Introduction
Mesenchymal tumors of the gastrointestinal tract are
uncommon, representing only a small percentage of gastrointestinal
neoplasms (1–7). The tumors are generally localized
within the submucosa as intramural nodules that can to lead to
obstruction, ulceration and bleeding (3,8,9). The
majority of these tumors, including leiomyomas, schwannomas and
neurofibromas, show benign behavior, even if their malignant
counterparts have been reported (2,7,8).
Nevertheless, among spindle cell tumors, gastrointestinal stromal
tumors (GISTs) represent the most commonly occurring event, but
they are characterized by a different prognosis and clinical
management, and therefore require a diagnostic distinction from the
other entities, mainly from leiomyomas (3,7,10–14).
The differential diagnosis between GISTs and gastrointestinal
leiomyomas offers certain difficulties, not only due to their
overlapping clinical and ultrasound presentations, but also due to
their cytological appearance, largely represented by spindle
cells.
Endoscopic ultrasound-guided fine-needle aspiration
cytology (EUS-FNAC) has proven itself to be a reliable method for
the diagnosis of GISTs (15) and
other gastrointestinal mesenchymal tumors, including true
leiomyomas (15). The present study
reports two cases of true intramural leiomyomas of the esophagus,
in which EUS-FNAC allowed the sampling of the submucosal lesions,
which are otherwise difficult to biopsy by traditional methods;
moreover, the immunophenotypic profile readily obtained from cell
blocks aided in the definition of these lesions, distinguishing
them from other gastrointestinal stromal or mesenchymal tumors.
Patients provided written informed consent.
Case reports
Case 1
A 43-year-old male presented with dysphagia that had
been apparent for 2 months. A physical examination revealed no
abnormalities, and the standard serum laboratory tests were in the
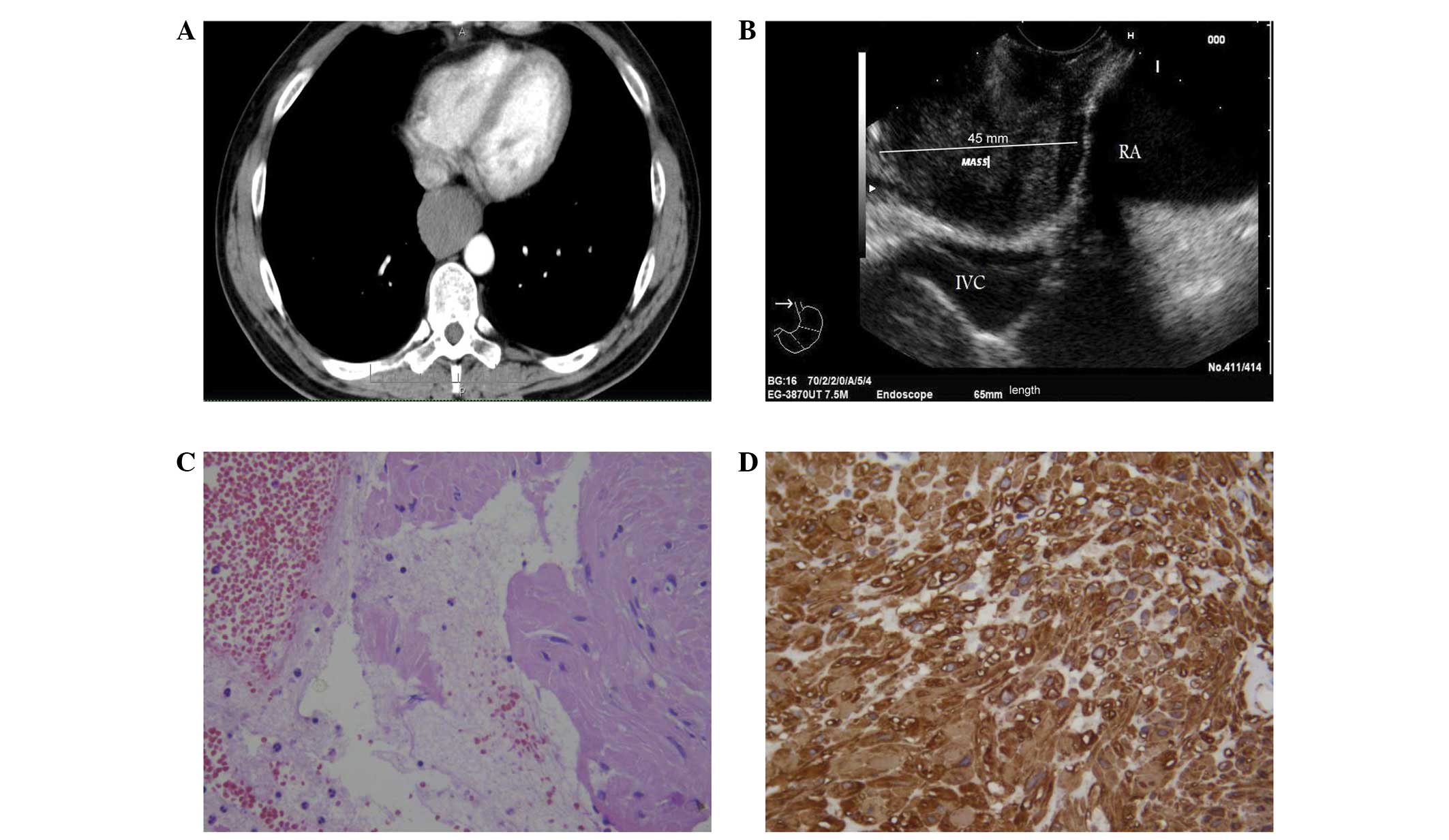
normal range. Computed tomography (CT) scans of the chest revealed
a hypodense mass developing in the distal esophagus and causing
substenosis of the lumen, which was extended to 65 mm in length,
with non-homogeneous contrast enhancement (Fig. 1A). EUS examination revealed a 45-mm
hypoechoic, round lesion with well demarcated margins, originating
from the muscle layer of the distal esophagus in contact with the
inferior caval vein and right atrium (Fig. 1B). EUS-FNAC was performed by using a
convex array echoendoscope (EG-3870 UTK; Pentax, Co., Ltd., Tokyo,
Japan) and by making two passes with a 22G needle. The specimens
were processed by an in-room cytopathologist and immediately
examined for adequate cellularity following staining by hematoxylin
and eosin. A second slide was immediately fixed in 98% ethanol and
stained with Papanicolaou. Any excess materials, including the
needle and syringe utilized in the procedure, were rinsed in 10 ml
50% ethanol in a specimen container. All content was centrifuged in
a 10-ml disposable centrifuge tube at 5,017 × g for 6 min to create
1 or 2 pellets; the supernatant fluid was decanted and the pelleted
material was immediately fixed in a freshly prepared solution of 4%
neutral buffered formalin for 45 min. The cell pellets were then
placed in a cassette and stored at 80% ethanol until ready for
processing in an automatic tissue processor (Leica TP1020; Leica,
Buckinghamshire, UK). The cell blocks obtained were embedded in
paraffin at 56°C, and 3-μm thick successive sections were cut and
routinely stained by hematoxylin and eosin; parallel serial
sections of the same thickness were mounted on silane-coated
glasses and submitted to immunohistochemical procedures, as
described previously (16,17).
Case 2
A 39-year-old female presented with dyspepsia and
esophageal reflux that had been apparent for 4 weeks. There was no
weight loss, but nausea and mild vomiting were occasionally
present. Upon physical examination, local peri-gastric discomfort
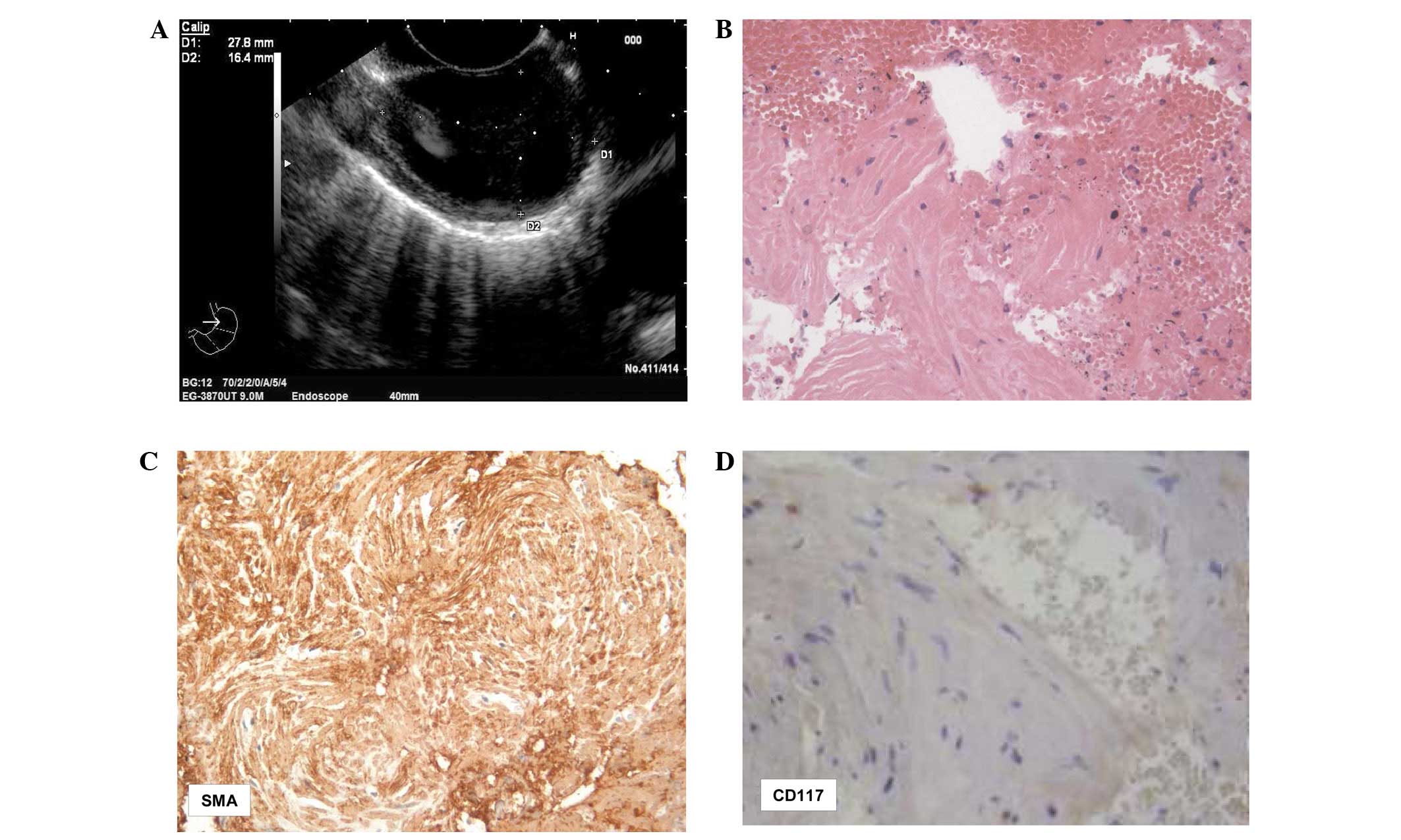
and pain were noted. EUS scanning showed a 27.8×16.4-mm ovoid,
homogeneous and hypoechoic well-delimited mass originating from the
esophageal sub-mucosa (Fig. 2A). No
lesions were evident elsewhere in the abdominal organs or lymph
nodes. EUS-FNAC was performed with the same procedure as utilized
in case 1; again, adequate cellularity and one cell block were
obtained.
Following the FNAC procedures, the two patients were
observed for a period of 48 h for any procedure-related
complications.
Cytological and immunocytochemical
findings
The smears from the two cases exhibited a
hemorrhagic background, with loose clusters or small aggregates of
spindle-shaped cells (Figs. 1C and
2B) that had elongated nuclei,
occasionally showing finely granular chromatin. No mitotic figures
were found. The corresponding cell blocks documented an equivalent
morphology characterized by small tissue fragments, with relatively
low to moderate cellularity composed of monomorphic-uniform spindle
cells, eosinophilic cytoplasm and vesicular nuclei (Figs. 1C and 2B). The nuclear chromatin was finely
granular and evenly dispersed, while micronucleoli were
inconspicuous. No atypia or mitoses were noted.
Immunohistochemical procedures were carried out on
the 3-μm serial sections, utilizing the following commercially
obtained antisera (all DakoCytomation, Copenhagen, Denmark):
Vimentin [working dilution (w.d.), 1:250], smooth muscle actin
(SMA; w.d. 1:200), desmin (w.d., 1:250), CD117 (w.d., 1:150), CD34
(w.d. 1:200), S-100 (w.d., 1:400) and Ki67 (MIB-1; w.d., 1:50). In
each of the two cases, strong and diffuse cytoplasmic
immunostaining was encountered for vimentin, desmin and SMA
(Figs. 1D and 2C). No immunostaining was recorded for
S100, CD34 and CD117 (Fig. 2D). The
growth fraction, determined using Ki67 as the MIB-1 labeling index,
was extremely low and quite inconspicuous, showing <1%
positively-labeled nuclei.
In light of the microscopic examination and
immunohistochemical findings, the two esophageal lesions were
diagnosed as intraparietal true leyomiomas, without atypia. The
patients refused surgical procedures, and were lost to follow-up
subsequent to a period of 12 months.
Discussion
It is well known that the diagnostic yield of
EUS-FNAC greatly depends on the site, size and characteristics of
the target tissues, as well as certain procedural aspects (9,15,18).
By contrast, although conventional endoscopy and CT scans may
identify esophageal lesions, these procedures cannot reveal the
nature, size or origin of sub-mucosal neoplasms (7,9).
However, the efficacy of EUS-FNAC as a main diagnostic procedure is
also largely dependent on the expertise, training and interaction
between the endosonographer and cytopathologist (15). In the present study, adequate
cellular smears and corresponding cell blocks were obtained using
the EUS-FNAC approach that is used on esophageal mesenchymal
tumors, particularly true leiomyomas. Even if the observed
spindle-shaped cells with elongated nuclei could also be confused
with other gastrointestinal non-epithelial tumors, the serial
immunohistochemical procedures performed on the cell blocks allowed
acquisition of the final diagnosis. In fact, the coexistence of
desmin and SMA strongly supported the smooth muscle nature of the
observed esophageal neoplastic lesions, while the constant
negativity for CD34, CD117 and S-100 excluded other diagnostic
hypotheses, including inflammatory fibroid polyps, GISTs and
schwannomas. Consequently, the availability of an adequate number
of serial sections obtained from tissue blocks appears to be an
additional diagnostic aid in order to perform the indicated
immunohistochemical algorithm, as described previously (15,19,20).
Finally, the low growth fraction, revealed by the Ki67 labeling
index in the present study, further indicates the benign nature of
leiomyomas, thus discounting the diagnostic hypotheses of highly
malignant neoplasms, including leiomyosarcomas, spindle-cell
amelanotic melanomas and undifferentiated sarcomatoid carcinomas
(14,15).
Esophageal leiomyomas are rare benign tumors, with a
frequent asymptomatic occurrence, that do not metastasize (21). In fact, patients with these tumors
more commonly seek care due to difficulty in swallowing or as a
result of the tumors being detected during the endoscopic workup
for other diseases, as documented in case 2 of the present study.
Moreover, the progression of these neoplasms shows a slow growing
phase and the size of the lesions remains stable during the first
year of follow-up. Therefore for those patients who refuse to
receive surgical excision, as in the present cases, a periodic
follow-up with EUS has been considered preferable and more accepted
(22,23). On the other hand, the surgical
treatment for esophageal leiomyomas depends on multiple factors,
including tumor size, location, gross morphology and the patient’s
symptoms and overall condition (21,24,25).
Furthermore, indications for surgical treatment include unremitting
symptoms, a progressive increase in tumor size, mucosal ulceration
or the requirement to achieve the histopathological diagnosis due
to an inconclusive EUS-FNAC procedure (25–27).
In summary, the present study provided further
indications that EUS-FNAC has great clinico-diagnostic pre-surgical
value, also allowing a correct differential diagnosis of other
esophageal mesenchymal/stromal neoplasias with unpredictable
biological behavior to be generated by immunohistochemistry.
References
|
1
|
Matsui M, Goto H, Niwa Y, Arisawa T,
Hirooka Y and Hayakawa T: Preliminary results of fine needle
aspiration biopsy histology in upper gastrointestinal submucosal
tumors. Endoscopy. 30:750–755. 1998.
|
|
2
|
Miettinen M, Sarlomo-Rikala M, Sobin LH
and Lasota J: Esophageal stromal tumors: a clinicopathologic,
immunohistochemical, and molecular genetic study of 17 cases and
comparson with esophageal leiomyomas and leiomyosarcomas. Am J Surg
Pathol. 24:211–222. 2000.
|
|
3
|
Miettinen M, Furlong M, Sarlomo-Rikala M,
Burke A, Sobin LH and Lasota J: Gastrointestinal stromal tumors,
intramural leiomyomas, and leiomyosarcomas in the rectum and anus:
a clinicopathologic, immunohistochemical, and molecular genetic
study of 144 cases. Am J Surg Pathol. 25:1121–33. 2001.
|
|
4
|
Wieczorek TJ, Faquin WC, Rubin BP and
Cibas ES: Cytologic diagnosis of gastrointestinal stromal tumor
with emphasis on the differential diagnosis with leiomyosarcoma.
Cancer. 93:276–287. 2001.
|
|
5
|
Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya
N, Nagasaka T and Hayakawa T: The diagnosis of GI stromal tumors
with EUS-guided fine needle aspiration with immunohistochemical
analysis. Gastrointest Endosc. 55:37–43. 2002.
|
|
6
|
Trupiano JK, Stewart RE, Misick C,
Appelman HD and Goldblum JR: Gastric stromal tumors: a
clinicopathologic study of 77 cases with correlation of features
with nonaggressive and aggressive clinical behaviors. Am J Surg
Pathol. 26:705–714. 2002.
|
|
7
|
Stelow EB, Stanley MW, Mallery S, Lai R,
Linzie BM and Bardales RH: Endoscopic ultrasound-guided fine-needle
aspiration findings of gastrointestinal leiomyomas and
gastrointestinal stromal tumors. Am J Clin Pathol. 119:703–708.
2003.
|
|
8
|
Stelow EB, Jones DR and Shami VM:
Esophageal leiomyosarcoma diagnosed by endoscopic ultrasound-guided
fine-needle aspiration. Diagn Cytopathol. 35:167–170. 2007.
|
|
9
|
Jenssen C and Dietrich CF: Endoscopic
ultrasound-guided fine-needle aspiration biopsy and trucut biopsy
in gastroenterology - An overview. Brest Pract Res Clin
Gastroenterol. 23:743–759. 2009.
|
|
10
|
Fletcher CD, Berman JJ, Corless C, et al:
Diagnosis of gastrointestinal stromal tumors: A consensus approach.
Hum Pathol. 33:459–465. 2002.
|
|
11
|
Blay JY, Bonvalot S, Casali P, et al; GIST
consensus meeting panelists. Consensus meeting for the management
of gastrointestinal stromal tumors. Report of the GIST Consensus
Conference of 20–21 March 2004, under the auspices of ESMO. Ann
Oncol. 16:566–578. 2005.
|
|
12
|
Nilsson B, Bümming P, Meis-Kindblom JM, et
al: Gastrointestinal stromal tumors: the incidence, prevalence,
clinical course, and prognostication in the preimatinib mesylate
era - a population-based study in western Sweden. Cancer.
103:821–829. 2005.
|
|
13
|
Blanke CD, Demetri GD, von Mehren M, et
al: Long-term results from a randomized phase II trial of standard-
versus higher-dose imatinib mesylate for patients with unresectable
or metastatic gastrointestinal stromal tumors expressing KIT. J
Clin Oncol. 26:620–625. 2008.
|
|
14
|
Maheshwari V, Alam K, Varshney M, Jain A,
Asif Siddiqui F and Bhargava S: Fine-needle aspiration diagnosis of
GIST: a diagnostic dilemma. Diagn Cytopathol. 40:834–838. 2012.
|
|
15
|
Todaro P, Crinò SF, Pallio S, Fazzari C,
Consolo P and Tuccari G: Gastrointestinal stromal tumors of the
stomach: Cytological and immunocytochemical diagnostic features of
two cases diagnosed by endoscopic ultrasound-guided fine needle
aspiration. Oncol Lett. 5:1862–1866. 2013.
|
|
16
|
Nathan NA, Narayan E, Smith MM and Horn
MJ: Cell block cytology. Improved preparation and its efficacy in
diagnostic cytology. Am J Clin Pathol. 114:599–606. 2000.
|
|
17
|
Barresi V, Cerasoli S, Paioli G, Vitarelli
E, Giuffrè G, Guiducci G, Tuccari G and Barresi G: Caveolin-1 in
meningiomas: expression and clinico-pathological correlations. Acta
Neuropathol. 112:617–626. 2006.
|
|
18
|
Yoshinaga S, Suzuki H, Oda I and Saito Y:
Role of endoscopic ultrasound-guided fine needle aspiration
(EUS-FNA) for diagnosis of solid pancreatic masses. Dig Endosc.
23(Suppl 1): 29–33. 2011.
|
|
19
|
Wong DW, Lupton SC, Bhatt L, Gross L,
Tanière P, Peake DR, Spooner D and Geh JI: Use of imatinib mesylate
in gastrointestinal stromal tumors: Pan-Birmingham Cancer Network
experience. Clin Oncol (R Coll Radiol). 20:517–522. 2008.
|
|
20
|
Reid R, O’Dywer P, MacDuff E, et al:
Guidelines for the management of gastrointestinal stromal tumors
(GIST) in Scotland. pp. 53–55. 2009, http://www.pathologyscotland.org/download/sgpg/guidelines/gist.pdf.
Accessed November 13, 2012
|
|
21
|
Jiang W, Rice TW and Goldblum JR:
Esophageal leiomyoma: experience from a single institution. Dis
Esophagus. 26:167–174. 2013.
|
|
22
|
Choi SH, Kim YT, Han KN, Ra YJ, Kang CH,
Sung SW and Kim JH: Surgical management of the esophageal
leiomyoma: lessons from a retrospective review. Dis Esophagus.
24:325–329. 2011.
|
|
23
|
Xu GQ, Qian JJ, Chen MH, Ren GP and Che
HT: Endoscopic ultrasonography for the diagnosis and selecting
treatment of esophageal leiomyoma. J Gastroenterol Hepatol.
27:521–525. 2012.
|
|
24
|
Hatch GF 3rd, Wertheimer-Hatch L, Hatch
KF, Davis GB, Blanchard DK, Foster RS Jr and Skandalakis JE: Tumors
of the esophagus. World J Surg. 24:401–411. 2000.
|
|
25
|
Lee LS, Singhal S, Brinster CJ, Marshall
B, Kochman ML, Kaiser LR and Kucharczuk JC: Current management of
esophageal leiomyoma. J Am Coll Surg. 198:136–146. 2004.
|
|
26
|
Maish M: Esophagus. Sabiston Textbook of
Surgery. Townsend CM Jr, Beauchamp RD, Ever BM and Mattox KL: 18th
edition. Saunders; Philadelphia, PA: pp. 1087–1088. 2007
|
|
27
|
Jiang G, Zhao H, Yang F, Li J, Li Y, Liu
Y, Liu J and Wang J: Thoracoscopic enucleation of esophageal
leiomyoma: a retrospective study on 40 cases. Dis esophagus.
22:279–283. 2009.
|