Introduction
Adenoid cystic carcinoma (ACC) is an uncommon form
of malignant neoplasm that arises most commonly in the major and
minor salivary glands of the head and neck (1), but rare in the esophagus. Only ~60
cases of esophageal ACC have previously been reported, which only
accounts for <1% of all primary esophageal carcinomas reviewed
(2). Usually a radical resection is
performed, if the tumor is resectable. A result of which is that
the small biopsy tissue samples may not display the characteristic
architectural patterns of the tumor, and therefore a precise
pre-operation diagnosis of ACC of the esophagus is difficult to
achieve. The prognosis of esophageal ACC is poor, with organ
mesastasis occurring more frequently with ACC than in other
carcinomas (3). However, to date,
ACC originating from the cardia has not been reported. As the
invasive site of ACC is close to the esophagus and cardia, and the
biological and pathological characteristics are similar, the
current study presents a rare case of cardial ACC and a review of
17 other cases of ACC of the esophagus that have been reported in
China so as to provide more information regarding the clinic
manifestations and operable treatments for ACC of the upper
digestive tract. Patient provided written informed consent.
Case report
Patient history and examination
A 53-year-old male was hospitalized at the Huashan
Hospital Affiliated to Fudan University (Shanghai, China) with a
four-month history of progressive dysphagia and proximal dull pain
in the upper abdomen. The physical examination did not show any
specific findings, and carcinoembryonic antigen, carbohydrate
antigen 19-9 and markers for gastrointestinal carcinoma were
normal. The findings from additional laboratory assessments were
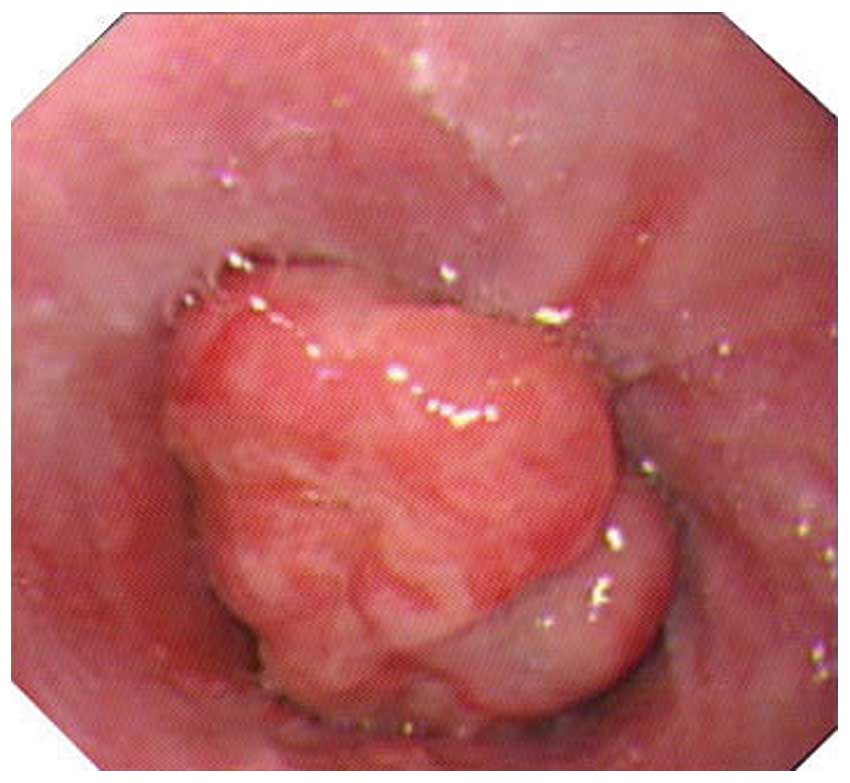
also normal. Endoscopy showed a protruded lesion with a shallow
depression on the surface of the cardia, ~40–45 cm away from the
patient’s incisors. The mucosa around the lesion was smooth, pink
and gray-white, and the capillaries were diastolic (Fig. 1). The enhanced computed tomography
(CT) scan showed that the stomach wall of the cardia was abnormally
thickened and the lymph nodes around the lesser curvature were
enlarged (Fig. 2). No apparent
abnormalities were identified in the chest CT scan and abdominal
B-mode ultrasound. A biopsy specimen indicated an adenocarcinoma
with a partial structure of ACC. During the exploratory laparotomy,
a 6×4-cm tumor was observed on the lesser curvature of the cardia,
invading into the stomach body. A pale nodule, 5 mm in diameter,
was identified in the left lateral lobe of the liver (potentially a
metastatic nodule). No apparent signs of metastasis were found in
the other organs in the abdomen. A local expanded resection of the
liver nodule was performed to ascertain its pathological type and
the frozen section results indicated that the nodule was an
adenomatous hyperplasia. Thus, a radical total gastrectomy with a
D2 lymphadenectomy and Roux-en-Y esophagojejunal anastomosis was
performed.
Treatment and follow-up
The resected tumor was a non-encapsulated
fungoid-shaped mass, measuring 6×4 cm, located in the cardia and 2
cm from the upper incisal margin. The cut surface of the tumor had
a hard, gray-white appearance (Fig.
3A). Microscopic examination of the lesion demonstrated an
infiltrative malignant neoplasm invading serosa without metastases
to the lymph nodes (pT4aN0M0, stage IIB according to the
tumor-node-metastasis; TNM staging system). Histologically, the
tumor primarily exhibited a cribriform pattern and partial tubular
and solid patterns (Fig. 3B). The
cells formed cylidromatous and tubule microscopic spaces, the lumen
of which contained eosinophilic or basophilic AB-positive
hyalinized materials. Specific lumens contained eosinophilic,
periodic acid-Schiff (PAS)-positive materials. The inner layer of
the tumor tissue was composed of a glandular epithelium, a layer of
cubic or columnar cells forming glandular tubes, with abundant
cytoplasm and enlarged hyperchromatic nuclei. By contrast, the
external layer was composed of basaloid myoepithelium, exhibiting a
uniformly small size, hyperchromatic, ovoid or spindle-shaped and
scant cytoplasm. Results of immunohistochemistry (IHC) that was
performed on the resected tumor are listed in Table I and demonstrated in Fig. 3C–F. These findings were consistent
with an ACC developing from the cardia. The patient was discharged
from hospital seven days following surgery and no signs of
recurrence have been detected during the 30 months of
follow-up.
 | Table IResults of IHC of the resected
tumor. |
Table I
Results of IHC of the resected
tumor.
| Biomarker | IHC result |
|---|
| Ki-67 | 10% + |
| p53 | + |
| C-erbB-2 | c |
| Cytokeratin
(AE1/AE3) | + |
| High-molecular
cytokeratin | + |
| Low-molecular
cytokeratin | + |
| Cytokeratin 5/6
Focal | + |
| Epithelial membrane
antigen | + |
| Vimentin | + |
| p63 | + |
| Calponin | + |
| Smooth muscle
actin | + |
| S-100 Focal | + |
| CD117 | + |
| CD56 | − |
| CD45/LCA | − |
| Carcinoembryonic
antigen | − |
| Estrogen
receptor | − |
| Progesterone
receptor | − |
| Chromogranin A | − |
| Synaptophysin | − |
Discussion
Cancers of the adenoid cystic type commonly
originate in the major salivary glands and account for 10–25% of
all salivary gland tumors. These are indolent, locally aggressive
tumors that only metastasize in the advanced cases. By contrast,
ACC rarely originates from the esophagus (3). Since the first study, reported in 1954
by Gregg and Stamler (4), there
have only been 17 cases reported in China and ~60 cases in other
countries. Petursson (5) reviewed
45 cases of esophageal ACC. The mean age of patients with
esophageal ACC was 65 years, and the male to female ratio was
3.4:1. Progressive dysphagia is the most common presenting symptom
of ACC, which is comparable to esophageal squamous cell carcinoma
(SCC); certain patients may also complain of upper abdominal
discomfort. Superficial carcinoma in the early stage may be
asymptomatic and identified during a routine gastroscopy
examination. ACC originates most commonly in the middle third of
the esophagus, less often in the lower third and rarely in the
upper third. The present case was a primary ACC of the gastric
cardia, involving the stomach body, which, to the best of our
knowledge, has not previously been reported. Myoepithelial cells
are rare in gastric glands. It was believed in the present case
that the tumor originated from the lower esophagus and infiltrated
into the cardia and the body of the stomach. As a result, the
following is a further discussion about esophageal ACC.
The clinical data of the present case and 17 other
cases of ACC of the esophagus documented in the literature are
listed in Table II (6–18). The
patients ranged in age from 42 to 68 years with an average age of
54.5 years. The gender ratio was 14 males to four females (3.5:1).
The middle third of the esophagus was the most commonly affected
region (10/18). The tumor appearance was protruded in nine cases
and ulcerative in three of the 12 cases, where the macroscopic
appearance was reported. These epidemiological results are
consistent with a study by Morisaki et al (3). A total of 12 cases adopted barium
radiography for preoperative diagnosis, seven cases of which
indicated an esophageal malignant tumor, three indicated a benign
tumor and one indicated cricopharyngeal achalasia. Although barium
radiography may provide a precise direction for the position of the
lesion, the topical diagnosis of esophageal ACC relies on
microscopic examination. In total, 11 cases reported the
performance of endoscopic biopsies for diagnosis, however, the
diagnostic results were poor. Only three of the 12 reported
biopsies indicated ACC, four indicated SCC, one indicated
adenocarcinoma, one indicated leiomyoma, one indicated focal mild
hyperplasia and the remaining biopsy was negative for any
abnormality. The biggest challenge in diagnosing esophageal ACC
from a biopsy specimen is associated with the small tissue samples,
which may not exhibit the primary characteristic structural pattern
of the tumor.
 | Table IIClinical data of the present case and
17 other cases of ACC of the esophagus reported in China. |
Table II
Clinical data of the present case and
17 other cases of ACC of the esophagus reported in China.
| Case (ref) | Age/gender | Location | Biopsy | Macroscopic
appearance | Depth of
invasion | Metastasis to LN | Distant
metastases | Treatment | Outcome |
|---|
| 1 (Present) | 53/M | Cardia | ACC | Protruding | Serosa | None | None | Surgery | Alive 30 mo. |
| 2 (6) | 47/M | Lower | SCC | Protruding | Muscule | None | None | Surgery +
chemotherapy (DCVU) | NS |
| 3 (7) | 48/M | Middle | Not done | Ulcerative | Submucosa | None | None | Surgery | Alive 17 mo. |
| 4 (8) | 59/M | Middle | Focal mild
hyperplasia of squamous epithelia | Protruding | Submucosa | None | None | Surgery | Alive 21 mo. |
| 5 (9) | 60/M | Lower | SCC | Protruding | Muscule | None | None | Surgery | Succumbed 22
days |
| 6 (10) | 64/F | Middle | Leiomyoma | Protruding | Muscule | None | None | Surgery | NS |
| 7–10 (11) | 42–62 (Mean, 51)/3M,
1F | 2 Middle | 1 ACC | Unknown | NS | NS | 1 Brain | 2 × surgery | NS |
| 1 Lower | 1 SCC | | | | | 2 ×biopsy | |
| 1 Upper | 1 Negative | | | | | | |
| 1 No biopsy | | | | | | |
| 11 (12) | 60/M | Middle | SCC | Protruding | Adventitia | None | NS | Surgery | NS |
| 12 (13) | 50/M | Lower | Not done | Ulcerative | Adventitia | None | None | Surgery | Alive 30 mo. |
| 13 (14) | 54/F | Middle | Not done | Protruding | Serosa | NS | NS | Surgery | NS |
| 14 (15) | 49/M | Middle | ACC | Protruding | Mucosa | None | None | Surgery | Alive 46 mo. |
| 15 (16) | 47/M | Lower | Not done | Protruding | Mucosa | None | None | Surgery | Alive 3 mo. |
| 16 (17) | 68/F | Lower | Adeno-carcinoma | Ulcerative | NS | NS | NS | Surgery | NS |
| 17 (18) | 60/M | Middle | Not done | Unknown | Muscule | Yes | NS | Surgery | Alive 2 mo. |
| 18 (18) | 58/M | Middle | Not done | Unknown | Sub-adventitia | Yes | NS | Surgery | Alive 37 mo. |
ACC is predominantly composed of glandular tube and
myoepithelial cells. Polymorphism is a key characteristic of the
tumor cells as they usually present in a cribriform, tubule or
solid pattern. The cyst lumen that is formed by tumor cells often
exhibits Alcian blue-positive or PAS-positive staining, which is
indicative of mucoid materials. Other malignant tumors may also
originate from the esophagus, including basaloid (B) SCC and
carcinoid tumors, and exhibit a polymorphism that is identical to
ACC. However, the biological behavior of the two tumors is
diverse.
BSCC was initially reported by Wain et al in
1986 (20) although the biological
characteristics of BSCC were not clearly understood until recently.
As a result of this, Tsang et al (21) proposed that the numerous ACC cases
that have been reported should have been diagnosed as BSCC. The
following assumptions are made in order to differentiate between
these two carcinomas (22–25): BSCCs are generally composed of dense
clusters of small cells with scant cytoplasm. Hyperchromatic nuclei
and occasionally polygonal nuclei are observed with pathological
mitotic figures that exhibit >10/10 high-power fields.
Furthermore, BSCC may occur with various differentiation degrees of
SCC, including carcinoma in situ and infiltrating carcinoma
that present with a solid-lobule pattern, in the center of which
may be an acne-like necrosis. The cells around the BSCC usually
form nests in a cribriform pattern and deposit basement
membrane-like material, which hyalinize between the nests. However,
the epithelial surface of the tumor is always malignant. By
contrast, squamous cells, central acne-like necrosis and mitotic
figures are rarely present in ACC. ACC is predominantly composed of
glandular epithelium and myoepithelial cells. IHC may aid with the
diagnosis of ACC, particularly when it is difficult to
differentiate from BSCC. BSCC only expresses cytokeratin, however,
ACC occasionally expresses p63, calponin, smooth muscle actin,
S-100 and cluster of differentiation (CD)117, and continuously
express cytokeratin (19,25–29).
In the present case, the tumor presented with a characteristic
adenoid cystic differentiation and positive IHC results of muscle
actin and S-100 protein provided additional support to the
diagnosis.
The macro-appearance of a submucosa carcinoid of the
cardia is usually protruded, which was comparable to what was
observed in the present case. Histologically, carcinoids lack the
typical cribriform pattern, while observation of a tubule or solid
pattern is common, therefore, a definitive differentiation for ACC
exhibiting a tubule/solid pattern is required. Thus, IHC is
considered to be necessary. Carcinoids often express neurosecretory
markers, including neuron specific enolase, CD56, chromogranin A
and synaptophysin (30), whereas
ACCs do not express such biomarkers.
Radical resection is generally the primary option
for treating esophageal ACC. The principles of surgery are the same
as those for esophageal carcinoma, including radical resection with
free margins and local lymphadenectomy (31). In the present case, the lesion was
in the cardia, which infiltrates the stomach body. The patient
underwent radical total gastrectomy with D2 lymphadenectomy and
Roux-en-Y esophagojejunal anastomosis, and recovered well.
Meanwhile, the therapeutic effect of radiotherapy and chemotherapy
for esophageal ACC remains controversial. The study by Petursson
(5) reported that a combination of
chemotherapy with cyclophosphamide, vincristine, adriamycin and
cisplatin completely remitted ACC. The TNM staging of the patient
in the present study was stage IIB. No chemotherapy was applied and
no signs of recurrence or metastasis occurred 30 months following
surgery.
The prognosis of esophageal ACC is poor due to early
lymphatic and distant metastasis (3,5,30). The
average overall survival is only seven months following clinical
diagnosis, and nine months subsequent to surgical resection. As was
reported, the higher metastatic rate compared with other types of
carcinoma was the predominant reason for the relatively poor
prognosis of ACC. In the 37 esophageal ACC patients in the study
reported by Morisaki et al (3), only one patient survived for five
years following surgery, 15 patients did not exhibit lymph node
metastasis and 11 were alive at the time when the study was
reported. The mode of postoperative recurrence was lymph node
metastasis in three patients, organ metastasis in four and a
combination of the two in five patients. Of the nine patients who
exhibited organ metastasis postoperatively, seven were found to
have lymph node metastasis during the surgery. These findings
indicate that a lack of lymph node metastasis is associated with an
improved prognosis for esophageal ACC. Specific relative
information regarding nine of the 17 cases that were reported in
China is as follows: One patient exhibited anastomotic leakage 10
days following surgery and succumbed to hematemesis on day 22,
postoperatively. The remaining eight were alive at the time when
the report was published, ~2–46 months following the surgery. In
the current case the tumor was relatively large and invaded all
layers of the stomach. However, all 23 lymph nodes detected were
negative for tumor metastasis. A suspected liver metastatic nodule
was identified during surgery; however, the pathological
examinations revealed that it was an adenomatous hyperplasia. As a
result, the prognosis of this patient may be good. Although the
patient has been free from recurrence during the 30 months since
surgery, continued regular follow-up is considered to be
necessary.
The incidence of adenoid cystic carcinoma derived
from the esophagus is relatively low and ACC of the cardia is even
rarer. Hence little is known regarding the manefestations and
operable treatments for this type of malignancy. The current study
presented a case of ACC of the cardia diagnosed by its typical
adenoid cystic differentiation and positive IHC results for muscle
actin and S-100 protein. BSCC and carcinoids should be carefully
differentiated through immunohistological examinations due to the
fact that they both exhibit a polymorphism that is identical to
ACC. Early lymphatic and distant metastasis are associated with
poor survival rates. The primary option for treatment is radical
resection, however, the efficacy of radiotherapy and chemotherapy
remain uncertain. Case series studies with large samples are
necessary in order to acquire more information regarding the
clinical manifestations and operable treatments for ACC of the
upper digestive tract.
References
|
1
|
Gondivkar SM, Gadbail AR, Chole R and
Parikh RV: Adenoid cystic carcinoma: a rare clinical entity and
literature review. Oral Oncol. 47:231–236. 2011.
|
|
2
|
Epstein JI, Sears DL, Tucker RS and Eagan
JW Jr: Carcinoma of the esophagus with adenoid cystic
differentiation. Cancer. 53:1131–1136. 1984.
|
|
3
|
Morisaki Y, Yoshizumi Y, Hiroyasu S, et
al: Adenoid cystic carcinoma of the esophagus: report of a case and
review of the Japanese literature. Surg Today. 26:1006–1009.
1996.
|
|
4
|
Gregg JB and Stamler FW: Unusual neoplasms
of the esophagus: review of literature and report of a case. AMA
Arch Otolaryngol. 59:159–169. 1954.
|
|
5
|
Petursson SR: Adenoid cystic carcinoma of
the esophagus. Complete response to combination chemotherapy.
Cancer. 57:1464–1467. 1986.
|
|
6
|
Ou LQ, Ren YF and Song GF: A case of
primary esophageal adenoid cystic carcinoma. Journal of Rare and
Uncommon Diseases (Han Shao Ji Bing Za Zhi). 9:39–40. 2002.(In
Chinese).
|
|
7
|
Wu CX and Chen SQ: A case of esophageal
adnoid cystit carcinoma. Acta Universitatis Medicinae Tongji
(Tongji Yi Ke Da Xue Xue Bao). 30:3962001.(In Chinese).
|
|
8
|
Jiang WX, Fu XN, Fu SL and Liao YD: A case
report of esophageal adnoid cystic carcinoma. Acta Med Univ Sci
Technol Huazhong. (Huazhong Ke Ji Da Xue Xue Bao, Yi Xue Ban).
41:115–116. 2012.(In Chinese).
|
|
9
|
Li CG, Gu HP and Hu HX: A case of
esophageal adnoid cystic carcinoma. Chin J Clin Oncol (Zhongguo
Zhong Liu Lin Chuang). 29:2572002.(In Chinese).
|
|
10
|
Wang HW, Li H and Lu JY: A case of primary
esophageal adnoid cystic carcinoma. Chin J Diagn Pathol (Zhongguo
Zhen Duan Bing Li Xue Za Zhi). 9:1922002.(In Chinese).
|
|
11
|
Zhang AL, Li RL and Gao Y: Adnoid cystic
carcinoma of the esophagus (analysis of 4 cases). J Pract Radiol
(Shi Yong Fang She Xue Za Zhi). 15:97–98. 1999.(In Chinese).
|
|
12
|
Yang ZH: A case of esophageal adenoid
cystic carcinoma. Journal of Luzhou Medical College (Luzhou Yi Xue
Yuan Xue Bao). 25:3002002.(In Chinese).
|
|
13
|
Guo YP and Zhan ZL: A case of esophageal
collision carcinoma (squamous cell carcinoma and adnoid cystic
carcinoma). Henan J Oncol (Henan Zhong Liu Xue Za Zhi).
13:2422000.(In Chinese).
|
|
14
|
Chen XH, Cui QW, Tong XD, et al: A case of
esophageal adnoid cystic carcinoma. Med J CASC (Zhongguo Hang Tian
Gong Ye Yi Yao). 2:112000.(In Chinese).
|
|
15
|
Ma MQ, Tang P and Zhao XH: A case of
adenoid cystic carcinoma of the esophagus: case report and
literature review. Ai Zheng. 26:798–799. 2007.(In Chinese).
|
|
16
|
Zhang L, Lv B, Fan YH, et al: Endoscopic
resection of esophageal adenoid cystic carcinoma: a case report.
Chin J Dig Endosc (Zhong Hua Xiao Hua Nei Jing Za Zhi). 26:276–278.
2009.(In Chinese).
|
|
17
|
Wang YP and Gao HT: A case of esophageal
adnoid cystic carcinoma. Mod J Intergr Tradit Chin Western Med
(Xian Dai Zhong Xi Yi Jie He Za Zhi). 11:10052002.(In Chinese).
|
|
18
|
Shi SS and Liu FS: Two case reports of
esophageal adnoid cystic carcinoma. J. Pract Oncol (Shi Yong Zhong
Liu Xue Za Zhi). 2:79–80. 1995.(In Chinese).
|
|
19
|
Wain SL, Kier R, Vollmer RT and Bossen EH:
Basaloid-squamous carcinoma of the tongue, hypopharynx, and larynx:
report of 10 cases. Hum Pathol. 17:1158–1166. 1986.
|
|
20
|
Tsang WY, Chan JK, Lee KC, et al:
Basaloid-squamous carcinoma of the upper aerodigestive tract and
so-called adenoid cystic carcinoma of the esophagus: the same tumor
type? Histopathology. 19:35–46. 1991.
|
|
21
|
Morice WG and Ferreiro JA: Distinction of
basaloid squamous cell carcinoma from adenoid cystic and small cell
undifferentiated carcinoma by immunohistochemistry. Hum Pathol.
29:609–612. 1998.
|
|
22
|
Barnes L, Ferlito A, Altavilla G, et al:
Basaloid squamous cell carcinoma of the head and neck:
clinicopathological features and differential diagnosis. Ann Otol
Rhinol Laryngol. 105:75–82. 1996.
|
|
23
|
Klijanienko J, el-Naggar A, Ponzio-Prion
A, et al: Basaloid squamous carcinoma of the head and neck.
Immunohistochemical comparison with adenoid cystic carcinoma and
squamous cell carcinoma. Arch Otolaryngol Head Neck Surg.
119:887–890. 1993.
|
|
24
|
Li TJ, Zhang YX, Wen J, et al: Basaloid
squamous cell carcinoma of the esophagus with or without adenoid
cystic features. Arch Pathol Lab Med. 128:1124–1130. 2004.
|
|
25
|
Shima I, Kakegawa T, Yamana H, et al:
Clinicopathologic studies on esophageal carcinoma with basaloid
features. Nihon Kyobu Geka Gakkai Zasshi. 41:2067–2074. 1993.(In
Japanese).
|
|
26
|
Emanuel P, Wang B, Wu MX and Burstein DE:
p63 immunohistochemistry in the distinction of adenoid cystic
carcinoma from basaloid squamous cell carcinoma. Mod Pathol.
18:645–650. 2005.
|
|
27
|
Nishimura W, Naomoto Y, Hamaya K, et al:
Basaloid-squamous cell carcinoma of the esophagus: diagnosis based
on immunohistochemical analysis. J Gastroenterol Hepatol.
16:586–590. 2001.
|
|
28
|
Tsubochi H, Suzuki T, Suzuki S, et al:
Immunohistochemical study of basaloid squamous cell carcinoma,
adenoid cystic and mucoepidermoid carcinoma in the upper
aerodigestive tract. Anticancer Res. 20:1205–1211. 2000.
|
|
29
|
Bordi C, Yu JY, Baggi MT, et al: Gastric
carcinoids and their precursor lesions. A histologic and
immunohistochemical study of 23 cases. Cancer. 67:663–672.
1991.
|
|
30
|
Cerar A, Jutersek A and Vidmar S: Adenoid
cystic carcinoma of the esophagus. A clinicopathological study of
three cases. Cancer. 67:2159–2164. 1991.
|
|
31
|
National Comprehensive Cancer Network.
clinical practice guidelines for esophageal cancer. http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf.
Accessed July 1, 2012
|