Introduction
Uterine leiomyosarcoma (LMS) is a rare and extremely
aggressive disease. In patients with advanced LMS, the five-year
survival rate is <20%. In addition, suboptimal primary surgery
that induces tumor injury and a cut-through of the uterine sarcoma
is associated with a worse prognosis compared with complete
resection of the uterine sarcoma (1,2).
In patients with advanced and unresected uterine
LMS, multidisciplinary therapy is the best treatment option for the
extension of life. For treatment using chemotherapy subsequent to
radiotherapy, numerous agents have been investigated in patients
with LMS, however, results have been unsatisfactory (Table I) (3–16). A
number of studies have shown that chemotherapy-based treatments may
have potential in patients with LMS through the simultaneous use of
hyperthermia (HT) and drugs (17–20).
However, to the best of our knowledge, there have been no studies
on the treatment with radiotherapy followed by a combination of
chemotherapy and HT in patients with locally-advanced, unresected
uterine LMS. To the best of our knowledge, the present study is the
first to report the use of these three modalities for the treatment
of locally-advanced, unresected uterine LMS.
 | Table IReview of the schedules of
chemotherapy followed by radiotherapy used to treat patients with
leiomyosarcoma of the uterus in previous studies. |
Table I
Review of the schedules of
chemotherapy followed by radiotherapy used to treat patients with
leiomyosarcoma of the uterus in previous studies.
| A, Single-agent |
|---|
|
|---|
| Drug | A/B | Schedule | Response rate, % | First author (year)
[ref] |
|---|
| Gemcitabine | 20/31 | 1,250
mg/m2 on days 1 and 8 in a 3-weekly schedule | 3.23 | Svancarova L et
al (2002) [3] |
| 15/29 | 1,250
mg/m2 every week × three, cycles repeated every 28
days | 3 | Okuno S et al
(2002) [4] |
| Paclitaxel | 15/48 | 135 mg/m2
for patients with prior radiotherapy every 3 weeks | 8.4 | Gallup DG et
al (2003) [5] |
| Trabectedin | 21/36 | A 24-h continuous iv
infusion at a dose of 1.5 g/m2 every 3 weeks | 8 | Garcia-Carbonera R
et al (2004) [6] |
| Trimetrexate | 7/23 | 5
mg/m2/day orally for 5 days every other week | 4.3 | Smith HO et al
(2002) [7] |
| Etoposide | 6/29 | 30–40
mg/m2/day for prior radiotherapy as a single dose for 21
days, every 28 days | 6.9 | Rose PG et al
(1998) [8] |
| 7/28 | 100 mg/m2
orally ond ays 1,3 and 5 | 11 | Slayton RE et
al (1987) [9] |
| Amonafide | 8/26 | 300 mg/m2
× 5 days every 3 weeks | 4 | Asbury R et al
(1998) [10] |
|
| B, Combination |
|
| Drug | A/B | Schedule | Response rate, % | First author (year)
[ref] |
|
| Dacarbazine +
Mitimycin + Doxorubicine + Cisplatin | 7/18 | Day 0 consisting of
dacarbazine 750 mg/m2 iv over 2 h, mitomycin 6
mg/m2 iv over 2–5 min, doxorubicin 40 mg/m2
iv over 2–5 min and cisplatin 60 mg/m2 iv over 2 h,
retreated at 4-week intervals | 27.8 | Long HJ III et
al (2005) [11] |
| Mitimycin +
Doxorubicine + Cisplatin | 8/35 | Mitomycin 8
mg/m2 and doxorubicin 40 mg/m2 each by iv
injection, followed by cisplatin 60 mg/m2 by 2-h iv at
3-week intervals | 23 | Edmonson JH et
al (2002) [12] |
| Hydroxyurea +
Dacarbazine + Etoposide | 11/32 | Hydroxyurea 2 g in
divided doses on day 1, 700 mg/m2 dacarbazine and 100
mg/m2 etoposide on day 2 and 100 mg/m2
etoposide on days 3 and 4 | 18 | Currie J et al
(1996) [13] |
| Ifosfamide +
Doxorubicin | 9/34 | Ifosfamide, 5.0
g/m2/24 h, and mesna, 6.0 g/m2/36 h, by
continuous iv infusion preceded by doxorubicin, 50 mg/m2
iv over 15 min
Each course of therapy was repeated every 3 weeks | 30.3 | Sutton G et al
(1996) [14] |
| Gemcitabine +
Docetaxel | 14/34 | Gemcitabine 900
mg/m2 iv on days 1 and 8 plus docetaxel 100
mg/m2 iv on day 8 delivered every 21 days (25% lower
doses if prior radiotherapy) | 53 | Hensley ML et
al (2002) [15] |
| Gemcitabine +
Docetaxel | 17/39 | Gemcitabine 900
mg/m2 iv on days 1 and 8 plus docetaxel 100
mg/m2 iv on day 8 delivered every 21 days | 27 | Hensley ML et
al (2008) [16] |
Case report
Patient presentation
A 41-year old female (gravida 2, para 2) presented
to the University of Fukui Hospital (Fukui, Japan) with prolonged
uterine bleeding and lower abdominal fullness. The patient had
previously been treated for a large symptomatic leiomyoma with
monthly injections of a long-acting gonadotropin-releasing hormone
(GnRH) agonist at the Fukui Aiiku Hospital (Fukui, Japan). Upon
examination, the patient appeared anemic and had a palpable
abdominal mass that was the same size as the head of a newborn
child. Magnetic resonance imaging revealed a large, heterogeneously
hyperintense mass in the T1- and T2-weighted images of the pelvic
cavity. Despite these abnormal findings, the patient selected
conservative treatment in order to preserve fertility and due to
the previous good response to GnRH agonist treatment. The patient
was administered monthly injections of 1.88 mg leuprolide acetate.
Prior to the third injection, an increase in the size of the
uterine mass was observed, and two weeks after the injection the
patient presented with abdominal pain, bleeding and leg edema. The
patient subsequently decided to undergo surgery.
Surgery
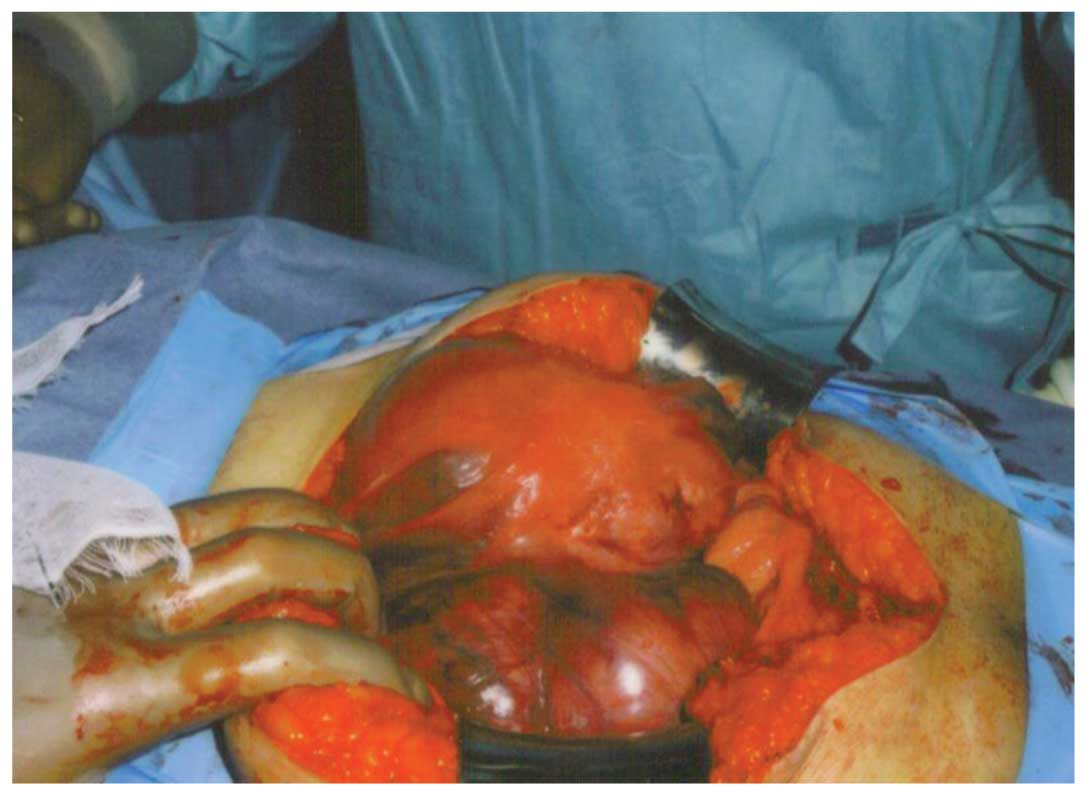
Exploratory laparotomy revealed a large tumor that
had extended from the pelvic wall to the outside of the pelvis and
then invaded the colon (Fig. 1).
Suboptimal debulking was performed, with a supra-hysterectomy and
resection of the metastatic masses. Large residual tumors remained
in the pelvis. Microscopically, the mass was composed of an LMS
component with nuclear atypia and a high mitotic index (Fig. 2) and a leiomyoma component.
Adjuvant chemotherapy and
radiotherapy
Following suboptimal debulking surgery, the patient
presented with lower abdominal pain and continuous vaginal
bleeding. A previous study showed that radiotherapy improves the
local status of uterine sarcomas (21), thus, subsequent to obtaining
informed consent, adjuvant radiotherapy was administered, followed
by chemotherapy at the University of Fukui Hospital. The patient
received 15-MV external-beam radiotherapy to the whole pelvis at a
dose of 50.4 Gy, in five fractions of 1.8 Gy per week, using a
four-field box technique according to our institutional protocol.
Computed tomography (CT) revealed stable disease (SD) according to
the Response Evaluation Criteria in Solid Tumors (RECIST), prior
and subsequent to radiotherapy. Two weeks after radiotherapy, the
patient received intravenous gemcitabine (900 mg/m2
administered over 90 min on days 1 and 8) and docetaxel (75
mg/m2 on day 8) with granulocyte growth factor support
on day 9 of a 21-day cycle to be scheduled every three weeks. Based
on the results of the previous radiotherapy (15), a 25% lower dose of docetaxel was
administered. Following three months of gemcitabine and docetaxel
chemotherapy, the patient was hospitalized due to grade II
pulmonary toxicity. The patient was treated with antibiotics and
showed marked improvement. Subsequent to a further three months of
receiving the same chemotherapy, the patient was readmitted with
grade II pulmonary toxicity. The patient was again treated with
antibiotics and showed marked improvement. A CT scan revealed the
SD status of the LMS according to the RECIST, prior and subsequent
to the six months of chemotherapy.
HT treatment
Based on the patient’s tumor response, toxicity and
performance status, HT was added to the chemotherapy regimen
subsequent to obtaining informed consent. Regional whole pelvis HT
was administered in the same week that the patient received
gemcitabine (900 mg/m2 administered over 90 min) and
docetaxel (75 mg/m2). Thermometry catheters were placed
in the rectum, bladder and vagina for thermal dose calculations.
Following appropriate adjustments to the treatment settings, the
power output was increased until the patient’s tolerance threshold
was reached. HT treatment was administered for 60 min after vaginal
measurements had reached 40°C. Subsequent to 6 weeks of HT with
chemotherapy, the patient was hospitalized with grade III burns and
subcutaneous fatty necrosis. Therefore, HT treatment was withdrawn.
CT revealed SD of the LMS according to the RECIST, prior and
subsequent to six months of receiving the combination of HT and
chemotherapy. While treatment was being administered for the grade
III/IV burns and subcutaneous fatty necrosis, the patient developed
multi-organ failure and succumbed.
Discussion
Adujuvant pelvic radiotherapy may improve local
pelvic control, but dose not improve patient survival (21). Thus, in patients with symptomatic
(for example exhibiting vaginal bleeding), unresected or advanced
LMS, radiotherapy is optimal.
In patients with advanced or unresected LMS,
systemic treatment is the best option for the extension of life.
Table I shows an overview of the
clinical trials of chemotherapy followed by radiotherapy used to
treat patients with advanced LMS in previous studies. Gemcitabine
and docetaxel are the most common agents used for non-primary
chemotherapy in patients with recurrent and advanced uterine
sarcoma. Hensley et al (15)
performed a phase II trial of gemcitabine and docetaxel in patients
with unresectable LMS. The study included patients who had received
prior pelvic radiation, those who had become worse following
doxorubicin-based therapy and those who had not received prior
chemotherapy. A 25% lower dose of the two agents was administered
to the patients who had received prior pelvic radiation. A complete
response or a partial response (PR) were observed in 53% of the
enrolled patients. Hematological toxicity was common, while
neutropenic fever and bleeding were rare (15). In the present case, the patient
received 25% lower doses of gemcitabine and docetaxel, as the
patient had received prior pelvic radiation treatment. Although PR
was not observed and the side-effects were acceptable, for patients
who have undergone prior treatment, particularly radiotherapy, the
use of 25% lower doses of gemcitabine and docetaxel should be
considered.
Research into LMS continues to focus on the
identification of active drugs and treatment combinations. A number
of studies have shown the potential for a response to chemotherapy
by the simultaneous use of HT and drugs (17–20).
Wiedemann et al (17)
reported that whole body hyperthermia (WBH) may enhance the
therapeutic index of specific chemotherapeutic agents, such as
ifosfamide, carboplatin and etoposide (ICE), resulting in a
response rate of 63% (17).
However, Pereira Arias et al (18) reported the development of an acute
systemic inflammatory response syndrome with multiple organ
dysfunction syndrome following administration of WBH in combination
with ICE. If chemotherapic agents and hyperthemia are selected
appropriately, for example, changing WBH to whole-pelvic HT, HT
with chemotherapy may be a safe and useful procedure for the
treatment of locally-advanced cervical cancer. Westerman et
al (19) reported that the
combination of full-dose radiotherapy, chemotherapy and HT is a
feasible and effective treatment strategy for patients with
advanced cervical carcinoma without concessions to radiotherapy,
chemotherapy or HT dose, compared with patients receiving single-
or combined-modality treatment. Additionally, Mohamed et al
(20) reported that HT increased
the cytotoxicity of docetaxel and gemcitabine in mouse
fibrosarcoma. Thus, in the present study, a compounding effect was
expected with the combination of docetaxel, gemcitabine and HT for
the patient with unresected LMS who had undergone prior
radiotherapy. In the present case, this combination had moderate
efficacy. The cytotoxicity of these drugs is synergized by heat,
but the timing between the chemotherapy and HT may not have been
adequate for the present patient. For the concomitant use of
radiotherapy, chemotherapy and HT for the treatment of patients
with cervical carcinoma, Westermann et al (19) advised that chemotherapy and HT
should be administered concurrently, preferentially 1 h, but no
more than 6 h, prior to radiotherapy. The optimal sequence of the
administration of these three therapeutic modalities for the
treatment of LMS has yet to be elucidated. The analysis of an
effective protocol is required for the administration of
chemotherapy, HT and radiotherapy in patients with unresected
LMS.
In the present case, the combination of HT with
chemotherapy was effective, however, grade III/IV burns and
subcutaneous fatty necrosis toxicity occurred. At present, no
consensus exists on the efficacy of treatment for locally-advanced,
unresected uterine LMS. The present case study described the
potential for a combination treatment using chemotherapy, HT and
radiotherapy. In conclusion, further investigation is required into
an effective protocol for the administration of chemotherapy, HT
and radiotherapy in patients with unresected LMS.
References
|
1
|
Park JY, Park SK, Kim DY, et al: The
impact of tumor morcellation during surgery on the prognosis of
patients with apparently early uterine leiomyosarcoma. Gynecol
Oncol. 122:255–259. 2011.
|
|
2
|
Leitao MM Jr, Zivanovic O, Chi DS, et al:
Surgical cytoreduction in patients with metastatic uterine
leiomyosarcoma at the time of initial diagnosis. Gynecol Oncol.
125:409–413. 2012.
|
|
3
|
Svancárová L, Blay JY, Judson IR, et al:
Gemcitabine in advanced adult soft-tissue sarcomas. A phase II
study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J
Cancer. 38:556–559. 2002.
|
|
4
|
Okuno S, Edmonson J, Mahoney M, et al:
Phase II trial of gemcitabine in advanced sarcomas. Cancer.
94:3225–3229. 2002.
|
|
5
|
Gallup DG, Blessing JA, Andersen W and
Morgan MA; Gynecologic Oncology Group Study. Evaluation of
paclitaxel in previously treated leiomyosarcoma of the uterus: a
gynecologic oncology group study. Gynecol Oncol. 89:48–51.
2003.
|
|
6
|
Garcia-Carbonero R, Supko JG, Manola J, et
al: Phase II and pharmacokinetic study of ecteinascidin 743 in
patients with progressive sarcomas of soft tissues refractory to
chemotherapy. J Clin Oncol. 22:1480–1490. 2004.
|
|
7
|
Smith HO, Blessing JA and Vaccarello L:
Trimetrexate in the treatment of recurrent or advanced
leiomyosarcoma of the uterus: a phase II study of the Gynecologic
Oncology Group. Gynecol Oncol. 84:140–144. 2002.
|
|
8
|
Rose PG, Blessing JA, Soper JT and Barter
JF: Prolonged oral etoposide in recurrent or advanced
leiomyosarcoma of the uterus: a gynecologic oncology group study.
Gynecol Oncol. 70:267–271. 1998.
|
|
9
|
Slayton RE, Blessing JA, Angel C and
Berman M: Phase II trial of etoposide in the management of advanced
and recurrent leiomyosarcoma of the uterus: a Gynecologic Oncology
Group Study. Cancer Treat Rep. 71:1303–1304. 1987.
|
|
10
|
Asbury R, Blessing JA, Buller R, et al:
Amonafide in patients with leiomyosarcoma of the uterus: a phase II
Gynecologic Oncology Group study. Am J Clin Oncol. 21:145–146.
1998.
|
|
11
|
Long HJ III, Blessing JA and Sorosky J:
Phase II trial of dacarbazine, mitomycin, doxorubicin, and
cisplatin with sargramostim in uterine leiomyosarcoma: a
Gynecologic Oncology Group study. Gynecol Oncol. 99:339–342.
2005.
|
|
12
|
Edmonson JH, Blessing JA, Cosin JA, et al:
Phase II study of mitomycin, doxorubicin, and cisplatin in the
treatment of advanced uterine leiomyosarcoma: a Gynecologic
Oncology Group study. Gynecol Oncol. 85:507–510. 2002.
|
|
13
|
Currie J, Blessing JA, Muss HB, Fowler J,
Berman M and Burke TW: Combination chemotherapy with hydroxyurea,
dacarbazine (DTIC), and etoposide in the treatment of uterine
leiomyosarcoma: a Gynecologic Oncology Group study. Gynecol Oncol.
61:27–30. 1999.
|
|
14
|
Sutton G, Blessing JA and Malfetano JH:
Ifosfamide and doxorubicin in the treatment of advanced
leiomyosarcomas of the uterus: a Gynecologic Oncology Group study.
Gynecol Oncol. 62:226–229. 1996.
|
|
15
|
Hensley ML, Maki R, Venkatraman E, Geller
G, Lovegren M, Aghajanian C, Sabbatini P, Tong W, Barakat R and
Spriggs DR: Gemcitabine and docetaxel in patients with unresectable
leiomyosarcoma: results of a phase II trial. J Clin Oncol.
20:2824–2831. 2002.
|
|
16
|
Hensley ML, Blessing JA, Degeest K,
Abulafia O, Rose PG and Homesley HD: Fixed-dose rate gemcitabine
plus docetaxel as second-line therapy for metastatic uterine
leiomyosarcoma: a Gynecologic Oncology Group phase II study.
Gynecol Oncol. 109:323–328. 2008.
|
|
17
|
Wiedemann GJ, Robins HI, Katschinski DM,
et al: Systemic hyperthermia and ICE chemotherapy for sarcoma
patients: rationale and clinical status. Anticancer Res.
17:2899–2902. 1997.
|
|
18
|
Pereira Arias AM, Wester JP, Blankendaal
M, et al: Multiple organ dysfunction syndrome induced by whole-body
hyperthermia and polychemotherapy in a patient with disseminated
leiomyosarcoma of the uterus. Intensive Care Med. 25:1013–1016.
1999.
|
|
19
|
Westermann AM, Jones EL, Schem BC, et al:
First results of triple-modality treatment combining radiotherapy,
chemotherapy, and hyperthermia for the treatment of patients with
stage IIB, III, and IVA cervical carcinoma. Cancer. 104:763–770.
2005.
|
|
20
|
Mohamed F, Marchettini P, Stuart OA, et
al: Thermal enhancement of new chemotherapeutic agents at moderate
hyperthermia. Ann Surg Oncol. 10:463–468. 2003.
|
|
21
|
Reed NS, Mangioni C, Malmström H, et al:
Phase III randomised study to evaluate the role of adjuvant pelvic
radiotherapy in the treatment of uterine sarcomas stages I and II:
an European Organisation for Research and Treatment of Cancer
Gynaecological Cancer Group Study (protocol 55874). Eur J Cancer.
44:808–818. 2008.
|