Introduction
A malignant peripheral nerve sheath tumor (MPNST) is
a rare tumor that accounts for ≤5% of soft-tissue sarcomas, often
arising from Schwann cells (1).
Approximately 50% of MPNSTs occur in patients with
neurofibromatosis type 1 (NF1), and 10% of these are
radiation-induced, while the remainder affect individuals without a
known genetic predisposition. The most common locations for MPNSTs
are the trunk, extremities and head and neck (2). Isolated studies have reported the
development of MPNST following irradiation and primary or
metastatic intradural MPNST of the spine (3–5).
Spinal MPNST usually develops from spinal nerve roots and causes
secondary bony changes. However, primary intraosseous MPNST is
extremely rare and, to the best of our knowledge, only five cases
in the spine have been reported in the literature (6–10). As
the tumors have a high malignancy and invasive natural course with
a common recurrence and distant metastases, they have a poor
prognosis, even with gross total resection.
An additional case of intraosseous MPNST of the
lumbar spine in a patient without NF1 is presented in the current
study, and the relevant literature on intraosseous MPNST is
reviewed. The study was conducted following a clinical research
review by the ethics committee of Toyama University Hospital
(Toyama City, Japan). Informed consent was obtained from the
patient, who was advised that the data from the case would be
submitted for publication.
Case report
Case summary
A 45-year-old female presented with a 1-month
history of severe lower back pain and pain radiating to the left
leg. No ‘café au lait’ spots or neurofibromas were present and the
patient confirmed there was no family history of NF1. The
neurological examination revealed a decreased sensation to a pin
prick in the left L4 area. There was no obvious motor weakness of
the leg, and bladder and bowel dysfunction was evident. Plain
radiographs of the lumbar spine showed an osteolytic lesion at the
L4 vertebral body. Computed tomography revealed compression of the
spinal canal resulting from destruction of the posterior elements
of L4 (Fig. 1). Magnetic resonance
imaging (MRI) showed a destructive lesion and extradural tumor at
the L4 level with dural compression. The lesion extending from the
L4 vertebral body to bilateral pedicles showed intermediate signal
intensity on T1-weighted images (Fig.
2A and C), and it was heterogeneous with mixed high and
intermediate signal intensities on T2-weighted images (Fig. 2B and D). No other tumors were
identified.
As the paralysis progressed rapidly, decompressive
laminectomies and extradural tumor resection were performed. At the
same time, posterior spinal fusion with instruments including a
percutaneous pedicle screw system (Mantis, Stryker Japan Co.,
Tokyo, Japan) was performed for maintenance of spinal stability and
prevention of tumor dissemination. The tumor appeared slightly
adherent to the dura. The tumor mass was not obviously connected
with the bilateral L4 spinal nerve roots or the dura. The boundary
between the tumor and the L4 vertebral body was unclear.
Histologically, the tumor consisted of coagulation necrosis and
sheets of tumor cells with alternating areas of hyper- and
hypocellularity. The majority of the tumor cells were atypical
spindle cells. The spindle cells exhibited mitotic figures and
pleomorphism. Immunohistochemically, the spindle cells were
positive for vimentin and smooth muscle actin and negative for
S-100, epithelial membrane antigen (EMA) and cluster of
differentiation 34 (CD34). The histological diagnosis was
undifferentiated pleomorphic sarcoma. Postoperatively, the symptoms
of the patient were dramatically relieved. However, they recurred
two weeks following the surgery, and the patient presented with
progressive hypoesthesia and motor weakness in the legs. A total
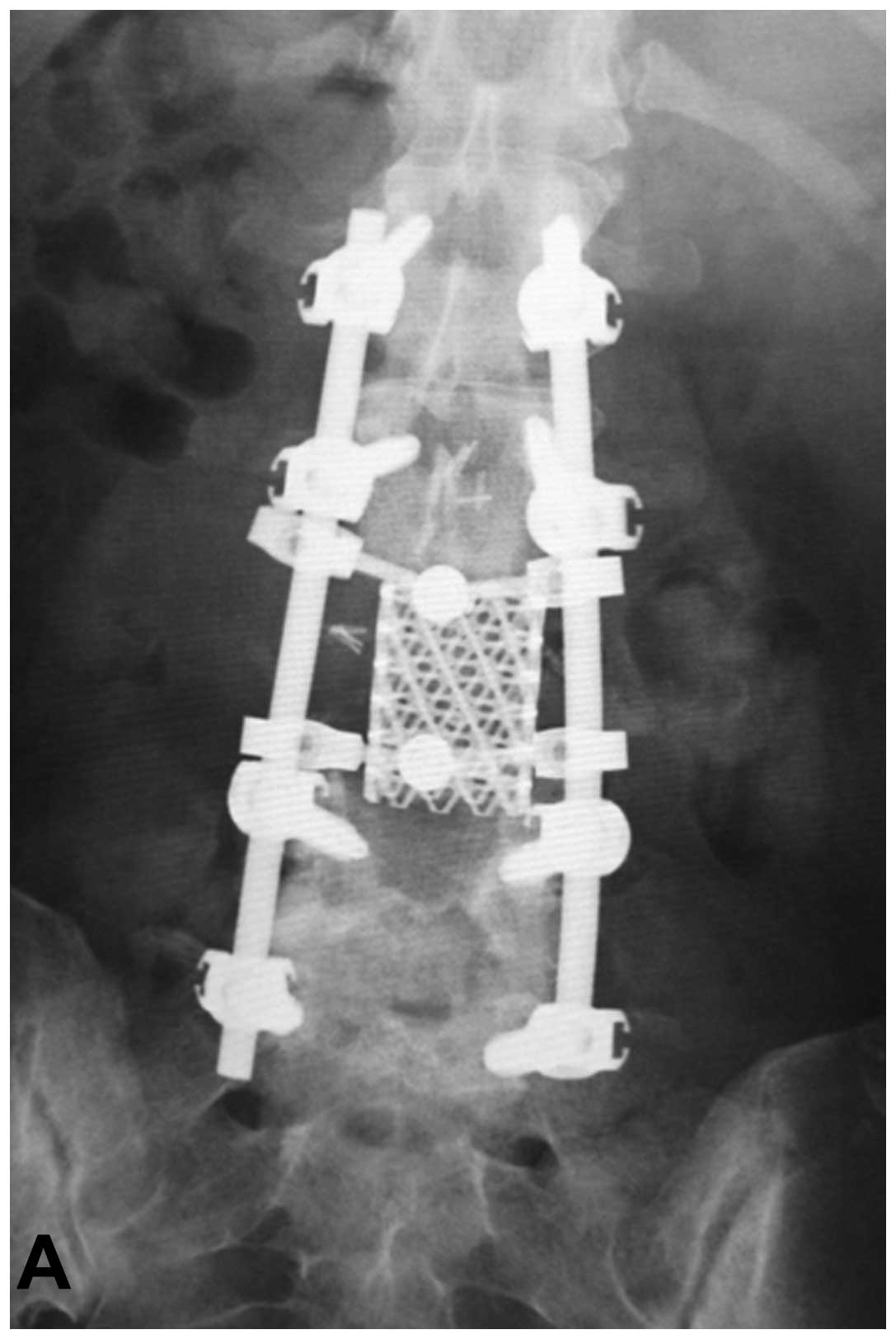
en bloc spondylectomy was performed according to a previous
study (Fig. 3) (11). Briefly, all posterior elements of
the spine (the spinous process, the superior and inferior articular
processes, the transverse process and the pedicle) were removed in
one section using a posterior approach. Subsequently, the anterior
elements of the spine (the vertebral body and psoas muscle) were
removed en bloc at the coincident level of the posterior
halves using an anterior midline transperitoneal approach. To
maintain stability of the tumor resection, posterior and anterior
instrumented fixation was performed.
Although multidrug adjuvant chemotherapy (30
mg/m2 doxorubicin, 1.5 g/m2 ifosfamide and
300 mg/m2 dacarbazine; 3 days] was administered
following the second surgery (12),
the patient succumbed to intramedullary dissemination and
carcinomatous meningitis 8 months following the initial
consultation.
Pathological study
The second postoperative histopathological
examination revealed a densely cellular area composed of spindle
cells with a fascicular growth pattern and coagulation necrosis.
Each of the spindle cells exhibited irregular contours with
abundant gross cellular atypia, mitotic figures and pleomorphism
(Fig. 4). On immunohistochemical
staining, the tumor cells were positive for vimentin and bcl-2,
focally positive for EMA, and negative for AE1/AE3, desmin, S-100,
CD99 and CD34.
Fluorescence in situ hybridization (FISH)
analysis
FISH was performed on an unstained,
paraffin-embedded tissue section using the Dual Color, Break Apart
Rearrangement Probe (Kreatech Diagnostics, Amsterdam, Netherlands)
according to the manufacturer’s instructions. Hybridization signals
were assessed in 200 interphase nuclei with strong, well-delineated
signals and distinct nuclear borders by two individuals, as
previously described (13). Dual
color FISH analysis involving chromosome 17q showed that >10% of
the cells from the tumor showed the deletion signal pattern of one
red (NF1 region probe on 17q11) and two green
[myeloperoxidase (MPO) gene region on 17q22 as the control
probe), demonstrating a deletion of the NF1 gene (Fig. 5). These histopathological and
cytomolecular findings confirmed the diagnosis of MPNST with focal
epithelioid features.
Discussion
MPNSTs are rare malignant tumors arising from
peripheral nerve sheath cells. These tumors typically present as an
enlarging mass originating from a peripheral nerve root of the
trunk (~50%), in the extremities (~30%), or the head and neck
region (~20%) (2). Secondary bony
infiltration in a paraspinal MPNST is a well-known entity. However,
a primary intraosseous MPNST is extremely rare. An intraosseous
MPNST may develop from minute, mainly unmyelinated nerve roots that
accompany nutrient vessels and ramify within Volkmann’s canals and
bone marrow (14–16). To the best of our knowledge, only 19
cases of intraosseous MPNSTs, including the present study, have
been reported in the literature to date (Table I) (6–10,17–29).
There were nine male and 10 female patients, and the age at
diagnosis ranged from 4–76 years. A total of eight of the 19 cases
(42%) occurred in the mandible or maxilla and six cases (32%)
involved a vertebral body of the spine. Although <50% of MPNSTs
arise in patients with NF1 (29),
intraosseous MPNST cases were not associated with the hereditary
NF1 syndrome, except for one case. The case of the present study
was also not associated with NF1.
 | Table ISummary of reported cases of
intraosseous MPNST. |
Table I
Summary of reported cases of
intraosseous MPNST.
| Case (ref.) | Age
(years)/Gender | Location | NF1 | Surgery | Adjuvant therapy | Outcome (months) |
|---|
| 1 (17) | 55/M | Ulna | - | CR | None | NED (20) |
| 2 (18) | 65/F | Mandible | - | NA | NA | NA |
| 3 (19) | 65/M | Mandible | - | Resection | RT | AWD (NA):
recurrence |
| 4 (20) | 4/F | Mandible | NA | NA | NA | NA |
| 5 (21) | 28/M | Ulna | - | NA | NA | NA |
| 6 (22) | 11/F | Mandible | - | Resection | None | NED (6) |
| 7 (23) | 76/F | Mandible | - | NA | NA | NA |
| 8 (24) | 61/F | Maxilla | - | NA | NA | NA |
| 9 (25) | 47/F | Maxilla | - | Resection | RT, CT | DOD (22) |
| 10 (26) | 50/M | Mandible | - | NA | RT | DOD (12) |
| 11 (27) | 28/M | Femur | - | Resection | None | DOD (1): pulmonary
metastasis |
| 12 (28) | 26/M | Femur | - | Resection | CT | DOD (15): pulmonary
metastasis |
| 13 (29) | 29/M | Ulna | - | Resection | None | DOD (36): pulmonary
metastasis |
| 14 (6) | 40/F | C2 | NA | STR, PSF | None | DOD (12): pulmonary
metastasis |
| 15 (7) | 59/F | T3 | - | STR, PSF | RT, CT | AWD (46): bone
metastasis |
| 16 (8) | 75/F | T7 | - | STR, PSF | RT | DOD (6): pulmonary
metastasis |
| 17 (9) | 41/M | C7 | - | CR, PSF | CT | NED (24) |
| 18 (10) | 75/M | L3 | - | STR | NA | NA |
| 19 (present) | 45/F | L4 | - | STR, PSF | CT | DOD (8):
carcinomatous meningitis |
Due to a wide morphologic spectrum and lack of
specific markers, the diagnosis of intraosseous MPNST is extremely
difficult, particularly in a case lacking the manifestations of NF1
and classic histopathological features. High-grade MPNSTs may
resemble other malignancies, including fibrosarcoma, synovial
sarcoma and malignant fibrous histiocytoma. In the present study,
although the initial histological diagnosis was an undifferentiated
pleomorphic sarcoma, the histological diagnosis of the recurrent
mass was MPNST with positive immunohistochemistry for vimentin and
bcl-2, focally positive for EMA, but negative for AE1/AE3, desmin,
S-100, CD99 and CD34. Numerous antigens are useful in the
identification of nerve sheath differentiation, including S-100
protein, Leu-7 and myelin basic protein. The protein S-100 is the
most commonly used antigen for neural differentiation, and it can
be identified in ~50% of MPNSTs, although the staining is typically
focal and limited to a small number of cells. As encountering an
MPNST with a strong and diffuse immunoreactivity for the S-100
protein is uncommon, such a staining pattern always indicates that
other benign diagnoses should be reconsidered, particularly
cellular schwannoma. The proteins myelin basic protein and Leu-7
have been identified in ~40 and ~50% of MPNSTs, respectively
(30).
The molecular pathogenesis of MPNST remains poorly
understood. In contrast to numerous other sarcomas, there is no
pathognomonic chromosomal translocation and conventional
cytogenetic studies typically yield complex, often near-triploid
karyotypes without specific aberrations (1). However, loss involving chromosome 17q,
the site of the NF1, has been detected in 25–50% of reported
sporadic and NF1-associated cases, often in the form of chromosomal
monosomy. Similarly, NF1 deletion in FISH analysis may aid
in distinguishing MPNSTs from other high-grade malignancies with
overlapping morphological features. Perry et al (31) demonstrated that NF1 deletions
were detectable in 76% of sporadic and NF1-associated MPNSTs by
FISH analysis and observed in five out of six high-grade MPNSTs
that lacked S-100 protein immunoreactivity, which is considered to
be a significant marker for Schwann cells. Additionally, it has
been reported that of eight cases with MPNST, NF1 deletion
was detected within the S-100-positive cellular populations of four
MPNSTs (50%), while S-100-negative nuclei were observed in all
eight MPNSTs. These results indicated the prevalence of NF1
deletion in MPNSTs, regardless of S-100 protein expression
(32). In the case of the present
study, immunohistochemistry for S-100 protein was negative in tumor
cells, and NF1 deletion by FISH was simultaneously detected.
Therefore, MPNST in the present case was believed to be a
high-grade malignancy from the viewpoint of the S-100
protein-negative cells, which represent dedifferentiated Schwann
cells.
Surgical resection is the treatment of choice for
MPNSTs. Complete tumor resection with negative margins remains the
most effective treatment. A study by Wong et al (33) reported that the rate of en
bloc resection in 128 patients with MPNSTs was 83% and, of
these patients, only 48% had negative surgical margins. However,
en bloc resection is often extremely complicated in cases of
spinal MPNST due to the surrounding spinal cord, dura mater and
large blood vessels. In previous studies, four out of the five
vertebral MPNSTs underwent subtotal resection, and the outcomes of
three cases included two who succumbed to disease and one who
remained with disease (Table I).
Adjuvant radiation therapy to a dose of >60 Gy improves local
control of this disease (34). The
effectiveness of chemotherapy for MPNST remains controversial.
Certain success has been reported with doxorubicin use alone or in
combination with other drugs (35).
However, the effects of chemotherapy and radiotherapy are
unclear.
In conclusion, the current study presents a case of
intraosseous MPNST arising in a lumbar vertebra. Although en
bloc resection of the tumor and adjuvant chemotherapy were
performed, the patient succumbed to carcinomatous meningitis. Since
the outcome remains poor, further studies on genetic therapy of the
tumor based on its molecular pathogenesis are required.
Acknowledgements
This study was supported in part by a Grant-in-Aid
for Scientific Research [(C) 24592227 (KAKENHI)]. The authors would
like to thank Professor Tadashi Hasegawa, Department of Surgical
Pathology, Sapporo Medical University (Japan) for providing
excellent FISH analysis. The abstract was presented at the
International Society of Limb Salvage 17th General Meeting
(abstract no. 396).
References
|
1
|
Weiss SW and Goldblum JR: Malignant tumors
of the peripheral nerves. Enzinger and Weiss’s Soft Tissue Tumors.
5th edition. Mosby; St. Louis, MO: pp. 903–916. 2007
|
|
2
|
Ducatman BS, Scheithauer BW, Piepgras DG,
Reiman HM and Ilstrup DM: Malignant peripheral nerve sheath tumors.
A clinicopathologic study of 120 cases. Cancer. 57:2006–2021. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amin A, Saifuddin A, Flanagan A, Patterson
D and Lehovsky J: Radiotherapy-induced malignant peripheral nerve
sheath tumor of the cauda equina. Spine (Phila Pa 1976).
29:E506–E509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baek WS, Pytel P, Undevia SD and Rubeiz H:
Spinal cord metastasis of a non-neurofibromatosis type-1 malignant
peripheral nerve sheath tumor: an unusual manifestation of a rare
tumor. J Neurooncol. 74:183–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Acharya R, Bhalla S and Sehgal AD:
Malignant peripheral nerve sheath tumor of the cauda equina. Neurol
Sci. 22:267–270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan RJ, Asgher J, Sohail MT and Chughtai
AS: Primary intraosseous malignant peripheral nerve sheath tumor: a
case report and review of the literature. Pathology. 30:237–241.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gnanalingham K, Bhattacharjee S and
O’Neill K: Intraosseous malignant peripheral nerve sheath tumor
(MPNST) of the thoracic spine: a rare cause of spinal cord
compression. Spine (Phila Pa 1976). 29:E402–E405. 2004. View Article : Google Scholar
|
|
8
|
Miyakoshi N, Nishikawa Y, Shimada Y, et
al: Intraosseous malignant peripheral nerve sheath tumor with focal
epithelioid differentiation of the thoracic spine. Neurol India.
55:64–66. 2007. View Article : Google Scholar
|
|
9
|
Moon SJ, Lee JK, Seo BR, et al: An
intraosseous malignant peripheral nerve sheath tumor of the
cervical spine: a case report and review of the literature. Spine
(Phila Pa 1976). 33:E712–E716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patnaik A, Mishra SS, Senapati SB, et al:
Primary intraosseous malignant peripheral nerve sheath tumor of
spine with a giant paraspinal and retrospinal subcutaneous
extension. Surg Neurol Int. 3:1572012. View Article : Google Scholar
|
|
11
|
Kawahara N, Tomita K, Murakami H, et al:
Total en bloc spondylectomy of the lower lumbar spine: a surgical
techniques of combined posterior-anterior approach. Spine (Phila Pa
1976). 36:74–82. 2011.PubMed/NCBI
|
|
12
|
Elias A, Ryan L, Sulkes A, et al: Response
to mesna, doxorubicin, ifosfamide, and dacarbazine in 108 patients
with metastatic or unresectable sarcoma and no prior chemotherapy.
J Clin Oncol. 7:1208–1216. 1989.PubMed/NCBI
|
|
13
|
Yasuda T, Perry KD, Nelson M, et al:
Alveolar rhabdomyosarcoma of the head and neck region in older
adults: genetic characterization and a review of the literature.
Hum Pathol. 40:341–348. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nannapaneni R and Sinar EJ: Intraosseous
schwannoma of the cervical spine. Br J Neurosurg. 19:244–247. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang CJ, Huang JS, Wang YC and Huang SH:
Intraosseous schwannoma of the fourth lumbar vertebra: case report.
Neurosurgery. 43:1219–1222. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Polkey CE: Intraosseous neurilemmoma of
the cervical spine causing paraparesis and treated by resection and
grafting. J Neurol Neurosurg Psychiatry. 38:776–781. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peers JH: Primary intramedullary
neurogenic sarcoma of the ulna: Report of a case. Am J Pathol.
10:811–820. 1934.PubMed/NCBI
|
|
18
|
Bell WE: Neurogenic sarcoma of the
mandible: report of a case. J Am Dent Assoc. 23:1351–1357.
1936.
|
|
19
|
Devore DT and Waldron CA: Malignant
peripheral nerve tumors of the oral cavity. Review of the
literature and report of a case. Oral Surg Oral Med Oral Pathol.
14:56–68. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ingram FL: Radiology of tumours of the
mandible. Clin Radiol. 13:47–53. 1962. View Article : Google Scholar
|
|
21
|
Bose KS, Thakur S, Chakrabarty S and
Banerjee S: Intra-osseous malignant schwannoma. J Indian Med Assoc.
54:328–329. 1970.PubMed/NCBI
|
|
22
|
Glass RT and Livingston RJ: Malignant
epithelioid schwannoma in a child: a case report. Quintessence Int
Dent Dig. 15:1047–1450. 1984.PubMed/NCBI
|
|
23
|
Shirasuna K, Fukuda Y, Kitamura, et al:
Malignant schwannoma of the mandible. Int J Oral Maxillofac Surg.
15:772–776. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kameyama Y, Maeda H, Nakane S, et al:
Malignant schwannoma of the maxilla in a patient without
neurofibromatosis. Histopathology. 11:1205–1208. 1987.PubMed/NCBI
|
|
25
|
Urade M, Fujimoto Y, Ogura T and Matsuya
T: Malignant schwannoma and melanoma occurring in the maxilla. J
Osaka Univ Dent Sch. 30:153–156. 1990.PubMed/NCBI
|
|
26
|
Bailet JW, Abemayor E, Andrews JC, et al:
Malignant nerve sheath tumors of the head and neck: a combined
experience from two university hospitals. Laryngoscope.
101:1044–1049. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bullock MJ, Bedard YC, Bell RS and Kandel
R: Intraosseous malignant peripheral nerve sheath tumor: report of
a case and review of the literature. Arch Pathol Lab Med.
11:367–370. 1995.PubMed/NCBI
|
|
28
|
Terry DG, Sauser DD and Gordon MD:
Intraosseous malignant peripheral nerve sheath tumor in a patient
with neurofibromatosis. Skeletal Radiol. 27:346–349. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kendi TK, Erakar A, Yildiz HY, Saglik Y
and Erekul S: Intraosseous malignant peripheral nerve sheath tumor
with local recurrence, lung metastases and death. Skeletal Radiol.
33:223–225. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wick MR, Swanson PE, Scheithauer BW and
Manivel JC: Malignant peripheral nerve sheath tumor: An
immunohistochemical study of 62 cases. Am J Clin Pathol.
87:425–433. 1987.PubMed/NCBI
|
|
31
|
Perry A, Kunz SN, Fuller CE, et al:
Differential NF1, p16, and EGFR patterns by interphase cytogenetics
(FISH) in malignant peripheral nerve sheath tumor (MPNST) and
morphologically similar spindle cell neoplasms. J Neuropathol Exp
Neurol. 61:702–709. 2002.
|
|
32
|
Perry A, Roth KA, Banerjee R, et al: NF1
deletions in S-100 protein-positive and negative cells of sporadic
and neurofibromatosis 1 (NF1)-associated plexiform neurofibromas
and malignant peripheral nerve sheath tumors. Am J Pathol.
159:57–61. 2001. View Article : Google Scholar
|
|
33
|
Wong WW, Hirose T, Scheithauer BW, et al:
Malignant peripheral nerve sheath tumor: analysis of treatment
outcome. Int J Radiat Oncol Biol Phys. 42:351–360. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Anghileri M, Miceli R, Fiore M, et al:
Malignant peripheral nerve sheath tumors: prognostic factors and
survival in a series of patients treated at a single institution.
Cancer. 107:1065–1074. 2006. View Article : Google Scholar
|
|
35
|
Goldman RL, Jones SE and Heusinkveld RS:
Combination chemotherapy of metastatic malignant schwannoma with
vincristine, adriamycin, cyclophosphamide, and imidazole
carboxamide: a case report. Cancer. 39:1955–1958. 1977. View Article : Google Scholar
|