Introduction
Adenocarcinoma is a type of tumor that originates
from the glandular epithelial cells. When it secretes more mucous
it is termed mucinous carcinoma. Mucinous carcinomas that have a
large number of papillary structures termed papillary
adenocarcinomas, and cystic carcinomas that have highly expanded
lumina are known as cystadenocarcinomas (1). Cystadenocarcinomas may be accompanied
by papillary growth, and are then termed papillary
cystadenocarcinomas (2). Papillary
cystadenocarcinomas usually occur in the pancreas, with an
incidence rate of <1% of all pancreatic tumor cases. The primary
clinical manifestation of papillary cystadenocarcinoma is abdominal
distension or pain, as well as occasional obstructive jaundice
(3,4). The carcinoma is a potentially
low-grade malignant tumor. Currently, radical surgical resection is
the only effective treatment, and a correct diagnosis is dependent
on histopathological examination (5). Patient provided written informed
consent.
Case report
Patient presentation
A 56-year old male patient presented with backache
that had persisted for more than seven months, with exacerbation of
the pain for half a month. Six months prior to this, the patient
felt pain in the back with no clear predisposing cause. In October
2011, the patient sought medical advice from the Affiliated
Hospital of Inner Mongolia Medical University (Hohhot, China). A
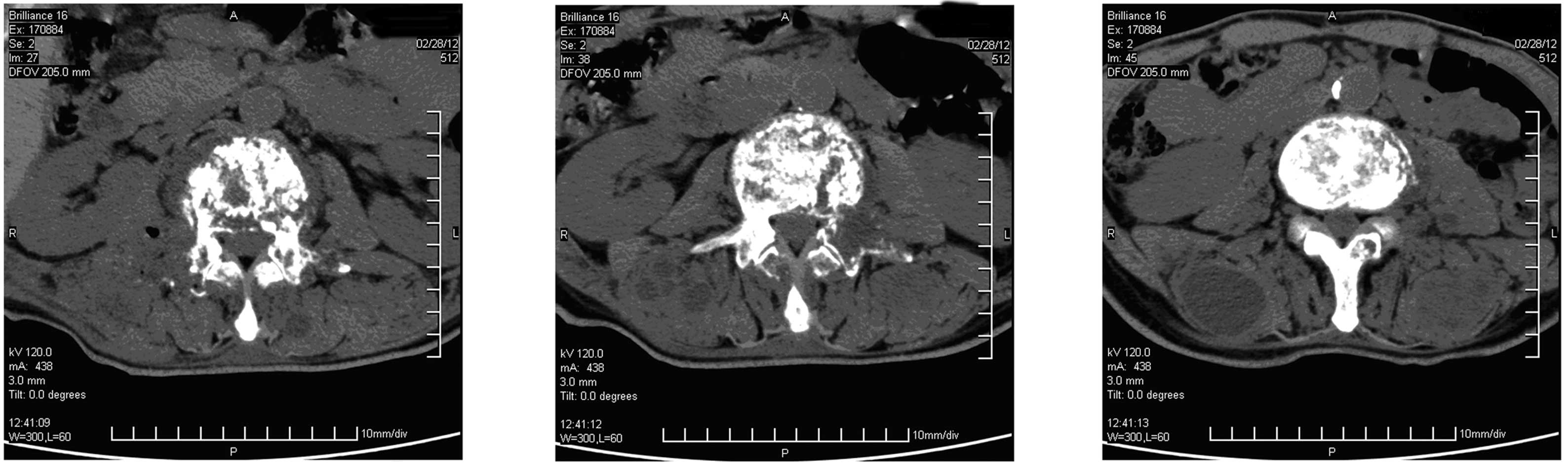
lumbar computed tomography (CT) scan revealed a lumbar disc bulging
between the third and fourth lumbar vertebrae. The patient received
Chinese medicinal pills (dosage and composition unknown) and
plaster external treatment; however, the results were not
effective. In January 2012, half a month prior to the current
admittance to hospital, the patient felt lumbar acid, with
exacerbated pain, weakness of the double lower limbs, activity
limitations and dark brown urine. The patient consulted The Second
Affiliated Hospital of Inner Mongolia Medical College (Hohhot,
China), and CT revealed some specific findings (Fig. 1).
In February 2012, in order for further diagnosis and
treatment, the patient was hospitalized in the Department of
Orthopedics of the Affiliated Hospital of Inner Mongolia Medical
University. Since the onset of the symptoms, the patient had
experienced alternating diarrhea and constipation, and had lost ~30
pounds in weight. The patient’s family history was not
contributory, and physical and library examinations revealed no
abnormalities.
Diagnosis
Lumbar vertebrae magnetic resonance imaging (MRI)
(Fig. 2) and thoracic MRI (Fig. 3) scans showed abnormal signals. The
CT and MRI findings revealed multiple myeloma and multiple bone
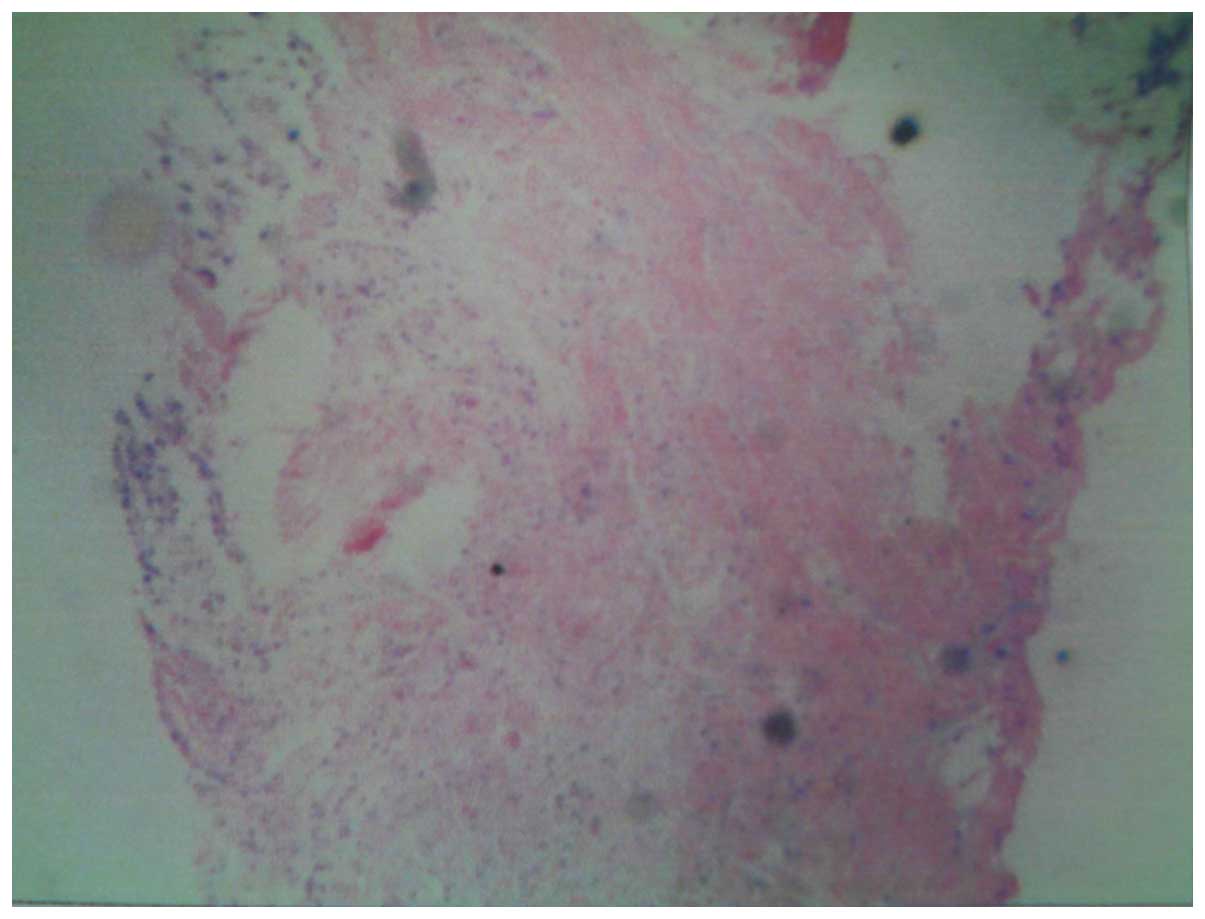
metastases. A Bence-Jones protein test and biopsy were performed,
with negative results. However, the pathological diagnosis was
positive (Fig. 4). Based on these
findings and the patient history, the patient was diagnosed with
malignant papillary mucinous cystadenocarcinoma.
When the imaging and pathological diagnoses were
combined, the source of the spinal multiple malignant tumors was
not clear. Malignant papillary mucinous cystadenocarcinoma is
primarily derived from glands, including those of the respiratory
and gastrointestinal tracts. Based on analyses of the respiratory
and digestive tracts, and other laboratory examinations and
imaging, the tumor was located in the body of the pancreas
(Fig. 5).
Patient outcome
In June 2013, the patient succumbed to the
condition. Retrospective analyses suggested that if the pancreatic
lesions had been detected and treated earlier, the prognosis may
have been improved.
Discussion
Solid cystic papillary tumor of the pancreas (SCPT)
is a rare form of pancreatic cancer and was first reported by
Frantz (4) in 1959. The disease is
also confusingly known as pancreatic solid and papillary tumor,
pancreatic solid and papillary epithelial tumor, pancreatic
papillary cystic solid tumor, pancreatic papillary
adenoma/carcinoma and pancreatic solid cystic papillary tumor
(6,7). The World Health Organization’s tumor
histological classification of 2004 classified the tumor as a solid
false papilloma; listed as a source of pancreatic exocrine tumors
(8).
SCPT often occurs in the pancreatic tail, its growth
is relatively slow and the clinical symptoms are insidious. The
tumors are round or oval in shape, with a clear boundary and a
fibrous capsule. SCPT has sections with the structure of solid and
cystic lesions, and is often is full of blood or gelatin. Under
light microscopy, the tumor cells appear consistent, with clear or
eosinophilic cytoplasm, round or oval nuclei, no obvious atypia and
with low numbers of mitotic figures. The tumor cells around the
vascular complex layer are arranged in papillary and solid areas
alternately, with different degrees of visible hemorrhage and
cystic change (9).
The origin of SCPT is controversial, as the tumor
can derived from outside or within the pancreas (10). Immunohistochemical studies have
reported the diversification of the tumor and have no specific
findings. SCPT has been reported to exhibit positive staining for
neuron-specific enolase (84.1%), antitrypsin (83.1%) and vimentin
(72.1%), and the positive rate of progesterone and estrogen
receptor expression is 35.1 and 5.1%, respectively (11).
It is difficult to distinguish between the imaging
findings from papillary cystadenoma and cystadenocarcinoma
(12). However, cystadenomas often
exhibit a clear boundary around the circular or oval cystic lesions
and do not generally involve the main pancreatic duct. The cyst
fluid has a low density on CT, high signal on T2-weighted imaging
(T2WI) and generally low signal on T1-weighted imaging (T1WI), with
no enhancement on dynamic enhanced scans. The cystic wall and
septum have soft tissue density on CT, occasionally with strip or
curved calcification. On MRI, the cystic wall and septum have
relatively low signals on T2WI, soft tissue signals on T1WI and
mild enhancement on enhanced scans. The mural nodules attached to
the wall or gap, have soft tissue density and signals, or mild to
severe enhancement on enhanced scans. Furthermore, the more solid
components are more likely to be borderline of malignant tumors.
Therefore, certain studies have hypothesized that cystadenoma and
cystadenocarcinoma reflect different stages of lesion development
(13). In the present case, the
patient initially presented with backache and lumbar discomfort,
with no bone destruction or other positive signs on CT. The doctors
were not familiar with the disease, therefore, it was difficult to
make a correct diagnosis, delaying the treatment.
Surgical resection, including local tumor resection,
resection of the pancreatic body and tail and pancreatectomy, is
the primary method for treating SCPT. SCPT has a good prognosis and
survival period, with a two-year survival rate of 97% and a
five-year survival rate of 95% (14).
In conclusion, due to the low incidence and
difficult diagnosis of SCPT, as well as the lack of specific
laboratory and imaging examinations, doctors should be aware of the
possibility of SCPT when the location or imaging features are not
typical, particularly if the first symptoms are metastases.
References
|
1
|
Salvia R, Festal L, Butturini G, et al:
Pancreatic cystic tumors. Minerva Chir. 59:185–207. 2004.(In
English and Italian).
|
|
2
|
Sahani D, Prasad S, Saini S and Mueller P:
Cystic pancreatic neoplasms evaluation by CT and magnetic resonance
cholangiopancreatography. Gastrointest Endosc Clin N Am.
12:657–672. 2002.
|
|
3
|
Hruban RH and Fukushima N: Pancreatic
adenocarcinoma update on the surgical pathology of carcinomas of
ductal origin and PanINs. Mod Pathol. 20:S61–S70. 2007.
|
|
4
|
Frantz VK: Tumors of the pancreas.
Anonymous Atlas of Tumor Pathology. 7. Armed Forces Institute of
Pathology; Washington, DC: pp. 32–33. 1959
|
|
5
|
Maehado MC, Maehado MA, Bacchella T, et
al: Solid pseudopapillary neoplasm of the pancreas: distinct
patterns of onset, diagnosis, and prognosis for male versus female
patients. Surgery. 143:29–34. 2008.
|
|
6
|
Klimstra DS, Wenig BM and Heffess CS:
Solid-pseudopapillary tumor of the pancreas: a typically cystic
carcinoma of low malignant potential. Semin Diagn Pathol. 17:66–80.
2000.
|
|
7
|
Notohara K, Hamazaki S, Tsukayama C, et
al: Solid-pseudopapillary tumor of the pancreas:
immunohistochemical localization of neuroendocrine markers and
CD10. Am J Surg Pathol. 24:1361–1371. 2000.
|
|
8
|
Zhang KZ, Jia HM, Shu H and Qiu F:
Pathological and clinical features of solid cystic papillary tumor
of pancreas. Chin J Cancer Prev Treat. 11:853–855. 2007.
|
|
9
|
Yong Yin, Zhao-Li Li, Qin Wang, et al:
Meta analysis of solid pseudopapillary tumors of the pancreas. Chin
J Pancreatol. 10:341–344. 2010.(In Chinese).
|
|
10
|
Basu A and Jha A: Solid and cystic tumor
arising from an extrapancreatic site - a case report. Nepal Med
Coll J. 5:107–108. 2003.
|
|
11
|
Feng YF, Rao HL, Wu QL, et al:
Clinicopathological and Immunohistochemical Analysis of
Solid-pseudopapillary Neoplasm of Pancreas. J Sun Yatsen Univ (Med
Sci). 30:200–204. 2009.(In Chinese).
|
|
12
|
Fernández-del Castillo C, Targarona J,
Thayer SP, et al: Incidental pancreatic cysts: clinicopathologic
characteristics and comparison with symptomatic patients. Arch
Surg. 138:427–434. 2003.
|
|
13
|
Yamao K, Nakamura T, Suzuki T, et al:
Endoscopic diagnosis and staging of mucinous cystic neoplasms and
intraductal papillary-mucinous tumors. J Hepatobiliary Pancreat
Surg. 10:142–146. 2003.
|
|
14
|
Papavramidis T and Papavramidis S: Solid
pseudopapillary tumors of the pancreas: review of 718 patients
reported in English literature. J Am Coil Surg. 200:965–972.
2005.(In Chinese).
|