Introduction
S100A4 protein, a polypeptide containing 101 amino
acids with a molecular weight of 11.5 kDa, is associated with
Ca2+-dependent regulation of intra- and extracellular
activities, such as protein phosphorylation, enzyme activity and
cell motility (1–4). Previous studies into the roles of
S100A4 have predominantly focused on the invasive growth and
metastasis of cancer (5–7). The increased expression of S100A4 is
correlated with breast, colorectal and gastric carcinoma, and it
has been suggested that S100A4 may be used in the early diagnosis
and prognosis of cancer as a complementary specific biomarker
(8,9). However, problems such as preparation
complexity and cross-reactivity of available antibodies impede
further investigation into the biochemical roles and distribution
of the S100A4 protein. Therefore, the possibility of generating
whole S100A4 via recombinant techniques may be advantageous in such
applications. In the present study, the construction and expression
of a synthetic gene that encodes S100A4 within Escherichia
coli, is described. The expressed S100A4 was highly soluble and
stable, as well as functionally active. Thus, the present study
provides a foundation for further investigation and for application
of S100A4 in clinical studies.
Materials and methods
Reagents
Cloning vector pEasy-T3, vector pBV-220 and E.
coli DH5α were preserved in the Performance Medicine Laboratory
of the Institute of Health and Environmental Medicine (Tianjin,
China). A gel extraction kit, plasmid extraction kit, BamHI,
EcoRI and T4 DNA ligase were purchased from Promega
Corporation (Madison, WI, USA). Taq DNA polymerase and Pfu DNA
polymerase were obtained from Tiangen Biotech (Beijing, China). Rat
S100A4 multifunctional antibody was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Acrylamide and N,
N′-methylenebisacrylamide were purchased from GE Healthcare Life
Sciences (Pittsburgh, PA, USA). SDS, 3,3′-diaminobenzidine
tetrahydrochloride and staphylococcal protein A (SPA) sepharose
CL-4b column chromatography equipment was procured from Sigma (St.
Louis, MO, USA). All other chemicals were obtained from Shanghai
Sangon Biological Engineering Technology and Service Co., Ltd.
(Shanghai, China). Cell culture inserts were purchased from
Millipore (Billerica, MA, USA) and 24-well cell culture plates were
acquired from Corning Co., Ltd. (Corning, NY, USA). Matrigel was
purchased from BD Biosciences (Franklin Lakes, NJ, USA) and
complete and incomplete Freund’s adjuvant was obtained from Beijing
Dingguo Biotechnology Co., Ltd. (Beijing, China). The remaining
chemical reagents used in the experiments were of analytical grade.
This study was approved by the ethics committee of the Institute of
Health and Environmental Medicine (Tianjin, China).
Construction of cloning vector pEasy-T3
S100A4
The gene fragment of rat S100A4 was constructed by
overlapping polymerase chain reaction (PCR) using 12 synthetic
oligonucleotides as primers (Table
I). The primers were synthesized based on the codon preference
within E. coli. The forward primer, pbf104;
5′-GCTGAATTCATGGCGCGTCCGCTGGAAG-3′ contained a site for
EcoRI (underlined), whereas the reverse primer, pbr104;
5′-GCAGGATCCTTAATGATGGTGGTG ATGATGC-3′
contained a site for BamHI (bold sequence), 6X His tags and
a stop codon (underlined). Standard PCR was used to amplify the
full-length coding sequence using the designed primers as
templates. The primer concentrations decreased from each end
towards the middle: Primers 1 and 12, with a concentration of 20
nmol/l; primers 2 and 11, with a concentration of 10 nmol/l;
primers 3 adn 10 with a concentration of 5 nmol/l; primers 4 and 9
with a concentration of 2..5 nmol/l; primers 5 and 8 with a
concentration of 1.25 nmol/l and primers 6 and 7, with a minimum
concentration of 0.625 nmol/l. PCR amplification was initially
performed over five cycles of predenaturation at 94°C for 3 min,
denaturation at 94°C for 30 sec, annealing at 45°C for 30 sec and
extension at 72°C for 30 sec, followed by a further 25 cycles of
denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec and
extension at 72°C for 30 sec. The products were digested with the
corresponding restriction enzymes and ligated into the pEasy-T3
vector. S100A4-pEasy plasmid was sequenced to verify the integrity
of S100A4 (Invitrogen Life Technologies, Carlsbad, CA, USA).
 | Table IOligonucleotide primers with mutual
overlaps. |
Table I
Oligonucleotide primers with mutual
overlaps.
| Primer | Primer sequence | Length of primers
(bp) |
|---|
| 1 | GCTCCATGGCGCGTCCGCTGGAAGAAGCGCTGGATGTGATTGT | 43 |
| 2 |
CATTGCCGCTATACTTATGAAAGGTGCTCACAATCACATCCAGCGCTTCT | 50 |
| 3 |
ACCTTTCATAAGTATAGCGGCAATGAGGGCGACAAATTCAAACTGAATAA | 50 |
| 4 |
GGGTCAGCAGTTCTTTCAGTTCGGTTTTATTCAGTTTGAATTTGTCGCCC | 50 |
| 5 |
GAACTGAAAGAACTGCTGACCCGTGAACTGCCGAGCTTTCTGGGCCGTCG | 50 |
| 6 |
TTCATCAGTTTCTGAAACGCCGCTTCATCGGTACGACGGCCCAGAAAGCT | 50 |
| 7 |
CGGCGTTTCAGAAACTGATGAATAATCTGGATAGCAATCGTGATAATGAA | 50 |
| 8 |
ACACGCAATATTCCTGAAAATCCACTTCATTATCACGATTGCTATCCAGA | 50 |
| 9 |
TGGATTTTCAGGAATATTGCGTGTTTCTTAGCTGCATTGCGATGATGTGC | 50 |
| 10 |
TTTATCCGGGCAGCCTTCGAAAAATTCATTGCACATCATCGCAATGCAGC | 50 |
| 11 |
CGAAGGCTGCCCGGATAAAGAACCGCGTAAAAAGCATCATCACCACCATC | 50 |
| 12 | GCCAAGCTTTTAATGATGGTGGTGATGATGCTTTTTAC | 38 |
| pbf104 |
GCTGAATTCATGGCGCGTCCGCTGGAAG | |
| pbr104 |
GCAGGATCCTTAATGATGGTGGTGATGATGC | |
Expression of rat recombinant S100A4
protein
The coding region of rat S100A4 in the cloning
vector was digested by EcoRI and BamHI and was
ligated into the PBV220 vector. The pBV220-S100A4 plasmid was
transferred into E. coli DH5α. A single bacterial colony was
inoculated in 5 ml Luria-Bertani (LB) media containing 50 μg/ml
ampicillin and was placed on a rotary shaker at 30°C overnight; the
seed cultures were subsequently transferred to 500 ml LB media
containing ampicillin. As the optical density (OD)-600 of the
culture reached 0.8, induction was initiated by heating; the
culture was induced for 4 h at 42°C and collected by centrifugation
at 5000 × g for 10 min. Thereafter, the culture was suspended in 1X
phosphate-buffered saline (PBS) containing 10 mM mercaptoethanol
and 1 mM phenylmethylsulfonyl fluoride. The culture was sonicated
in an ice bath, the lysate was centrifuged at 5000 × g for 15 min,
and the supernatant was determined by SDS-PAGE and stained with
Coomassie Brilliant Blue, to confirm the S100A4 expression. The
fusion protein quantity was assessed through comparison with the
bands formed by the standard protein.
Purification of rat recombinant
S100A4
Metal-chelate affinity chromatography (5 ml HiTrap
HP; GE Healthcare Life Sciences) was used to purify the rat
recombinant S100A4 using the Amersham fast-protein liquid
chromatography (FPLC) purification system (Harlow Scientific,
Arlington, MA, USA). The supernatant was diluted five times with 1X
binding buffer (containing 0.1 mol/l guanidinium hydrochloride)
prior to filtering and the generated liquids were discarded after
the column was balanced to the baseline, according to the
manufacturer’s instructions. Step gradient elution was subsequently
conducted with the elution buffer and the recombinant proteins were
collected and confirmed via SDS-PAGE.
Western blot analysis
The purified protein was transferred to a
nitrocellulose membrane following SDS-PAGE, using a semi-dry
electrophoretic transfer device (Jim-X Biotechnology Co., Ltd.,
Dalian, China). The membrane was blocked with 3% bovine serum
albumin (BSA) in PBS containing 0.5% Tween-20 and was incubated
with rabbit polyclonal antibody against rat S100A4 (Santa Cruz
Biotechnology, Inc.) and horseradish peroxidase (HRP)-coupled goat
anti-rabbit IgG secondary antibody (Abcam (Hong Kong) Ltd., Hong
Kong, China)
Protein assay
The protein concentrations in the samples were
determined using a Bradford protein assay kit (Sangon Biotech,
Shanghai Co., Ltd, Shanghai, China) with BSA at the standard
concentration (10).
SDS-PAGE analysis
SDS-PAGE analysis was performed under denaturing
conditions using the method described by Laemmli (11). The concentrations of the stacking
and resolving gels were 5 and 15%, respectively.
Bioactivity of the recombinant
protein
Recombinant protein bioactivity was identified by
Transwell migration and invasion assays (12). Transwell invasion chambers were
coated with Matrigel and the assays were conducted according to the
manufacturer’s instructions. HeLa cells (1×105) with 50
μg/ml recombinant S100A4 protein were placed in the top chambers
and served as the experimental group, while HeLa cells
(1×105) without S100A4 protein were used as the control
group. The cells were incubated for 24 h at 37°C and the motile
cells at the top of each chamber were removed with cotton swabs.
The cells at the bottom of each chamber were fixed with 0.1%
glutaraldehyde for 30 min, rinsed briefly with PBS and stained with
0.2% crystal violet for 20 min. The chambers were washed thoroughly
with PBS and the number of migrating cells or invasive cells was
tallied using ×200 magnification (Olympus CKX31, Olympus, Tokyo,
Japan); the mean number of cells per chamber was also determined.
The results were calculated as the migration/invasion rate relative
to the parental control cells. Each experimental condition was
duplicated and repeated three times.
Polyclonal antibody preparation
Antibodies against rat S100A4 protein were raised
within New Zealand white rabbits obtained from the Experimental
Animal Center of the Institiue of Health and Environmental Medicine
Research (Heping, China). The immunization procedure was
implemented as follows: On day one, 200 μg purified antigen was
mixed with an equal volume of complete Freund’s adjuvant, which was
multipoint injected subcutaneously into the back of each rabbit.
The rabbits were then boosted subcutaneously four times with 200 μg
recombinant protein in incomplete Freund’s adjuvant at 10 day
intervals. The antiserum was collected and determined 10 days
subsequent to the last injection. Purification of rabbit IgG was
performed according to the methods described by Moro et al
(13) with minor modifications. The
IgG fraction was purified by precipitation with 100% saturated
(NH4)2SO4 and by passing the IgG
fraction through the SPA-sepharose CL-4b column chromatograph.
Antibody titer determination
Antiserum titers were examined using indirect ELISA.
The wells of the polystyrene microtiter plates were coated with an
antigen and, following overnight incubation at 4°C, the coated
microtiter plates were blocked with BSA. The wells were incubated
for 15 min in the dark at room temperature, with polyclonal
antibodies against S100A4 with different deliquations (from 1:100
to 1:100,000) and HRP-conjugated goat anti-rabbit IgG (dilution,
1:3,000); the peroxidase substrate was tetramethyl benzidine.
Following incubation, the reaction was stopped using 2 M
H2SO4 and the absorbance at was measured at
an OD of 450 nm, using a microplate reader (Multiskan MK3;
Thermoscientific, Waltham, MA, USA).
Statistical analysis
All results are expressed as the means ± SD. Data
were analyzed using SPSS version 17 (SPSS, Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Design, cloning and analysis of the
S100A4 synthetic gene
The natural gene sequence (GenBank accession no.
NM012618) was used as the template for designing and optimizing
S100A4 according to the E. coli codon usage. Thirty-four
rare codons of the natural gene were replaced with synonymous
high-frequency codons in a synthetic gene that encodes S100A4. A
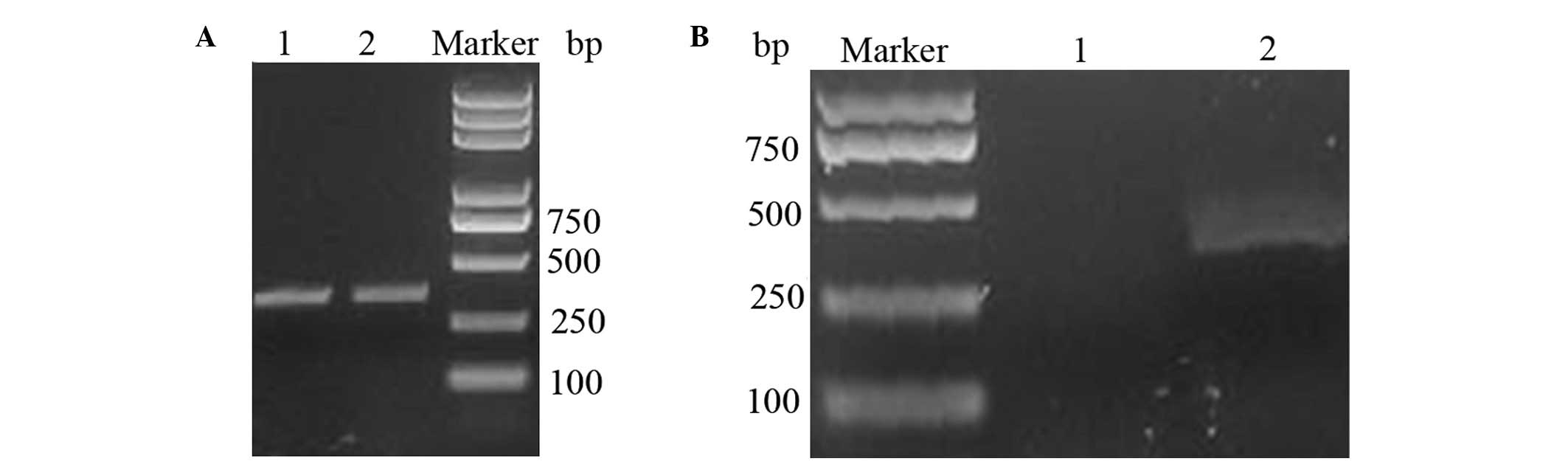
300-bp fragment was synthesized using three-step PCR (Fig. 1A). The PCR-synthesized sequence
cloned into the pEasy-T3 vector was digested by EcoRI and
BamHI, thereby producing the expected band size.
Construction of the expression vector,
pBV220-S100A4
The pEasy-S100A4 plasmid was sequenced to verify the
integrity of S100A4. Following confirmation by DNA sequencing and
EcoRI and BamHI digestion, the S100A4 gene was
successfully cloned into the expression vector, pBV220-S100A4.
Agarose gel analysis of the digestion of pBV220 is shown in
Fig. 1B.
Recombinant S100A4 protein
expression
The pBV220-S100A4 plasmid was transferred into E.
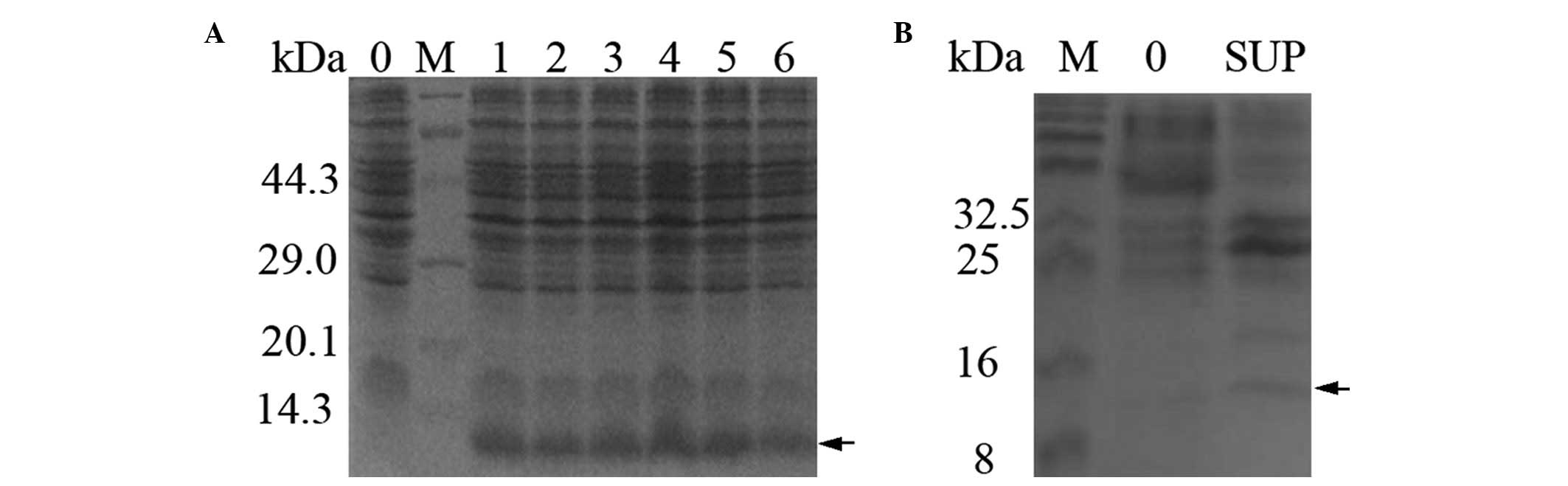
coli DH5α and was induced using heat shock. The 11.5 kDa S100A4
fusion protein was expressed at a high level, ~20–30% of the total
protein of the cell, as analyzed by Total Lab 2.01 software
(Newcastle, UK; Fig. 2A). The
expected 11.5 kDa band was apparent in the supernatant and absent
in the negative control (Lane 0; Fig.
2B). The SDS-PAGE analysis indicated that the S100A4 protein
was expressed in a soluble form.
Recombinant S100A4 purification
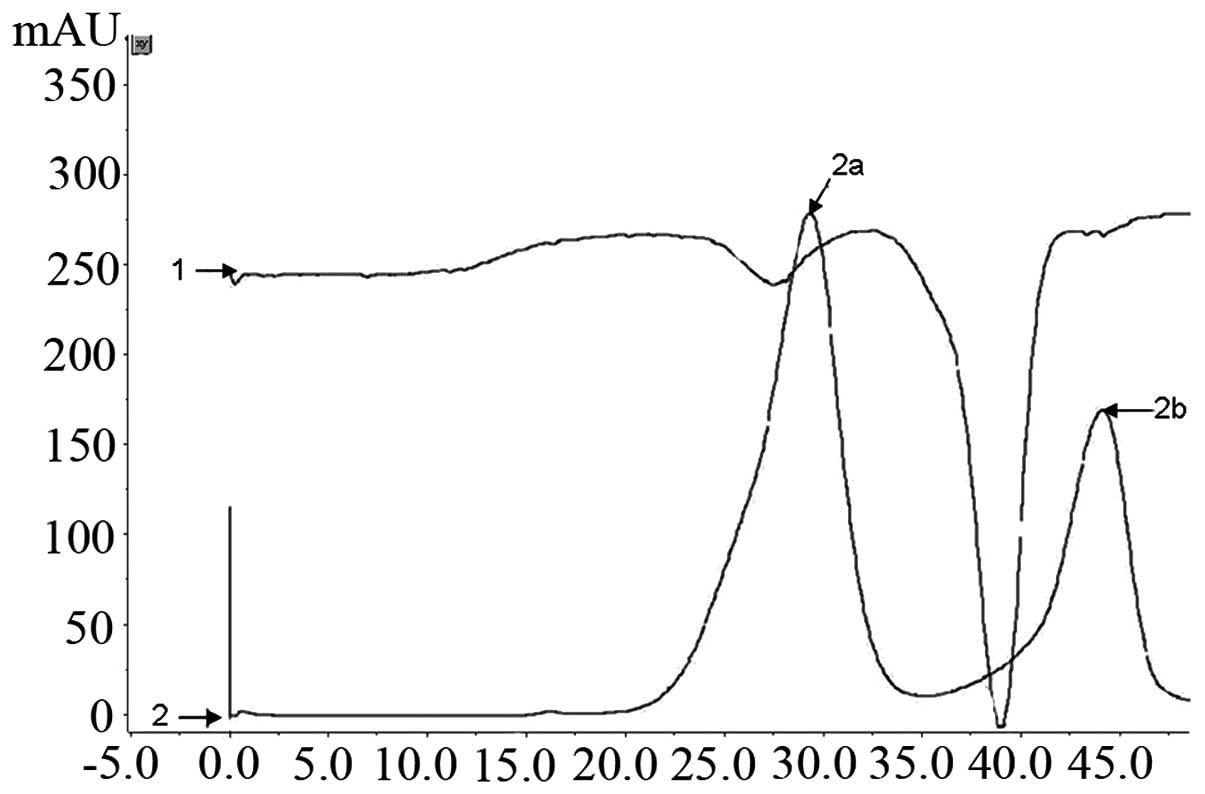
The 6X His-tagged S100A4 protein was one-step
purified by the Ni2+ affinity chromatography column
using the FPLC purification system. The column was washed with 200
mmol/l imidazole gradient elution buffer at 3 ml/min and ~15 ml
concentrated eluted fluid was obtained (Fig. 3). The SDS-PAGE analysis identified
that the protein remained intact and the purity of the final S100A4
was 95% following desalination with HiTrap Desalting (GE Healthcare
Life Sciences; Fig. 4B). The
yielded purified recombinant protein was ~50 mg/l of the induced
culture.
Western blot analysis of recombinant
S100A4
The rabbit polyclonal antibody was capable of
recognizing natural S100A4 and recombinant S100A4 protein, and the
western blot analysis identified that the recombinant S100A4 was
functionally expressed (Fig.
4A).
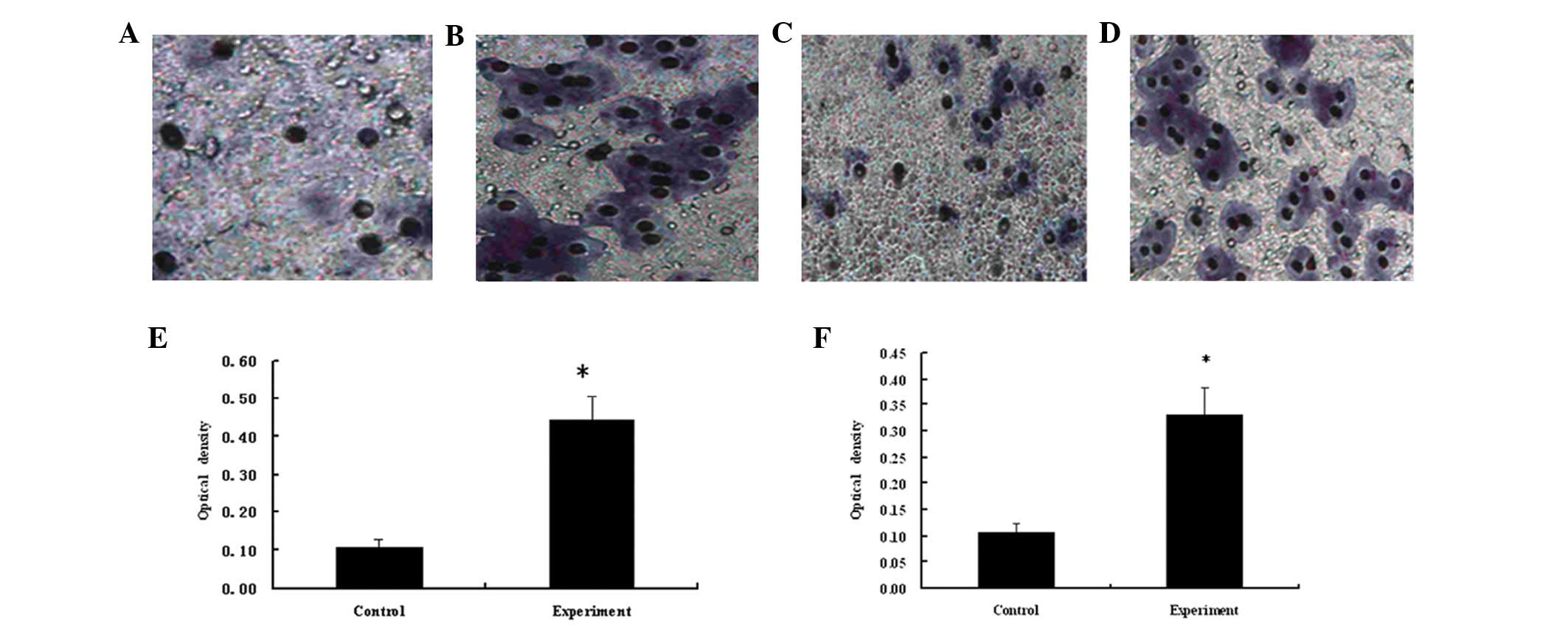
Migration and invasion of HeLa cells
The Transwell migration and invasion assays
identified that the number of cells in the experimental group was
greater than that of the control group (Fig. 5A–D), thus identifying that the
recombinant protein was capable of improving the motility and
invasiveness of cancer cells. A four-fold increase was observed in
the number of migrating cells in the experimental group compared
with the control group (P<0.05; Fig.
5E). The invasive ability of the HeLa cells in the experimental
group was determined using the Matrigel invasion assay, which
identified that the invasive ability of HeLa cells in the
experimental group was three-fold greater than that of the control
group (P<0.05; Fig. 5F). This
therefore indicated that the recombinant protein levels promoted
the motility and invasiveness of cancer cells.
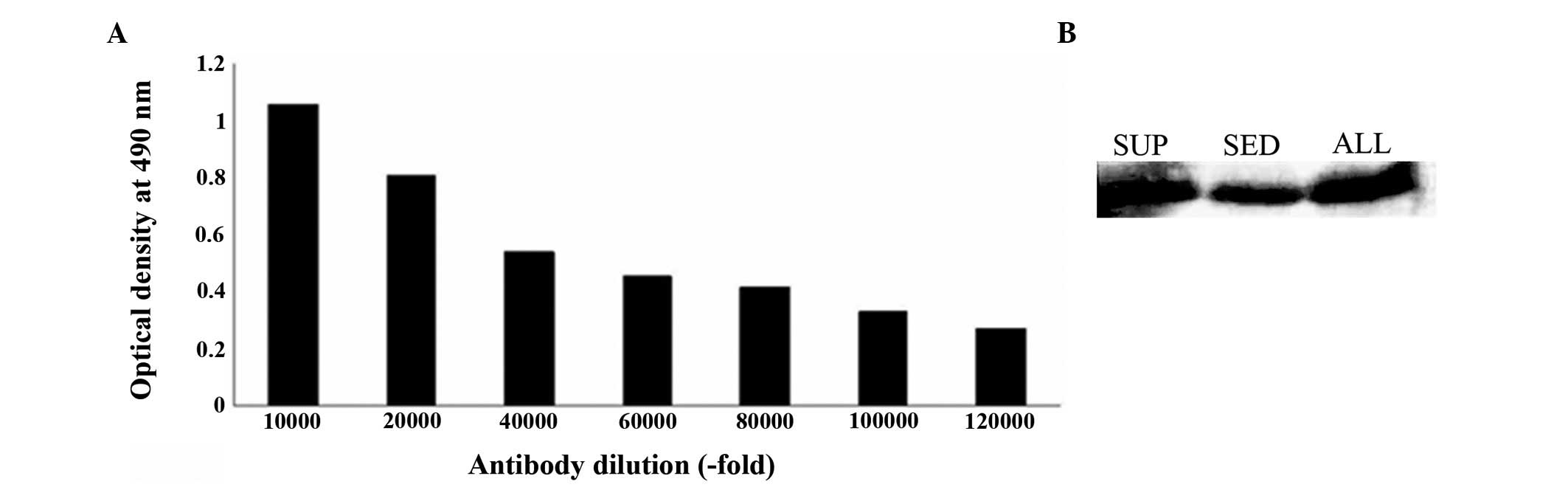
Titer and specificity of polyclonal
antibody
Following rabbit immunization with recombinant
S100A4, the antiserum was purified by protein G affinity
chromatography. The anti-S100A4 antibody concentration was 0.78
mg/ml and the antibody titer, determined using ELISA, was
~1:120,000 (Fig. 6A). The antiserum
obtained was specific to the recombinant protein according to the
western blot analysis (Fig.
6B).
Discussion
In the present study, a pBV220-S100A4 vector was
successfully constructed based on an optimized S100A4 gene. The
soluble S100A4 protein was expressed at an increased level
following transferal of the constructed expression vector into
E. coli DH5α and induction via heat shock. The purity of the
S100A4 protein was improved following purification via revised Ni
affinity chromatography. Additional proteins with similar
structures that belong to the same protein family were also
obtained, such as S100A1 and S100B, using the same methodology. The
present study identified that the expression system was efficient
and stable (15).
It has been shown that the synthesis of a
full-length gene may contribute to the expression DH5α in E.
coli by the optimized gene (16,17).
There was an initial attempt to synthesize the S100A4 gene by
one-step PCR; however, following several cloning procedures, it was
not continued as one or two frameshift or lethal mutations occurred
in the synthesized sequence. These mutations may have been a result
of the mismatch between overlapping primers. Therefore, two short
fragments were synthesized by PCR and subsequently used to obtain
the full-length gene by performing an additional PCR. This process
was conducted to reduce the mismatch by decreasing the quantity of
overlapping primers, which was reasonable and aided synthesis of
long DNA fragments via PCR.
Studies have shown that pET30a(+) was used as the
expression vector of the S100A4 protein (17,18).
However we used plasmid pBV220 as the expression vector in our
experiment. The results of the present study demonstrated that the
soluble S100A4 protein was expressed at a high level and accounted
for >20% of the total protein levels, which was greater than
that observed in pET30a(+). Plasmid pBV220, which was constructed
at the National Institute for Viral Disease Control and Prevention,
is an efficient cloning and expression vector that is regularly
used in China (Beijing, China) (14). The temperature, induced by
PRPL double promoters, controls the pBV220
plasmid. The gene was inserted into pBV220 and was highly expressed
in E. coli DH5α, which exhibits rapid growth and
reproduction, low nutrition requirements and is readily
manipulated. E. coli DH5α is an optimal expression system
for proteins that are capable of biological activity in
vitro. The present study identified that soluble S100A4 protein
was expressed at a high level and accounted for >20% of the
total protein.
The present study also aimed to obtain increasingly
purified S100A4 proteins. In the initial purification
investigation, soluble S100A4 was not successfully combined with
certain metal ions and the penetrating fluids contained numerous
target proteins. This may be due to the inability of the 6X His tag
to be fully exposed during the protein folding process. Therefore,
the common Ni affinity chromatography method was revised and
following the addition of 0.1 mol/l guanidine hydrochloride into
the binding buffers, the binding capacity was significantly
improved and the protein was further purified. Thus suggesting that
guanidine hydrochloride, or other denaturing agents at an
appropriate concentration, are capable of effectively improving the
binding as a result of increased exposure of the His tag to the
target protein.
The Transwell migration and invasion assays showed
that the recombinant protein levels promoted the motility and
invasiveness of the cancer cells. Antiserum was obtained from
immunized rabbits using S100A4 as the immunogen; the polyclonal
antibody obtained was identified as possessing highly specific and
sensitive affinities to the protein.
As anti-S100A4 antiserum was able to bind as
strongly to recombinant S100A4 as to natural S100A4, the basic
biological activity of recombinant S100A4 was retained. Thus, the
expression vector, pBV220-S100A4, was successfully constructed
based on the 6X His-tagged N-terminal S100A4 gene. The 6X
His-tagged recombinant pBV220-S100A4 protein was purified using
revised Ni affinity chromatography. In conclusion, the production
of the active recombinant S100A4 protein within E. coli may
aid with further investigation and applications of S100A4.
Acknowledgements
The authors would like to acknowledge the financial
support of the National Natural Science Foundation of China (grant
nos. 81373108, 81202164 and 30971421) and the Tianjin Research
Program of Application Foundation and Advanced Technology (grant
no. 10JCYBJC10200).
References
|
1
|
Yammani RR, Long D and Loeser RF:
Interleukin-7 stimulates secretion of S100A4 by activating the
JAK/STAT signaling pathway in human articular chondrocytes.
Arthritis Rheum. 60:792–800. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ismail TM, Zhang S, Fernig DG, Gross S, et
al: Self-association of calcium-binding protein S100A4 and
metastasis. J Biol Chem. 285:914–922. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen M, Sinha M, Luxon BA, et al: Integrin
alpha6beta4 controls the expression of genes associated with cell
motility, invasion, and metastasis, including S100A4/metastasin. J
Biol Chem. 284:1484–1494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oslejsková L, Grigorian M, Hulejová H, et
al: Metastasis-inducing S100A4 protein is associated with the
disease activity of rheumatoid arthritis. Rheumatology (Oxford).
48:1590–1594. 2009.PubMed/NCBI
|
|
5
|
Xie R, Schlumbrecht MP, Shipley GL, et al:
S100A4 mediates endometrial cancer invasion and is a target of
TGF-beta1 signaling. Lab Invest. 89:937–947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sherbet GV: Metastasis promoter S100A4 is
a potentially valuable molecular target for cancer therapy. Cancer
Lett. 280:15–30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sack U and Stein U: Wnt up your mind -
intervention strategies for S100A4-induced metastasis in colon
cancer. Gen Physiol Biophys. 28:F55–F64. 2009.PubMed/NCBI
|
|
8
|
Boye K and Maelandsmo GM: S100A4 and
metastasis: a small actor playing many roles. Am J Pathol.
176:528–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ai KX, Lu LY, Huang XY, et al: Prognostic
significance of S100A4 and vascular endothelial growth factor
expression in pancreatic cancer. World J Gastroenterol.
14:1931–1935. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao XN, Tang SQ and Zhang XF: S100A4
antisense oligodeoxynucleotide suppresses invasive potential of
neuroblastoma cells. J Pediatr Surg. 40:648–652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moro A, Yoshitake T, Ogawa T, et al:
Single-step purification of pepsin-derived monoclonal antibody
fragments from crude murine ascitic fluids by ceramic
hydroxyapatite high-performance liquid chromatography. J Biochem.
144:733–739. 2008. View Article : Google Scholar
|
|
14
|
Tang X, Tan Y, Zhu H, et al: Microbial
conversion of glycerol to 1,3-propanediol by an engineered strain
of Escherichia coli. Appl Environ Microbiol. 75:1628–1634.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu ZQ, Chen YY, Wang TH, et al:
Expression, purification, and identification of human S100A1
protein. J Prev Med Chin PLA. 29:240–244. 2011.
|
|
16
|
Han L, He HL, Guan WL, et al: Prokaryotic
expression and purification of bioactive human S100A4. Genomics and
Applied Biology. 32:142–148. 2013.(In Chinese).
|
|
17
|
Marlatt NM, Spratt DE and Shaw GS: Codon
optimization for enhanced Escherichia coli expression of
human S100A11 and S100A1 proteins. Protein Expr Purif. 73:58–64.
2011.
|
|
18
|
He H, Han L, Guan W, et al: An efficient
expression and purification strategy for the production of S100
proteins in Escherichia coli. Bioengineered. 4:55–58. 2013.
View Article : Google Scholar : PubMed/NCBI
|