Introduction
Extravasation is the leakage or direct infiltration
of a chemotherapeutic agent from a vessel into the surrounding
tissues (1) and has been reported
to occur in 0.1–6.5% of chemotherapy infusions (2). Docetaxel is an antineoplastic agent
widely used in the treatment of numerous solid tumors, including
breast, lung, ovarian and prostate carcinomas (3). The well-known adverse effects of
docetaxel include neutropenia, fluid retention, neuropathy,
hypersensitivity reaction, alopecia, mucositis or nail changes
(4). However, extravasation of
docetaxel has been reported to cause significant local tissue
injury, despite the fact that it was commonly classified as a
non-vesicant chemotherapeutic agent (5). This report presents the case of a
breast cancer patient that exhibited a significantly delayed skin
reaction one day after extravasation and relapsed one week
following docetaxel extravasation. The patient provided written
informed consent.
Case report
A 47-year-old female diagnosed with pathological
stage IC triple negative breast infiltrative ductal carcinoma
received a modified radical mastectomy of the left breast in
October 2012. The patient was admitted to the Chang Gung Memorial
Hospital (Keelung, Taiwan) where they received the first cycle of
docetaxel in March 2013, in an adjuvant setting. The chemotherapy
was administered via a portacath inserted into the left subclavian
vein at a dose of 75 mg/m2 docetaxel diluted in 250 ml
of 0.9% saline. Once ~250 ml was administered, the patient noticed
a wet sensation and swelling in a small area around the cannula due
to an accidental extravasation; the infusion was immediately
discontinued. An aspiration of docetaxel was not possible,
therefore, the cannula line was withdrawn. No immediate
abnormalities were noted at the infiltration site and local cooling
was initiated. However, on the following day the symptoms worsened
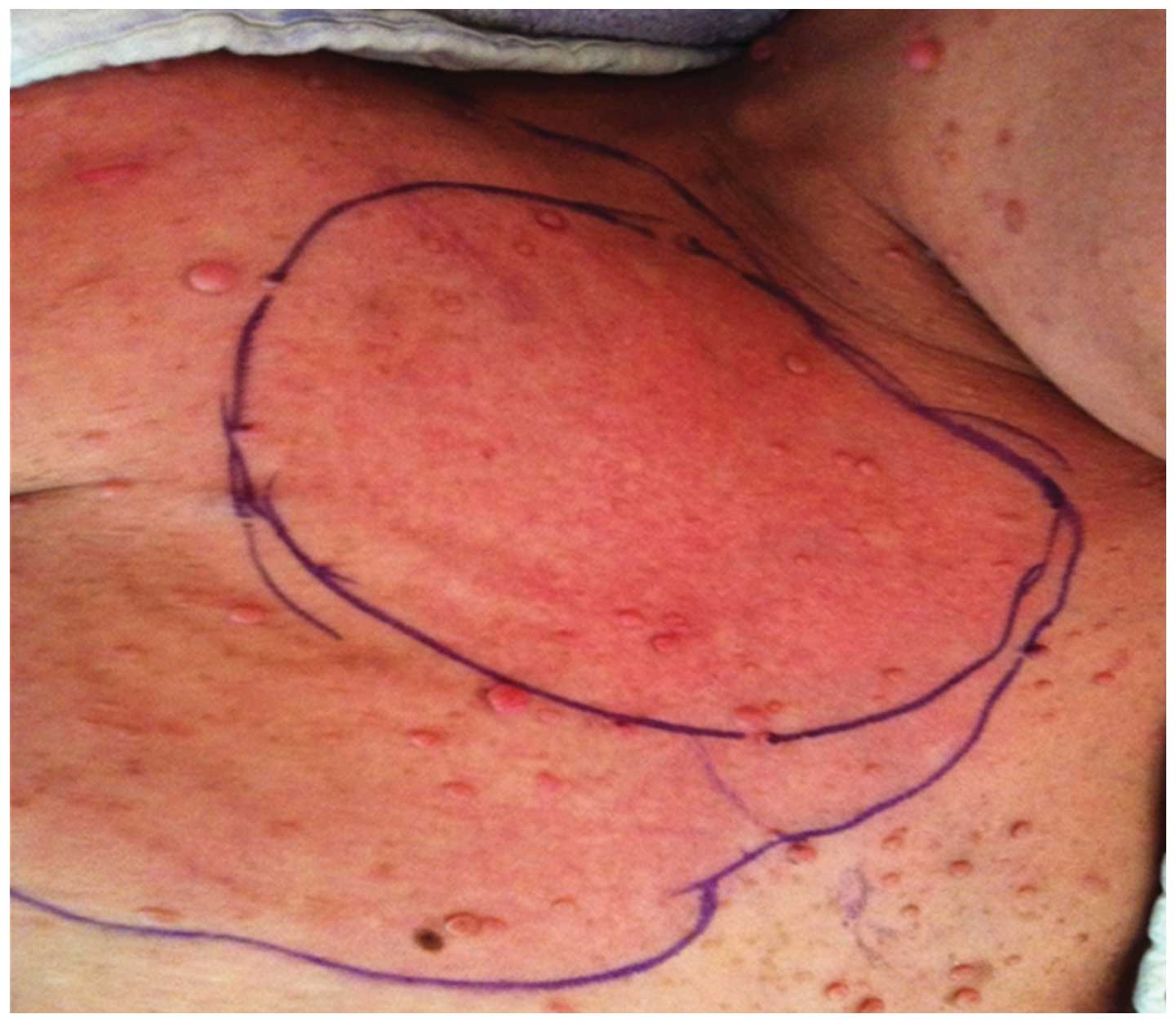
with marked swelling, erythema and pruritus of the right chest
wall, spanning an area of ~17×11 cm (Fig. 1). The range of motion of the left
shoulder was limited by the tenderness and edema. The patient was
treated with subcutaneous methylprednisolone around the site of
extravasation; in addition, 5 mg intravenous dexamethasone and 5 mg
chlorpheniramine were administered two times per day for three
days. The symptoms of pain and swelling improved thereafter.
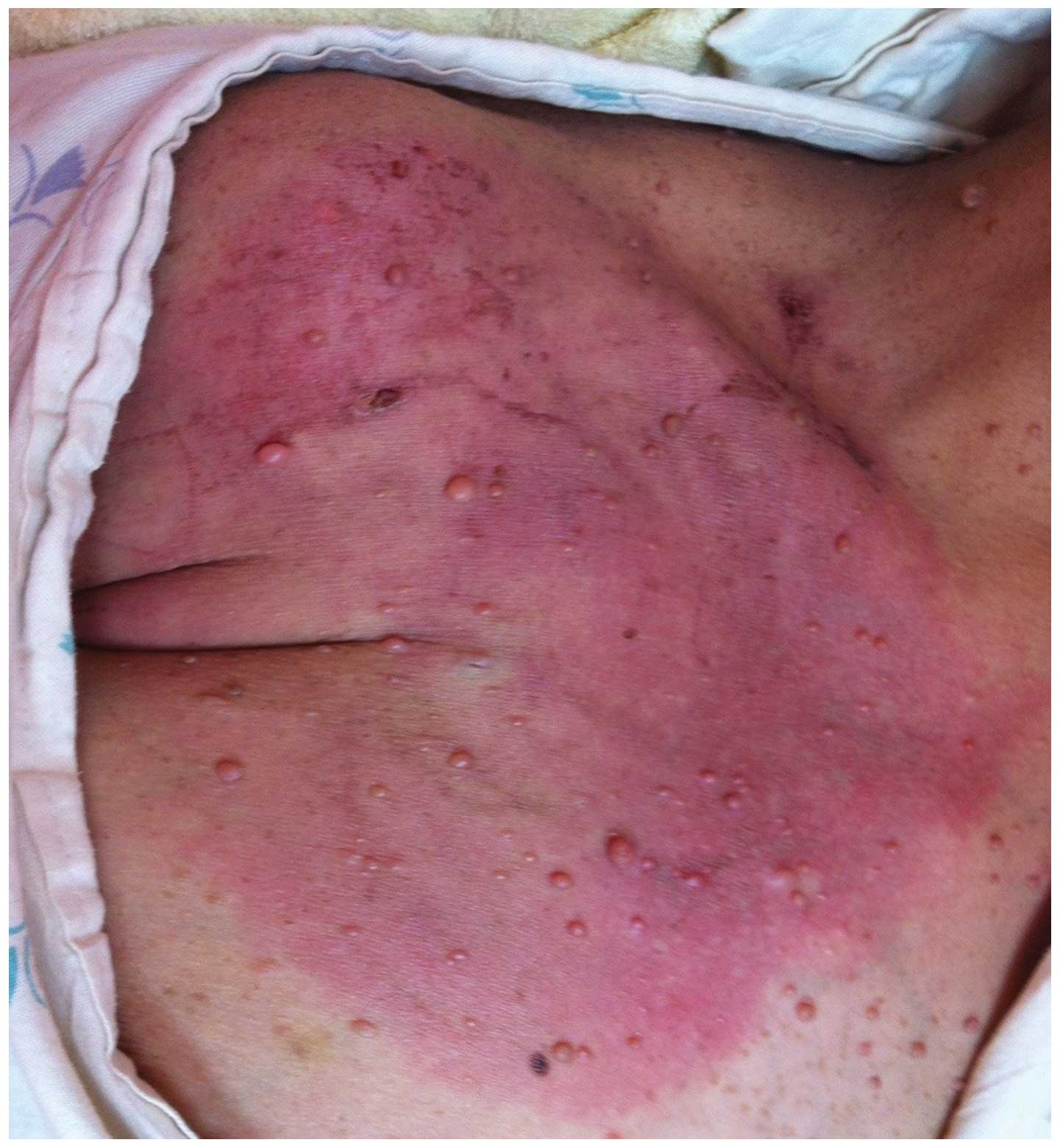
Unfortunately, the erythema relapsed and progressed to 18×15 cm in
size one week following extravasation (Fig. 2). Topical steroid ointment and
systemic dexamethasone was prescribed and the erythema gradually
improved. Three weeks after extravasation, desquamation of the
affected skin occurred and slight erythema was observed. There was
only a mild hyperpigmentation and the range of motion returned to
normal in the left shoulder. Furthermore, an angiography
examination of the portacath revealed no abnormalities. The patient
received an additional three cycles of docetaxel infusion without
experiencing further skin reactions.
Discussion
Extravasation has been reported to occur in 0.1–6.5%
of chemotherapy infusions (2). The
increasing number of different cytotoxic agents that patients are
exposed to may increase the prevalence of this complication. There
are various chemotherapeutic agents, which may be classified as
either irritants or vesicants depending on the reaction that is
produced following extravasation onto the skin (3). Irritant agents cause pain at the
injection site or along the vein, and may or may not result in an
inflammatory reaction. Vesicant agents are more significant as they
may induce a wide spectrum of lesions varying from mild erythema,
swelling, and formation of blisters to tissue destruction,
including ulceration and necrosis (3). Paclitaxel is a member of the taxoid
family that has been recognized as a clear vesicant agent (4). By contrast, docetaxel, also a member
of this family, is increasingly used to treat a wider spectrum of
solid tumors and has been widely classified as an irritant;
however, its vesicant potential should not be ignored (3,5).
The early manifestations of docetaxel extravasation
may be subtle. Furthermore, the clinical symptoms may immediately
appear following a leakage or may be delayed for weeks. Raley et
al (3) reported that a patient
developed clinical symptoms of extravasation, including erythema,
pain and blisters, six days after a docetaxel extravasation. By
contrast, our patient did not present with symptoms or signs of
extravasation immediately; the symptoms developed after one day.
The patients’ symptoms markedly improved following medical
management, however, the skin erythema relapsed and progressed one
week later. The differential diagnoses of skin lesions include
recall dermatitis and fixed drug eruptions. The combination of
persistent skin lesions following an extravasation as well as
aggravation of the symptoms within seven days are unlikely to
result in a diagnosis of recall dermatitis (6). As the skin lesions developed only at
the extravasation site and there was no previous history of
docetaxel treatment, an extravasation reaction was the favored
diagnosis, rather than a fixed drug eruption. Although there is no
consensus regarding the optimum treatment for extravasation
injuries, cooling with ice, and the administration of topical
steroids and antibiotics appear to provide good results (7). In addition, anti-inflammatory agents
may be administered to relieve pain; however, to the best of our
knowledge, plastic surgery has not been used to treat docetaxel
extravasation in the published literature to date.
In conclusion, this report presented a rare case of
a delayed skin reaction and a relapse of symptoms following a
docetaxel extravasation injury. This unusual clinical presentation
indicates that practitioners should carefully monitor additional
skin reactions in the weeks following an extravasation to observe
whether delayed or relapsed reactions occur. In addition, clinical
practitioners should be more aware of this potential complication
so as to better diagnose and promptly treat the symptoms, and avoid
sequelae (8); a better outcome
would be to avoid injury by improving the use of venipunctures and
infusions. Patients should also be made aware of the possibility of
an extravasation occurring, so that it may be reported promptly to
minimize the extravasation-related symptoms.
References
|
1
|
Bronner AK and Hood AF: Cutaneous
complications of chemotherapeutic agents. J Am Acad Dermatol.
9:645–663. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Albanell J and Baselga J: Systemic therapy
emergencies. Semin Oncol. 27:347–361. 2000.
|
|
3
|
Raley J, Geisler JP, Buekers TE and
Sorosky JI: Docetaxel extravasation causing significant delayed
tissue injury. Gynecol Oncol. 78:259–260. 2000. View Article : Google Scholar
|
|
4
|
Ener RA, Meglathery SB and Styler M:
Extravasation of systemic hemato-oncological therapies. Ann Oncol.
15:858–862. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ho CH, Yang CH and Chu CY: Vesicant-type
reaction due to docetaxel extravasation. Acta Derm Venereol.
83:467–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Susser WS, Whitaker-Worth DL and
Grant-Kels JM: Mucocutaneous reactions to chemotherapy. J Am Acad
Dermatol. 40:367–398. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uña E, Cuadrillero F and López-Lara F:
Drug extravasation: a dreaded complication. BMJ Case Rep.
2009:bcr09.2008.0887. 2009.PubMed/NCBI
|
|
8
|
Ascherman JA, Knowles SL and Attkiss K:
Docetaxel (taxotere) extravasation: a report of five cases with
treatment recommendations. Ann Plast Surg. 45:438–441. 2000.
View Article : Google Scholar
|