Introduction
Hyperplastic polyps (HPs) are the most common type
of lesion among polypoid lesions of the stomach. The reported
incidence of focal malignancy in these polypoid lesions differs
significantly between 0.8 and 7.1%. Although, HPs are considered to
be relatively harmless in their natural course, a general
understanding which has gained acceptance following a number of
previous follow-up studies (1–4).
However, certain authors have previously reported a few cases of
the malignant transformation of gastric HPs (5–7);
although, the incidence rate of malignant change has been reported
to be relatively low, with an average of only ~2.1% in large series
(6). With the recent prevalence of
endoscopic treatments for gastric polyps, including polypectomy and
mucosal resection, an increased number of cases of dysplasia or
carcinoma arising in gastric HP have been reported (6,8–13).
Previously, Yao et al proposed four characteristics of the
malignantly transformed lesions associated with gastric hyperplasia
polyps: i) lesions are predominantly of the well-differentiated
type, although, a small number of lesions of the poorly
differentiated type have also been reported; ii) malignant
transformation has been suggested to be associated with dysplasia,
although, involvement of intestinal metaplasia remains unknown;
iii) the mucin phenotype appears to be of the gastric type in a
number of cases; and iv) p53 may be important in the malignant
transformation (14).
Previous histochemical and immunohistochemical
analyses for mucins have indicated that differentiated
adenocarcinomas may be classified into gastric and intestinal
phenotypes as in Lauren’s classification (15). A number of markers have been
reported to distinguish between gastric and intestinal mucins;
however, a set of markers that are able to completely distinguish
between the two mucin phenotypes has not yet been reported
(16–19).
Tight, adherent and gap junctions, as well as
desmosomes, are well-known cell membrane structures that are
involved in cell-to-cell interactions. Adhesion tight junctions,
present in epithelial and endothelial cell membranes, form a
component of the intercellular junctional complexes and are
important in barrier function, cell polarity and cell signaling
pathways (20). Claudins are major
tight junction constituents and exhibit four transmembrane domains.
To date, 24 members of the claudin family have been identified
(20). From this family of
proteins, claudin-4 is reported to be highly expressed in gastric
intestinal-type adenocarcinoma and several previous studies have
shown that claudin-4 is involved in gastric cancer (21–23).
For example, it has been previously reported that Helicobacter
pylori has the ability to increase paracellular permeability by
occludin, claudin-4 and claudin-5 (24). It has also been shown that caudal
type homeobox 2 (Cdx2) is important in the regulation of intestinal
claudin expression, not only in gastric mucosa with intestinal
metaplasia, but also in gastric carcinoma (25). Furthermore, the involvement of
specific claudin factors in Epstein-Barr virus-associated gastric
cancer (26) and claudin-18 in
signet ring cell cancer has previously been shown (27). In order to further clarify the
mechanism of malignant transformation of gastric HPs, the present
study analyzed four cases of cancer-bearing HPs using
immunohistochemistry.
Materials and methods
Patients and specimens
In total, four patients with gastric polyps in the
body of the stomach (three cases), antrum (two cases) and residual
stomach (one case) were treated at the Department of Diagnostic
Pathology, Graduate School of Medicine and Pharmaceutical
Sciencies, University of Toyama, (Toyama, Japan), the Department of
Surgical and Molecular Pathology, Dokkyo Medical University School
of Medicine, (Tochigi, Japan) and the Department of Pathology,
Ibaraki Preifectural Central Hospital (Ibaraki, Japan). Of these
cases, four underwent endoscopic resection of the lesions and two
cases underwent surgical removal of the lesions. The criteria for
HPs was defined as hyperplastic foveolar epithelium without atypia
and neoplastic epithelium was classified according to the Vienna
classification (28). All patients
provided written informed consent and the present study was
approved by the ethics committee of our institute.
Immunohistochemistry
Routinely-processed, formalin-fixed and
paraffin-embedded tissue blocks were selected and 5-μm serial
sections were prepared from the cut surface of the blocks. The
antibodies, MUC1 (clone Ma695), MUC2 (clone Ccp58), MUC5AC (clone
CLH2), MUC6 (clone CLH5; 1:200 dilution; Novocastra, Milton Keynes,
UK), claudin-3 (polyclonal), claudin-4 (polyclonal), claudin-18
(polyclonal; 1:50 dilution; Zymed Laboratories, South San
Franscisco, CA, USA), Cdx2 (clone CDX2–88; 1:500 dilution; BioGenex
Laboratories, Freemont, CA, USA), p53 (clone DO-7; 1:100 dilution;
Dako, Carpinteria, CA, USA) and Ki-67 (clone MIB-1; 1:100 dilution;
Immunotech, Marseille, France) were used. Immunoperoxidase
reactions were performed using the Ventana BenchMark® LM
automated immunostainer (Ventana Medical Systems, Tucson, AZ, USA)
according to the manufacturer’s instructions. All cases were
reviewed by two investigators, who arrived at a consensus on the
pathological diagnoses and the assessment of immunoreactivity.
Statistical analysis
Statistical analysis was performed using Student’s
t-test for the comparisons of the p53 and Ki-67 labeling indices
between each component. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinicopathological observations
The patients consisted of four males and two
females, ranging in age between 65 and 78 years (mean age, 70
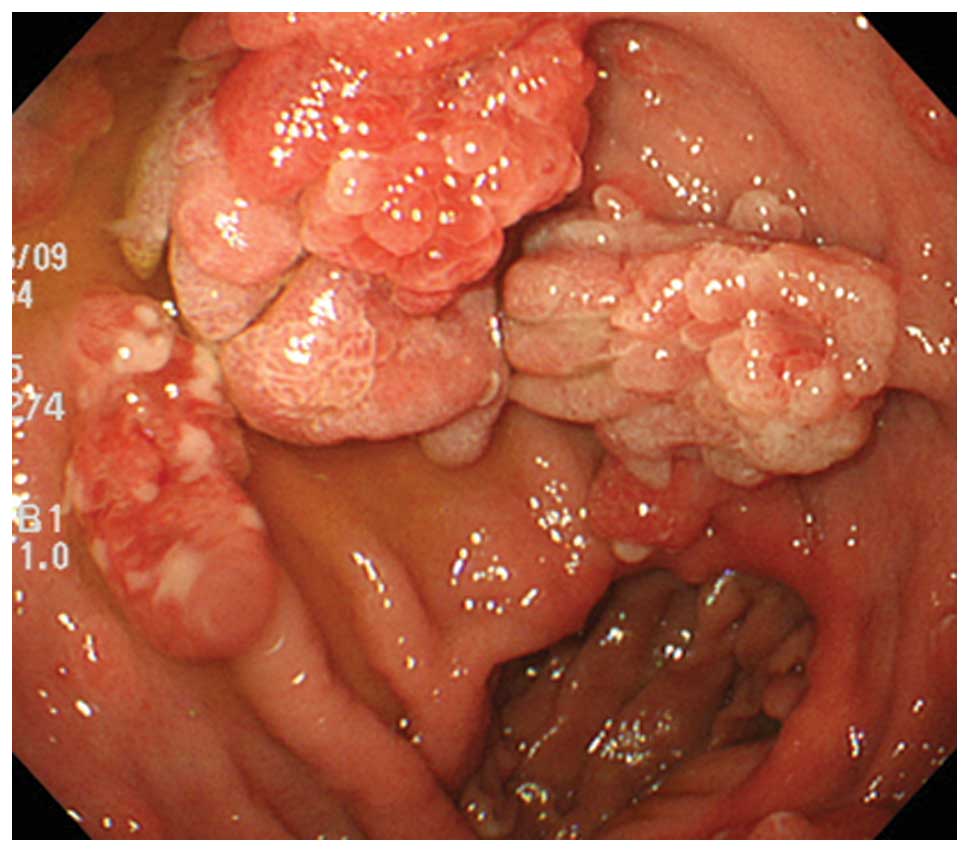
years). Endoscopic assessment revealed solitary (four cases) and
multiple (two cases) pedunculated polyps in the gastric mucosa
(Fig. 1). The patient
characteristics are summarized in Table
I.
 | Table IList of patient characteristics. |
Table I
List of patient characteristics.
| Patient |
|---|
|
|
|---|
| Variable | 1 | 2 | 3 | 4 | 5 | 6 |
|---|
| Age, years | 69 | 78 | 65 | 68 | 75 | 66 |
| Gender | M | F | M | M | F | M |
| Location | RS | B | A | B | A | B |
| Lesion | M | S | S | S | S | M |
| Size, cm | 4.0 | 2.5 | 3.0 | 1.0 | 2.5 | 2.6 |
Pathological observations
The resected polyps ranged in size between 10 and 40
mm in longitudinal diameter (mean size, 26 mm). All polyps were
regionally composed of intermingled components of plural lesions in
varied proportions. The lesions were basically composed of
hyperplastic foveolar epithelium and intramucosal neoplasia, the
latter of which was categorized as dysplasia and adenocarcinoma.
The carcinomatous components were mainly differentiated as tubular
adenocarcinoma. Although the border between each component was
distinct, the transitional zone was relatively undefined (Fig. 2).
Immunohistochemical observations
The present immunohistochemical study found that
MUC5AC was immunopositive in the hyperplastic, dysplastic and
carcinoma regions of the polyps; while this marker was detected in
the hyperplastic and dysplastic areas only, but not in the
carcinomatous component, in one case. These observations suggested
that the lesions were mostly of the gastric mucin type (Fig. 3). Furthermore, MUC2 expression was
not observed in any of the specimens and goblet cells were also
undetected, thus, the lesions were unlikely to be of the intestinal
mucin type. Immunoreactivity for MUC1 in three cases and MUC6 in
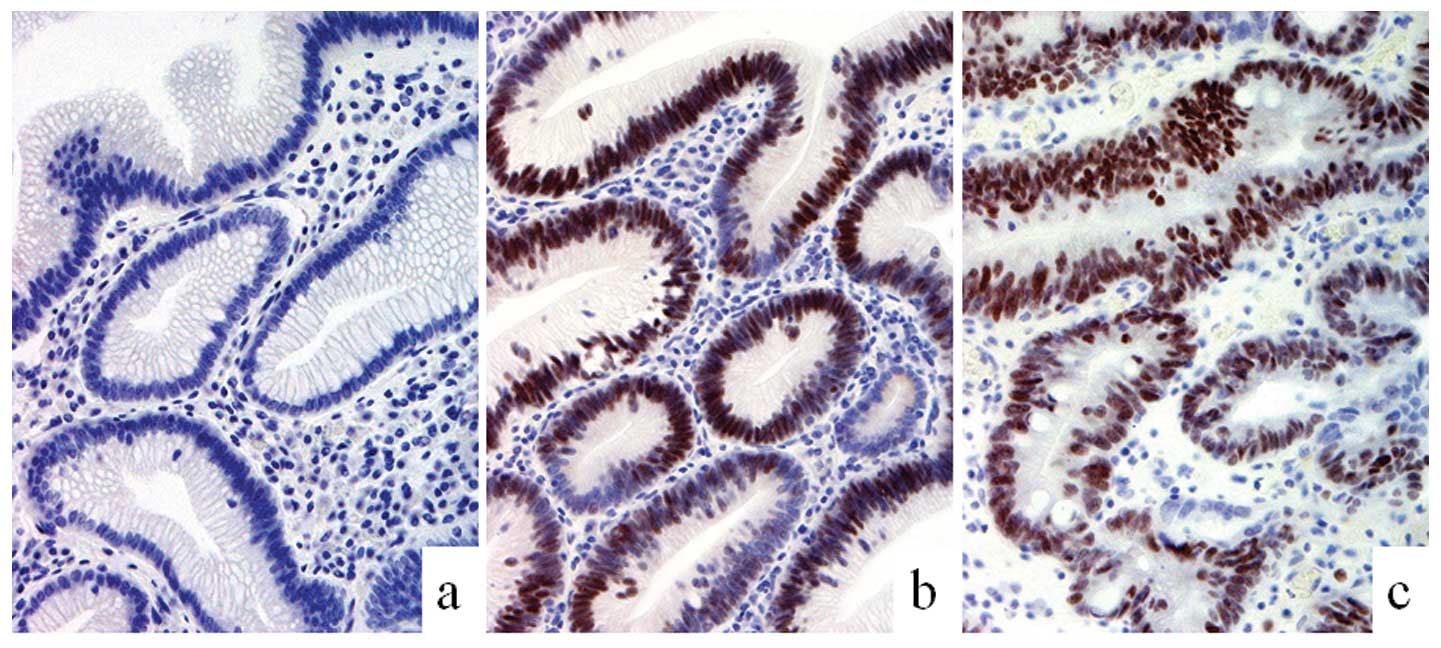
all cases were negative. Expression of Cdx2 in the nucleus of the
cells of the intestinal epithelium was found in the dysplastic and
carcinomatous components of all cases with the exception of one
case (Fig. 4). The tight junction
factor, claudin-3, was completely absent in the hyperplasia area,
but was immunopositive in the dysplastic and carcinomatous
components. By contrast, expression of claudin-4 was observed in
the dysplastic and carcinomatous component of all cases (Fig. 5). In addition, expression of
claudin-18 was observed in the hyperplastic, dysplastic and cancer
components of all cases. The frequency of cells with abnormal
nuclear accumulation of p53 was 12% in the hyperplastic area in one
case while that of the other cases was <5%. By contrast, the
frequencies ranged between 85–90% (mean, 88%) and 24–80% (mean,
64%) in the cancerous and dysplastic components, respectively. The
percentage of Ki-67-positive cells was 10–25% (mean, 15%) in the
hyperplastic areas, 55–70% (mean, 62%) in the dysplastic regions
and 80–90% (mean, 84%) in the carcinomas. A statistically
significant difference was observed between hyperplasia and each
neoplastic (dysplasia and carcinoma) component (P<0.01). The
immunohistochemical observations are summarized in Table II.
 | Table IIResults of immunohistochemical
analysis. |
Table II
Results of immunohistochemical
analysis.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|
|
|
|
|
|
|
|
|---|
| Markers | H | D | C | H | D | C | H | D | C | H | D | C | H | D | C | H | D | C |
|---|
| MUC1 | − | − | − | − | − | − | − | + | + | − | + | + | − | − | − | − | + | + |
| MUC2 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| MUC5AC | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| MUC6 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Cdx2 | − | − | − | − | + | ++ | + | ++ | ++ | − | ++ | ++ | − | ++ | ++ | − | ++ | ++ |
| Claudin-3 | − | + | + | − | + | + | − | − | + | − | − | − | − | − | + | − | − | + |
| Claudin-4 | ++ | ++ | ++ | − | ++ | ++ | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Claudin-18 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| p53, % | <5 | 24 | 85 | 12 | 80 | 90 | <5 | 80 | 90 | <5 | 60 | 90 | <5 | 75 | 85 | <5 | 65 | 90 |
Discussion
The current study immunohistochemically analyzed
polyp samples from six patients that all exhibited regions of
hyperplasia, dysplasia and carcinoma. From these observations, it
was suggested that the malignant transformation of gastric HPs may
occur by multistep carcinogenesis and these neoplastic cells may
acquire various phenotypes during this process. As described in
previous studies, a large polyp size is considered a risk factor
for malignancy or may be a sign of malignant transformation
(5,29). In the present study, all the polyp
samples exceeded 10 mm in diameter (mean diameter, 26 mm) and
consisted of hyperplasia, dysplasia and carcinoma, thus, consistent
with the observations of previous studies. All the carcinomatous
components were essentially composed of well-differentiated
adenocarcinomas, and poorly differentiated adenocarcinomas were not
included in the current series. Although certain cases of poorly
differentiated adenocarcinomas in HPs have been previously
reported, differentiated adenocarcinoma is considered to be the
most common histological type of carcinoma based on previous
large-scale studies (5,30).
With regard to the phenotype of the HPs, it appeared
to be of the gastric type, since MUC5AC was detected not only in
all the hyperplastic components, but also in all the dysplastic and
carcinomatous lesions. Thus, it was suggested that the neoplastic
lesions in all cases of the current series retained the gastric
phenotype even following malignant transformation. However,
previous studies have presented arguments with regard to the
intestinalization of gastric epithelial neoplasia; although, the
evidence is inconclusive, largely due to the reason that accurate
and specific markers of intestinalization are not yet available
(5,7,14,30).
However, since MUC2 expression is positive in goblet cells and CD10
is detected in the brush border, these markers may be important for
the acquisition of the intestinalization phenotype in neoplastic
cells and, thus, may be good candidate markers for this process
(16–18). Cdx2 is a transcription factor
involved in the differentiation of the intestinal epithelium and,
by immunohistochemistry, it was revealed that this protein was
expressed in the nucleus (31). The
expression of Cdx2 often parallels that of MUC2, but these two
proteins are not necessarily positively correlated. Cdx2 appears to
be expressed at the stage of the precursory intestinal epithelium
(31); therefore, this protein may
be more useful as a marker to detect the early stages of the
intestinalization phenotype.
In the current study, claudin-4 was immunopositive
in regions of dysplasia and/or carcinoma in all cases. It has
previously been reported that claudin-4 is expressed only in
cancer, whereas it is not expressed in the normal foveolar
epithelium (21–23). The present study also obtained
similar observations for claudin-3 in five of the cases. According
to a previous study by Shinozaki et al, claudin-3 appears to
be expressed in intestinal metaplasia in a similar expression
pattern as that of MUC2 and CD10 (26). However, claudin-3 appears to be
expressed during the early stage of intestinalization, similar to
Cdx2 expression in the present study. By contrast, the expression
pattern of claudin-18 was similar to that of MUC5AC. These results
indicated that the neoplastic lesions show an extremely similar
phenotype to gastric foveolar epithelium (26).
p53 protein is considered to be one of the most
important gene products during the carcinogenesis of various
malignancies, including gastric cancer (32). In the current study, nuclear
accumulation of p53 was highly detected in the neoplastic lesions
(dysplasia and carcinoma), but was absent or extremely low in the
areas of hyperplasia, with the exception of one case. Furthermore,
it was found that the Ki-67 labeling index gradually increased from
hyperplasia to dysplasia to carcinoma. These observations provided
evidence to support the importance of the
hyperplasia-dysplasia-carcinoma sequence during malignant
transformation of HPs (8).
In conclusion, during the malignant transformation
of gastric HPs, cancer may spontaneously occur in the lesion
through multistep carcinogenesis, such as the hyperplasia-adenoma
(dysplasia)-adenocarcinoma sequence. The neoplastic cells may
acquire various phenotypes during this process. Since it appears
that several gastric and intestinal phenotypes are admixed in a
complex manner within these polyps, the malignant transformation of
the cells may not necessarily undergo simple differentiation.
Acknowledgements
The authors would like to thank Mr. Yoshiaki Uchida,
Ms. Kaori Abe, Ms. Nozomi Nagashima, Ms. Midori Katayama, Ms.
Takako Ono and Ms. Chiaki Matsuyama for technical assistance with
the immunohistochemical analysis.
References
|
1
|
Ueno K, Oshiba S, Yamagata S, et al:
Histo-clinical classification and follow-up study of gastric polyp.
Tohoku J Exp Med. 118(Suppl): S23–S38. 1976. View Article : Google Scholar
|
|
2
|
Kozuka S, Masamoto K, Suzuki S, et al:
Histogenetic types and size of polypoid lesions in the stomach,
with special reference to cancerous change. Gann. 68:267–274.
1977.
|
|
3
|
Laxén F, Sipponen P, Ihamäki T, et al:
Gastric polyps; their morphological and endoscopical
characteristics and relation to gastric carcinoma. Acta Pathol
Microbiol Immunol Scand A. 90:221–228. 1982.PubMed/NCBI
|
|
4
|
Hattori T: Morphological range of
hyperplastic polyps and carcinomas arising in hyperplastic polyps
of the stomach. J Clin Pathol. 38:622–630. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Daibo M, Itabashi M and Hirota T:
Malignant transformation of gastric hyperplastic polyps. Am J
Gastroenterol. 82:1016–1025. 1987.
|
|
6
|
Zea-Iriarte WL, Sekine I, Itsuno M, et al:
Carcinoma in gastric hyperplastic polyps. A phenotypic study. Dig
Dis Sci. 41:377–386. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Orlowska J and Kupryjanczyk J: Malignant
transformation of gastric hyperplastic polyps. Am J Clin Pathol.
117:165–166. 2002.PubMed/NCBI
|
|
8
|
Ajiki T, Nakamura T, Suehiro I, et al:
Increased immunohistochemical labeling indices as indicators of
malignant transformation of a gastric hyperplastic polyp. Am J
Gastroenterol. 97:211–212. 2002. View Article : Google Scholar
|
|
9
|
Bosseckert H, Kratzsch KH, Machnik G, et
al: Hyperplasiogenic polyps and stomach carcinoma risk-experiences
following 1074 polypectomies and follow-up studies. Schweiz Rundsch
Med Prax. 79:537–539. 1990.(In German).
|
|
10
|
Gschwantler M, Pulgram T, Feichtenschlager
T, et al: Gastric carcinoma arising from a hyperplasiogenic polyp
with a diameter of less than 2 centimeters. Z Gastroenterol.
33:610–612. 1995.
|
|
11
|
Joffe N and Antonioli DA: Atypical
appearances of benign hyperplastic gastric polyps. AJR.
131:147–152. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orlowska J, Jarosz D, Pachlewski J and
Butruk E: Malignant transformation of benign epithelial gastric
polyps. Am J Gastroenterol. 90:2152–2159. 1995.PubMed/NCBI
|
|
13
|
Remmele W and Kolb EF: Malignant
transformation of hyperplasiogenic polyps of the stomach - case
report. Endoscopy. 10:63–65. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao T, Kajiwara M, Kuroiwa S, et al:
Malignant transformation of gastric hyperplastic polyps: alteration
of phenotypes, proliferative activity, and p53 expression. Hum
Pathol. 33:1016–1022. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tatematsu M, Ichinose M, Miki K, et al:
Gastric and intestinal phenotypic expression of human stomach
cancers as revealed by pepsinogen immunohistochemistry and mucin
histochemistry. Acta Pathol Jpn. 40:494–504. 1990.
|
|
16
|
Chang SK, Dohrman AF, Basbaum CB, et al:
Localization of mucin (MUC2 and MUC3) messenger RNA and peptide
expression in human normal intestine and colon cancer.
Gastroenterology. 107:28–36. 1994.PubMed/NCBI
|
|
17
|
Morohara K, Tajima Y, Nakao K, et al:
Gastric and intestinal phenotypic cell marker expressions in
gastric differentiated-type carcinomas: association with E-cadherin
expression and chromosomal changes. J Cancer Res Clin Oncol.
132:363–375. 2006. View Article : Google Scholar
|
|
18
|
Trejdosiewicz LK, Malizia G, Oakes J, et
al: Expression of the common acute lymphoblastic leukaemia antigen
(CALLA gp100) in the brush border of normal jejunum and jejunum of
patients with coeliac disease. J Clin Pathol. 38:1002–1006. 1985.
View Article : Google Scholar
|
|
19
|
Weiss AA, Babyatsky MW, Ogata S, et al:
Expression of MUC2 and MUC3 mRNA in human normal, malignant, and
inflammatory intestinal tissues. J Histochem Cytochem.
44:1161–1166. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsukita S, Furuse M and Itoh M:
Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol.
2:285–293. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsuda Y, Semba S, Ueda J, et al: Gastric
and intestinal claudin expression at the invasive front of gastric
carcinoma. Cancer Sci. 98:1014–1019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Resnick MB, Gavilanez M, Newton E, et al:
Claudin expression in gastric adenocarcinomas: a tissue microarray
study with prognostic correlation. Hum Pathol. 36:886–892. 2005.
View Article : Google Scholar
|
|
23
|
Cunningham SC, Kamangar F, Kim MP, et al:
Claudin-4, mitogen-activated protein kinase kinase 4, and stratifin
are markers of gastric adenocarcinoma precursor lesions. Cancer
Epidemiol Biomarkers Prev. 15:281–287. 2006. View Article : Google Scholar
|
|
24
|
Fedwick JP, Lapointe TK, Meddings JB, et
al: Helicobacter pylori activates myosin light-chain kinase to
disrupt claudin-4 and claudin-5 and increase epithelial
permeability. Infect Immun. 73:7844–7852. 2005. View Article : Google Scholar
|
|
25
|
Satake S, Semba S, Matsuda Y, et al: Cdx2
transcription factor regulates claudin-3 and claudin-4 expression
during intestinal differentiation of gastric carcinoma. Pathol Int.
58:156–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shinozaki A, Ushiku T, Morikawa T, et al:
Epstein-Barr virus-associated gastric carcinoma: a distinct
carcinoma of gastric phenotype by claudin expression profiling. J
Histochem Cytochem. 57:775–785. 2009. View Article : Google Scholar
|
|
27
|
Sentani K, Oue N, Tashiro T, et al:
Immunohistochemical staining of Reg IV and claudin-18 is useful in
the diagnosis of gastrointestinal signet ring cell carcinoma. Am J
Surg Pathol. 32:1182–1189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schlemper RJ, Riddell RH, Kato Y, et al:
The Vienna classification of gastrointestinal epithelial neoplasia.
Gut. 47:251–255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hizawa K, Fuchigami T, Iida M, et al:
Possible neoplastic transformation within gastric hyperplastic
polyp. Application of endoscopic polypectomy. Surg Endosc.
9:714–718. 1995.
|
|
30
|
Kushima R and Hattori T: Histogenesis and
characteristics of gastric-type adenocarcinomas in the stomach. J
Cancer Res Clin Oncol. 120:103–111. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park do Y, Srivastava A, Kim GH, et al:
CDX2 expression in the intestinal-type gastric epithelial
neoplasia: frequency and significance. Mod Pathol. 23:54–61.
2010.PubMed/NCBI
|
|
32
|
Lauwers GY, Wahl SJ, Melamed J and
Rojas-Corona RR: p53 expression in precancerous gastric lesions: an
immunohistochemical study of PAb 1801 monoclonal antibody on
adenomatous and hyperplastic gastric polyps. Am J Gastroenterol.
88:1916–1919. 1993.
|