2730/0.0650 |
−0.5190/0.0002a |
| Serum soluble
interleukin-2 receptor (pg/ml) |
−0.3620/0.0140a |
−0.3940/0.0060a |
−0.4290/0.0029a |
Discussion
Measurement of RTPs is essential in order to
accurately assess nutritional status (3,4). Among
these proteins, RBP, PA and TF are biologically stable and easy to
measure (3,4). Cancer growth and development are
associated with stimulation of the immune system, including
enhanced IL-2R expression in immune cells and subsequent shedding
into the circulation. A number of studies have demonstrated
critical connections between clinical symptoms, survival and
markers of inflammation (8–11). This study demonstrated that the
circulating RTP levels were decreased in advanced stages of ovarian
cancer, and that there were significant inverse correlations
between RTP levels and serum CRP and NLR levels. CRP levels were
also correlated with serum levels of VEGF and sIL-2R, which have
been reported to be closely associated with immunosuppression and
inflammation. Moreover, NLR, VEGF and sIL-2R levels, and IL-17
production, were all inversely correlated with RTP levels.
Although a causal relationship between inflammation
and the innate immunity of cancer is more widely accepted today
than it has been in the past, many of the precise cellular
mechanisms mediating this relationship remain unclear. Increased
neutrophils and decreased lymphocytes are occasionally observed in
patients with advanced cancer, and NLR has been used as one of the
easiest and most effective markers of chronic inflammation and
related immune suppression in these patients (8,9).
Malignant diseases have been found to be associated with impairment
of T-cell-mediated immunity, and sIL-2R, reported to be produced
primarily by lymphoid cells, appears to be crucial in this process
(11). We recently noted that
sIL-2R appears to be an inhibitory marker of cell-mediated immunity
and nutrition (12). It was
previously proposed that tumor growth and metastasis depend on
angiogenesis, and blockade of angiogenesis may thus provide one
strategy for inhibiting tumor growth (19). VEGF has been reported to be
important in the progression of malignant neoplasms, and to induce
the activity of myeloid-derived suppressor cells that appear in
cancer and inflammation (18).
Elevated VEGF levels are reportedly associated with advanced-stage
melanoma, as well as negative immune reactions, including Th2 (type
2 helper T cell) dominance and impaired dendritic cell function.
Previously reported results indicate that suppression of
cell-mediated immune reactions is closely related with nutritional
status, and that this appears to be involved in the development of
cancer cachexia (20,21). It has recently been reported that
IL-17 is important in the pathogenesis of inflammatory bowel
diseases, including Crohn’s disease and ulcerative colitis. In
human cancer, chronic inflammation involving IL-17 is believed to
be important in the development of disease-advancement indicators,
such as immune suppression or cachexia (17).
It appears that chronic inflammation may be
associated with compromised immune function, such as an impaired
T-cell response, via various inflammatory proteins including
sIL-2R, VEGF and IL-17. It has been reported that the key
mechanisms leading to cancer cachexia, in which nutritional
impairment is a major clinical issue, are mostly immune reactions
caused by chronic inflammation, and that treatment with a
cyclooxygenase-2 inhibitor, or a specific nutrient formula, is
effective (22,23). Further studies are warranted to
explore this possibility and to increase the understanding of this
field of medicine.
Acknowledgements
The authors wish to thank Professor Hitoshi Ohto,
Department of Blood Transfusion and Transplantation Immunology,
Fukushima Medical University, for the excellent immunological
suggestions and Mrs. Hideko Taguchi, Department of Organ Regulatory
Surgery, Fukushima Medical University, for the management of the
experimental equipment and funding.
References
|
1
|
Ministry of Health, Labour and Welfare.
Annual Health, Labour and Welfare Report 2011–2012. http://www.mhlw.go.jp/toukei/.
Accessed September 6, 2012
|
|
2
|
Asher V, Lee J and Bali A: Preoperative
serum albumin is an independent prognostic predictor of survival in
ovarian cancer. Med Oncol. 29:2005–2009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blackburn GL, Bistrian BR, Maini BS,
Schlamm HT and Smith MF: Nutritional and metabolic assessment of
the hospitalized patient. J Parenter Enteral Nutr. 1:11–22. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inoue Y, Nezu R, Matsuda H, Takagi Y and
Okada A: Rapid turnover proteins as a prognostic indicator in
cancer patients. Surg Today. 25:498–506. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valandingham S, Spiekerman AM and Newmark
SR: Prealbumin: a parameter of visceral protein levels during
albumin infusion. J Parenter Enteral Nutr. 6:230–231. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Delpeuch F, Cornu A and Chevalier P: The
effect of iron-deficiency anaemia on two indices of nutritional
status, prealbumin and transferrin. Br J Nutr. 43:375–379. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mears E: Outcome of continuous process
improvement of a nutritional care program incorporating serum
prealbumin measurements. Nutrition. 12:479–484. 1996. View Article : Google Scholar
|
|
8
|
McMillan DC, Canna K and McArdle CS:
Systemic inflammatory response predicts survival following curative
resection of colorectal cancer. Br J Surg. 90:215–219. 2003.
View Article : Google Scholar
|
|
9
|
Pierce BL, Ballard-Barbash R, Bernstein L,
Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland
FD, Sorensen BE, McTiernan A and Ulrich CM: Elevated biomarkers of
inflammation are associated with reduced survival among breast
cancer patients. J Clin Oncol. 27:3437–3444. 2009. View Article : Google Scholar
|
|
10
|
Kodama J, Miyagi Y, Seki N, Tokumo K,
Yoshinouchi M, Kobashi Y, Okuda H and Kudo T: Serum C-reactive
protein as a prognostic factor in patients with epithelial ovarian
cancer. Eur J Obstet Gynecol Reprod Biol. 82:107–110. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Witkowska AM: On the role of sIL-2R
measurements in rheumatoid arthritis and cancers. Mediators
Inflamm. 3:121–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gonda K, Shibata M, Shimura T, Machida T,
Suzuki S, Nakamura I, Ohki S, Sakurai K, Ohto H, Tomira R and
Takenoshita S: Serum soluble interleukin-2 receptor is increased in
malnourished and immunosuppressed patients with gastric and
colorectal cancer: Possible influence of myeloid-derived suppressor
cells. World J Oncol. 3:158–164. 2012.
|
|
13
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q and Dong C: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Z, Tato CM, Muul L, Laurence A and
O’Shea JJ: Distinct regulation of interleukin-17 in human T helper
lymphocytes. Arthritis Rheum. 56:2936–2946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Santarlasci V, Maggi L, Capone M, Frosali
F, Querci V, De Palma R, Liotta F, Cosmi L, Maggi E, Romagnani S
and Annunziato F: TGF-beta indirectly favors the development of
Th17 cells by inhibiting Th1 cells. Eur J Immunol. 39:207–215.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kolls JK and Lindén A: Interleukin-17
family members and inflammation. Immunity. 21:467–476. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yazawa T, Shibata M, Gonda K, Machida T,
Suzuki S, Kenjo A, Nakamura I, Tsuchiya T, Koyama Y, Sakurai K, et
al: Increased IL-17 production correlates with both
immunosuppression involving MDSC and nutritional impairment in
patients with various gastrointestinal cancers. Mol Clin Oncol.
1:675–679. 2013.
|
|
18
|
Watanabe T, Shibata M, Nishiyama H, Soeda
S, Furukawa S, Gonda K, Takenoshita S and Fujimori K: Elevated
serum levels of vascular endothelial growth factor is effective as
a marker for malnutrition and inflammation in patients with ovarian
cancer. Biomed Rep. 1:197–201. 2013.PubMed/NCBI
|
|
19
|
Goldmann E: The growth of malignant
disease in man and the lower animals, with special reference to the
vascular system. Proc R Soc Med. 1:1–13. 1908.PubMed/NCBI
|
|
20
|
Rubin H: Cancer cachexia: its correlations
and causes. Proc Natl Acad Sci USA. 100:5384–5389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deans C and Wigmore SJ: Systemic
inflammation, cachexia and prognosis in patients with cancer. Curr
Opin Clin Nutr Metab Care. 8:265–269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mantovani G, Macciò A, Madeddu C, Serpe R,
Antoni G, Massa E, Dessì M and Panzone F: Phase II nonrandomized
study of the efficacy and safety of COX-2 inhibitor celecoxib on
patients with cancer cachexia. J Mol Med (Berl). 88:85–92. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fearon KC, Von Meyenfeldt MF, Moses AG,
Van Geenen R, Roy A, Gouma DJ, Giacosa A, Van Gossum A, Bauer J,
Barber MD, et al: Effect of a protein and energy dense N-3 fatty
acid enriched oral supplement on loss of weight and lean tissue in
cancer cachexia: a randomised double blind trial. Gut.
52:1479–1486. 2003. View Article : Google Scholar
|