Introduction
Colorectal cancer (CRC) is the second most common
cause of cancer-associated mortality worldwide. Overall, ~50% of
patients diagnosed with CRC succumb to the disease, due to
complications associated with distant metastasis (1). The incidence of CRC in China has
increased over recent years, particularly in the more developed
areas (2). The tumor-host
interaction at the invasive margin of CRC is a crucial interface
where tumor progression and tumor cell dissemination ensue
(3). Epithelial-mesenchymal
transition (EMT), a dynamic process of colorectal carcinoma cell
dedifferentiation, occurs at the invasive tumor front (4). The biological behavior of cancer is
considered to be more accurately reflected by the histologic
features present at the invasive front rather than those observed
at the tumor center.
β-catenin is a component of the Wingless/Wnt
signaling pathway. Dysfunction of the Wnt signaling pathway is
important in colorectal carcinogenesis and results in the nuclear
accumulation of β-catenin (5).
Membranous beta-catenin forms a complex with E-cadherin, a critical
mediator of cell-cell adhesion, and is responsible for the
maintenance of cell polarity (6).
The membranous expression of beta-catenin and E-cadherin
characterizes the epithelial phenotype whereas the loss of this
membranous expression is indicative of a switch toward a more
mesenchymal phenotype. The nuclear translocation of β-catenin
induces EMT and pro-invasive gene expression (7). Therefore, the differential
intracellular distribution of β-catenin exerts a marked impact on
the phenotype and behavior of tumor cells (8).
In the present study, the expression of the
EMT-associated marker, β-catenin at the tumor invasive front and
tumor center was investigated using immunohistochemical (IHC)
analysis, and the correlations among β-catenin differential
expression patterns, and clinicopathological characteristics and
prognosis in CRC cases were determined.
Materials and methods
Patients and specimens
A total of 181 CRC tissue samples were obtained from
patients diagnosed with CRC (as determined by histopathological
evaluation) and subjected to surgical resection at the First
Hospital of China Medical University (Shenyang, China) between 2000
and 2001. None of the patients had been treated with preoperative
chemotherapy or radiation. Two senior pathologists reviewed the
tissue sections from all of the cases. Tumor histological
classification was assessed according to the World Health
Organization criteria (9) and
classified using the seventh edition of the tumor, node, metastasis
(TNM) staging manual produced by the International Union Against
Cancer/American Joint Committee on Cancer (2010) (10). All patients were followed up via
telephone enquiry or questionnaire. The follow-up time ranged
between 1.5 and 71 months (median, 51 months). The Ethics Committee
of China Medical University approved the use of tissue samples in
this study. Written informed consent was obtained from all of the
patients.
Antibodies and reagents
The primary antibodies used were monoclonal rabbit
anti-human β-catenin (Abcam, Cambridge, UK).
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections (4-μm
thick) were prepared from the CRC samples. The tissue sections were
deparaffinized and rehydrated via sequential washing with xylene,
graded ethanol and phosphate-buffered saline (PBS). Following
deparaffinization and rehydration, the tissue sections were
subjected to high temperature-induced epitope retrieval by briefly
steaming in target retrieval solution (10 mM citrate buffer; pH
6.0; (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China). Subsequently, the sections were treated with
normal goat serum blocking solution (Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd.) and then incubated with β-catenin
primary antibody (dilution, 1:500) overnight at 4°C. Antibody
binding was detected using an SP reagent kit (Zhongshan Chemical,
Beijing, China) following the manufacturer’s instructions. PBS
replaced the primary antibody in the negative control and samples
that were known to express β-catenin served as the positive
controls. All sections were counterstained with hematoxylin,
dehydrated and mounted.
Evaluation of staining
The degree of IHC staining in the tissue sections
was scored independently by two pathologists who were blinded to
the clinical and pathological data. Staining intensity was graded
using a scale of 0–3 as follows: 0, No staining; 1, weak staining;
2, moderate staining; and 3, strong staining (11). The extent of staining was graded on
a scale as follows: 0, ≤5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; or 4,
76–100% according to the percentage of the section exhibiting
positive staining, relative to the entire carcinoma-involved area
(12,13). The intensity and extent scores were
multiplied to generate the immunoreactivity score (IS; range, 0–12)
for each case (12,13). β-catenin immunoreactivity was
separately analyzed for the tumor center and the tumor invasive
front. Specimens were rescored if there were discrepancies in the
IS obtained by the two pathologists, until a consensus was reached.
Membranous expression of β-catenin was classified as preserved when
>80% of the cell membrane was stained; otherwise, the sample was
classified as exhibiting reduced expression (14). High cytoplasmic and nuclear
β-catenin expression grades were defined as >50% reactivity of
the tumor cells (15).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). The
paired-samples t-test was used to compare the differential
β-catenin expression levels between the tumor center and the tumor
invasive front. The statistical significance of the associations
between β-catenin expression levels and the patient
clinicopathological parameters was assessed using χ2
tests. Kaplan-Meier survival curves were plotted to analyze the
distribution of CRC-specific survival times and intergroup
differences were determined using the log-rank test. A multivariate
Cox regression model through a stepwise selection procedure was
applied to examine the independence of the significant factors
identified in the univariate analysis. Cox proportional hazards
regression was used to calculate the mortality hazard ratios
according to various clinicopathological features and protein
markers. Two-sided P<0.05 was considered to indicate a
statistically significant difference.
Results
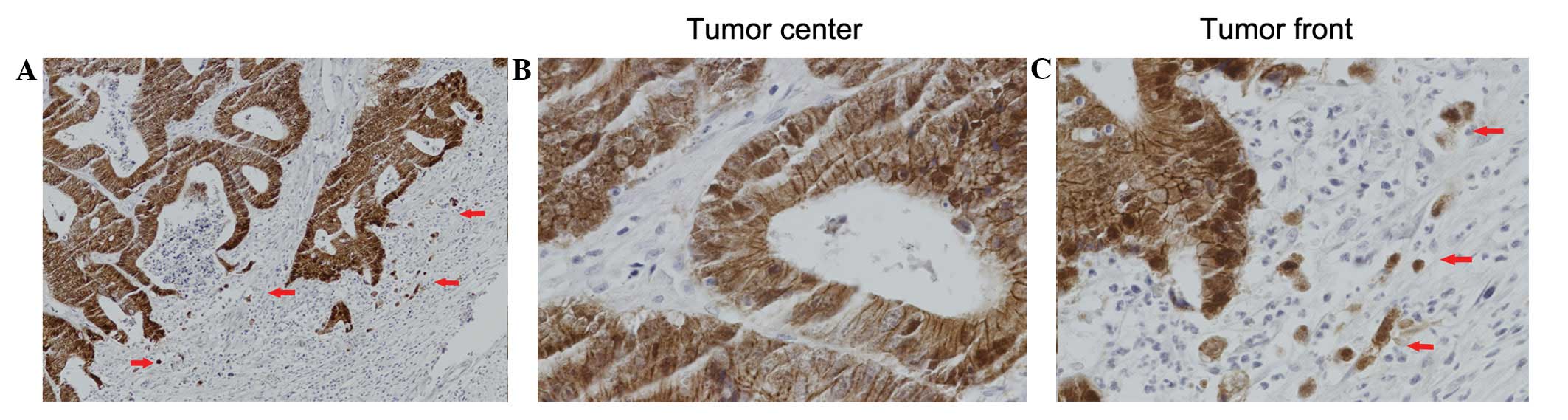
Expression of β-catenin in CRC
β-catenin protein expression was evaluated in the
CRC sections via IHC analysis. As shown in Fig. 1, β-catenin staining was observed
predominantly at the cell membrane, and marginally in the cell
cytoplasm and nucleus. Membranous β-catenin expression was
identified to be significantly higher in the tumor center than at
the tumor invasive front (IS: 5.36±3.812 versus 0.42±1.252,
respectively; P<0.001). However, reduced membranous β-catenin
expression levels in the tumor center were identified in 107
(59.1%) of the 181 patients. Nuclear β-catenin expression levels
were significantly lower at the tumor center than at the tumor
invasive front (IS: 0.05±0.303 versus 2.18±3.917, respectively;
P<0.001) as shown in Fig. 2 and
Table I. High nuclear β-catenin
expression levels at the tumor invasive front were observed in 30
(16.6%) of the 181 patients.
 | Table IPaired sample comparison (t-test) of
β-catenin expression levels between the tumor center and tumor
invasive front. |
Table I
Paired sample comparison (t-test) of
β-catenin expression levels between the tumor center and tumor
invasive front.
| Immunoreactivity
score |
|---|
|
|
|---|
| Variable | No. | Mean ± SD | P-value |
|---|
| Membranous
β-catenin |
| Tumor center | 181 | 5.36±3.812 | <0.001 |
| Tumor front | 181 | 0.42±1.252 | |
| Nuclear
β-catenin |
| Tumor center | 181 | 0.05±0.303 | <0.001 |
| Tumor front | 181 | 2.18±3.917 | |
Correlation between β-catenin expression
levels and the clinicopathological characteristics of CRC
The reduced membranous β-catenin expression levels
at the tumor center were identified to be significantly associated
with the occurrence of lymph node metastasis (P=0.002) and the TNM
stage (P=0.002). However, no associations between reduced
membranous β-catenin expression levels and age/gender at diagnosis,
tumor site or size, invasion depth, presence or absence of tumor
deposits, histological differentiation, or lymphatic or venous
invasion were evident. In addition, no statistically significant
correlations between cytoplasmic or nuclear expression levels of
β-catenin and the above-mentioned clinicopathological
characteristics were observed (Table
II).
 | Table IIβ-catenin expression levels at the
tumor center in association with patient clinicopathological
variables. |
Table II
β-catenin expression levels at the
tumor center in association with patient clinicopathological
variables.
| | Membranous
expresssion levels | Cytoplasmic
expression levels | Nuclear expression
levels |
|---|
| |
|
|
|
|---|
| Variable | Total | Preserved | Reduced | P-value | Low | High | P-value | Low | High | P-value |
|---|
| Age (years) |
| ≤60 | 65 | 21 | 44 | 0.079 | 47 | 18 | 0.248 | 62 | 3 | 0.255 |
| >60 | 116 | 53 | 63 | | 92 | 24 | | 114 | 2 | |
| Gender |
| Male | 105 | 43 | 62 | 0.982 | 82 | 23 | 0.626 | 103 | 2 | 0.408 |
| Female | 76 | 31 | 45 | | 57 | 19 | | 73 | 3 | |
| Tumor size (cm) |
| ≤5 | 94 | 40 | 54 | 0.635 | 71 | 23 | 0.675 | 91 | 3 | 0.714 |
| >5 | 87 | 34 | 53 | | 68 | 19 | | 85 | 2 | |
| Tumor site |
| Colon | 72 | 27 | 45 | 0.425 | 59 | 13 | 0.182 | 69 | 3 | 0.349 |
| Rectum | 109 | 47 | 62 | | 80 | 29 | | 107 | 2 | |
| T stage |
| pT1–pT2 | 61 | 30 | 31 | 0.106 | 52 | 9 | 0.055 | 60 | 1 | 0.511 |
| pT3–pT4 | 120 | 44 | 76 | | 87 | 33 | | 116 | 4 | |
| N stage |
| pN0 | 108 | 54 | 54 | 0.002a | 88 | 20 | 0.069 | 104 | 4 | 0.347 |
| pN1–pN2 | 73 | 20 | 53 | | 51 | 22 | | 72 | 1 | |
| TNM stage |
| I–II | 107 | 54 | 53 | 0.002a | 87 | 20 | 0.084 | 103 | 4 | 0.335 |
| III–IV | 74 | 20 | 54 | | 52 | 22 | | 73 | 1 | |
| Tumor deposit |
| Absent | 152 | 65 | 87 | 0.239 | 117 | 35 | 0.897 | 147 | 5 | 0.322 |
| Present | 29 | 9 | 20 | | 22 | 7 | | 29 | 0 | |
|
Differentiation |
| Well, mod | 132 | 57 | 75 | 0.302 | 98 | 34 | 0.182 | 127 | 5 | 0.167 |
| Por, muc | 49 | 17 | 32 | | 41 | 8 | | 49 | 0 | |
| Lymph invasion |
| Negative | 167 | 70 | 97 | 0.329 | 128 | 39 | 0.870 | 162 | 5 | 0.511 |
| Positive | 14 | 4 | 10 | | 11 | 3 | | 14 | 0 | |
| Venous
invasion |
| Negative | 178 | 73 | 105 | 0.789 | 136 | 42 | 0.337 | 173 | 5 | 0.768 |
| Positive | 3 | 1 | 2 | | 3 | 0 | | | 3 | 0 |
At the tumor invasive front, the detection of high
nuclear expression levels of β-catenin was significantly correlated
with lymph node metastasis (P=0.016), the TNM stage (P=0.020) and
histological differentiation (P=0.006), however, not with
age/gender at diagnosis, tumor site or size, invasion depth,
presence or absence of tumor deposits, or lymphatic or venous
invasion. In addition, high cytoplasmic expression levels of
β-catenin were significantly correlated with histological
differentiation (P=0.001) and tumor site (P=0.004). No
statistically significant association was observed between the
presence of high cytoplasmic expression of β-catenin and age/gender
at diagnosis, tumor size, invasion depth, lymph node metastasis,
TNM stage, presence or absence of tumor deposits, or lymphatic or
venous invasion. Furthermore, no statistically significant
correlation was detected between the detection of reduced
membranous expression levels of β-catenin and the above-mentioned
clinicopathological characteristics (Table III).
 | Table IIIβ-catenin expression levels at the
tumor invasive front in association with patient
clinicopathological variables. |
Table III
β-catenin expression levels at the
tumor invasive front in association with patient
clinicopathological variables.
| | Membranous
expression levels | Cytoplasmic
expression levels | Nuclear expression
levels |
|---|
| |
|
|
|
|---|
| Variable | Total | Reduced | Preserved | P-value | Low | High | P-value | Low | High | P-value |
|---|
| Age (years) |
| ≤60 | 65 | 52 | 13 | 0.151 | 37 | 28 | 0.080 | 57 | 8 | 0.248 |
| >60 | 116 | 102 | 14 | | 81 | 35 | | 94 | 22 | |
| Gender |
| Male | 105 | 91 | 14 | 0.482 | 66 | 39 | 0.438 | 90 | 15 | 0.330 |
| Female | 76 | 63 | 13 | | 52 | 24 | | 61 | 15 | |
| Tumor size
(cm) |
| ≤5 | 94 | 83 | 11 | 0.207 | 60 | 34 | 0.689 | 76 | 18 | 0.333 |
| >5 | 87 | 71 | 16 | | 58 | 29 | | 75 | 12 | |
| Tumor site |
| Colon | 72 | 58 | 14 | 0.165 | 56 | 16 | 0.004a | 62 | 10 | 0.430 |
| Rectum | 109 | 96 | 13 | | 62 | 47 | | 89 | 20 | |
| T stage |
| pT1–pT2 | 61 | 54 | 7 | 0.354 | 40 | 21 | 0.939 | 54 | 7 | 0.188 |
| pT3–pT4 | 120 | 100 | 20 | | 78 | 42 | | 97 | 23 | |
| N stage |
| pN0 | 108 | 91 | 17 | 0.705 | 73 | 35 | 0.410 | 96 | 12 | 0.016a |
| pN1–pN2 | 73 | 63 | 10 | | 45 | 28 | | 55 | 18 | |
| TNM stage |
| I–II | 107 | 90 | 17 | 0.659 | 72 | 35 | 0.476 | 95 | 12 | 0.020a |
| III–IV | 74 | 64 | 10 | | 46 | 28 | | 56 | 18 | |
| Tumor deposit |
| Absent | 152 | 130 | 22 | 0.701 | 99 | 53 | 0.968 | 129 | 23 | 0.232 |
| Present | 29 | 24 | 5 | | 19 | 10 | | 22 | 7 | |
|
Differentiation |
| Well, mod | 132 | 109 | 23 | 0.120 | 77 | 55 | 0.001a | 104 | 28 | 0.006a |
| Por, muc | 49 | 45 | 4 | | 41 | 8 | | 47 | 2 | |
| Lymph invasion |
| Negative | 167 | 143 | 24 | 0.476 | 110 | 57 | 0.510 | 139 | 28 | 0.811 |
| Positive | 14 | 11 | 3 | | 8 | 6 | | 12 | 2 | |
| Venous
invasion |
| Negative | 178 | 151 | 27 | 0.465 | 116 | 62 | 0.957 | 148 | 30 | 0.436 |
| Positive | 3 | 3 | 0 | | 2 | 1 | | 3 | 0 | |
Survival analysis
Patients with reduced membranous expression levels
of β-catenin at the tumor center had significantly lower
cancer-specific five-year survival rates (58.5%), compared with
patients that exhibited preserved membranous expression of
β-catenin at the tumor center (78.1%; log-rank, P=0.028; Fig. 3A). The difference in cancer-specific
survival rates between patients with high-grade nuclear expression
of β-catenin (five-year survival rate, 52.6%) and low-grade nuclear
expression of β-catenin (five-year survival rate, 70.1%) at the
tumor invasive front was also identified to be statistically
significant (log-rank test, P=0.020; Fig. 3B).
Univariate analysis revealed that the T stage
(P<0.001), N stage (P<0.001), TNM stage (P<0.001), the
presence of lymphatic invasion (P<0.001), the presence of tumor
deposits (P<0.001), reduced membranous expression levels of
β-catenin at the tumor center (P=0.028) and high-grade nuclear
expression of β-catenin at the tumor invasive front (P=0.020) were
significant prognostic factors. However, age, gender, tumor
location and size, tumor differentiation and venous invasion were
not significantly associated with patient survival (Table IV).
 | Table IVUnivariate and multivariate analyses
of survival rates in colorectal cancer patients. |
Table IV
Univariate and multivariate analyses
of survival rates in colorectal cancer patients.
| | Univariate
analysis | Multivariate
analysis |
|---|
| |
|
|
|---|
| Variable | Patients (n) | Five-year survival
rate (%) | P-value | HR | 95% CI | P-value |
|---|
| Age (years) |
| ≤60 | 65 | 69.7 | 0.254 | | | |
| >60 | 116 | 66.0 | | | | |
| Gender |
| Male | 105 | 69.9 | 0.151 | | | |
| Female | 76 | 64.0 | | | | |
| Tumor size
(cm) |
| ≤5 | 94 | 64.2 | 0.814 | | | |
| >5 | 87 | 70.6 | | | | |
| Tumor site |
| Colon | 72 | 65.2 | 0.266 | | | |
| Rectum | 109 | 68.3 | | | | |
| T stage |
| pT1–pT2 | 61 | 85.7 | <0.001a | | | |
| pT3–pT4 | 120 | 58.5 | | | | |
| N stage |
| pN0 | 108 | 92.5 | <0.001a | 10.729 | 1.336–86.14 | 0.026a |
| pN1–pN2 | 73 | 32.3 | | | | |
| TNM stage |
| I–II | 107 | 93.4 | <0.001a | 0.009 | 0.001–0.082 | <0.001a |
| III–IV | 74 | 31.8 | | | | |
| Tumor deposit |
| Absent | 152 | 76.8 | <0.001a | 0.368 | 0.208–0.651 | 0.001a |
| Present | 29 | 17.2 | | | | |
|
Differentiation |
| Well, mod | 132 | 68.7 | 0.984 | | | |
| Por, muc | 49 | 62.8 | | | | |
| Lymph invasion |
| Negative | 167 | 70.1 | <0.001a | | | |
| Positive | 14 | 35.7 | | | | |
| Venous
invasion |
| Negative | 178 | 67.9 | 0.086 | | | |
| Positive | 3 | 33.3 | | | | |
| Tumor center
membranous β-catenin |
| Preserved | 74 | 78.1 | 0.028a | 1.132 | 0.627–2.046 | 0.681 |
| Reduced | 107 | 58.5 | | | | |
| Tumor front nuclear
β-catenin |
| Low-grade | 151 | 70.1 | 0.020a | 0.708 | 0.384–1.705 | 0.268 |
| High-grade | 30 | 52.6 | | | | |
Multivariate analysis using Cox regression analysis
demonstrated that the TNM stage (P<0.001), presence of tumor
deposits (P=0.001) and lymph node metastasis (P=0.026) were
independent prognostic factors in CRC patients (Table IV). In addition, multivariate
analysis revealed that β-catenin levels were not a significant
prognostic factor.
Discussion
Despite significant advancements in CRC diagnosis
and treatment, the prognosis for patients with advanced CRC remains
poor. EMT, the switch from the polarized epithelial cell phenotype
to a migratory mesenchymal phenotype, is increasingly recognized as
a central event during malignant tumor progression and metastasis
(16,17). β-catenin maintains cell-to-cell
adhesion along with E-cadherin. However, β-catenin also acts as a
transcription factor in the Wnt signal transduction pathway
(5). The dual role of β-catenin in
cadherin-mediated cell-cell adhesion and in Wnt signaling, where it
is a key effector, renders β-catenin an ideal target for analyzing
the molecular basis of EMT and malignant cancer formation. The
accumulation and aberrant activation of β-catenin signaling, as
well as the transcription of target genes (hypothesized to
contribute to various stages in tumor development) result from
mutations in the adenomatous polyposis coli protein that abolish
its capacity to bind β-catenin or mutations in the β-catenin
phosphorylation motif at the N-terminus. The target genes include
predominant regulators of EMT, for example Slug (18), which inhibits E-cadherin
transcription. The release of β-catenin from cell-cell junctions
following the dismantling of cell-cell adhesions, which contain
E-cadherin, during EMT and the consequent activation of
β-catenin-mediated transactivation are also important in EMT
regulation (19).
However, the prognostic significance of β-catenin
expression levels in patients with CRC remains controversial.
Certain studies have shown that nuclear β-catenin expression is
associated with high tumor budding and a poor prognosis (15,20,21),
however, other studies did not detect this association (22–24).
Additionally, a recent study revealed that β-catenin overexpression
was correlated with a favorable prognosis (25).
Therefore, in the present study, the expression
levels of the EMT-associated marker, β-catenin were investigated at
the tumor invasive front and in the tumor center of CRC tissue
specimens. Long-term clinical follow-up of the CRC patients was
conducted and a large number of cases were included in the study,
thus, the results are considered to be meaningful.
The levels of β-catenin protein expression in serial
paraffin-embedded sections obtained from 181 human CRC samples were
examined using IHC staining. The correlations between the β-catenin
differential expression patterns and clinicopathological
characteristics and prognosis were also assessed. The results
showed that membranous β-catenin expression levels in the samples
were significantly reduced at the tumor invasive front, compared
with in the tumor center; however, nuclear β-catenin expression
levels were significantly increased at the tumor invasive front,
compared with in the tumor center. The dynamic changes in the
intracellular distribution of β-catenin and the changes in tumor
cell phenotype revealed a process that is reminiscent of EMT. The
presence of reduced membranous expression levels of β-catenin at
the tumor center was significantly associated with lymph node
metastasis and the TNM stage. At the tumor invasive front,
high-grade nuclear expression of β-catenin was significantly
correlated with lymph node metastasis, TNM stage and histological
differentiation.
Further analysis demonstrated that patients with
reduced membranous expression levels of β-catenin had significantly
lower cancer-specific five-year survival rates, compared with those
patients exhibiting preserved membranous expression of β-catenin at
the tumor center. The five-year survival rate of patients with
high-grade nuclear β-catenin expression was significantly lower
than that of patients with low-grade nuclear expression of
β-catenin at the tumor invasive front. However, this did not
persist as an independent prognostic factor following Cox
multivariate analysis. The undifferentiated tumor cells at the
tumor invasive front may undergo β-catenin-mediated EMT, which may
result in the dissemination of tumor cells, and subsequently induce
tumor invasion and metastasis.
In conclusion, the results of the present study
indicate that changes in β-catenin expression levels are associated
with aggressive morphological features, EMT and a poor prognosis in
patients with CRC. IHC staining of β-catenin is considered to be a
useful marker to predict the prognosis in CRC. However, large,
well-designed prospective studies are required to further
investigate the accurate prognostic significance of β-catenin.
Acknowledgements
The authors would like to thank the department of
Surgical Oncology of the First Hospital of China Medical University
(Shenyang, China) for providing the human colorectal cancer tissue
samples and the College of China Medical University (Shenyang,
China) for technical assistance during the experimental
procedures.
References
|
1
|
de Krijger I, Mekenkamp LJ, Punt CJ and
Nagtegaal ID: MicroRNAs in colorectal cancer metastasis. J Pathol.
224:438–447. 2011.
|
|
2
|
Xu AG, Yu ZJ, Jiang B, et al: Colorectal
cancer in Guangdong Province of China: a demographic and anatomic
survey. World J Gastroenterol. 16:960–965. 2010.
|
|
3
|
Zlobec I and Lugli A: Invasive front of
colorectal cancer: dynamic interface of pro-/anti-tumor factors.
World J Gastroenterol. 15:5898–5906. 2009.
|
|
4
|
Natalwala A, Spychal R and Tselepis C:
Epithelial-mesenchymal transition mediated tumourigenesis in the
gastrointestinal tract. World J Gastroenterol. 14:3792–3797.
2008.
|
|
5
|
Brabletz T, Jung A, Hermann K, et al:
Nuclear overexpression of the oncoprotein beta-catenin in
colorectal cancer is localized predominantly at the invasion front.
Pathol Res Pract. 194:701–704. 1998.
|
|
6
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007.
|
|
7
|
Sánchez-Tilló E, de Barrios O, Siles L,
Cuatrecasas M, Castells A and Postigo A: Beta-catenin/TCF4 complex
induces the epithelial-to-mesenchymal transition (EMT)-activator
ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci USA.
108:19204–19209. 2011.
|
|
8
|
Jang TJ: Expression of E-cadherin and
β-catenin is altered at tumor budding sites, whose number is
associated with the progression of colorectal carcinoma. Korean J
Pathol. 43:523–527. 2009.
|
|
9
|
International Agency for Research on
Cancer. World Health Organization classification of tumours.
Pathology and genetics of tumours of the digestive system. IARC
Press; Lyon, France: 2000
|
|
10
|
Edge SBD and Compton C: AJCC Cancer
Staging Manual. 7th edition. JAMA. 304. pp. 1726–1727. 2010
|
|
11
|
Ru GQ, Wang HJ, Xu WJ and Zhao ZS:
Upregulation of Twist in gastric carcinoma associated with tumor
invasion and poor prognosis. Pathol Oncol Res. 17:341–347.
2011.
|
|
12
|
Sinicrope FA, Ruan SB, Cleary KR, et al:
bcl-2 and p53 oncoprotein expression during colorectal
tumorigenesis. Cancer Res. 55:237–241. 1995.
|
|
13
|
Zhu JL, Song YX, Wang ZN, et al: The
clinical significance of mesenchyme forkhead 1 (FoxC2) in gastric
carcinoma. Histopathology. 62:1038–1048. 2013.
|
|
14
|
Boo YJ, Park JM, Kim J, et al: L1
expression as a marker for poor prognosis, tumor progression, and
short survival in patients with colorectal cancer. Ann Surg Oncol.
14:1703–1711. 2007.
|
|
15
|
Baldus SE, Mönig SP, Huxel S, et al: MUC1
and nuclear beta-catenin are coexpressed at the invasion front of
colorectal carcinomas and are both correlated with tumor prognosis.
Clin Cancer Res. 10:2790–2796. 2004.
|
|
16
|
Grünert S, Jechlinger M and Beug H:
Diverse cellular and molecular mechanisms contribute to epithelial
plasticity and metastasis. Nat Rev Mol Cell Biol. 4:657–665.
2003.
|
|
17
|
Hugo H, Ackland ML, Blick T, et al:
Epithelial-mesenchymal and mesenchymal-epithelial transitions in
carcinoma progression. J Cell Physiol. 213:374–383. 2007.
|
|
18
|
Conacci-Sorrell M, Simcha I, Ben-Yedidia
T, et al: Autoregulation of E-cadherin expression by
cadherin-cadherin interactions: the roles of beta-catenin
signaling, Slug, and MAPK. J Cell Biol. 163:847–857. 2003.
|
|
19
|
Gavert N and Ben-Ze’ev A:
Epithelial-mesenchymal transition and the invasive potential of
tumors. Trends Mol Med. 14:199–209. 2008.
|
|
20
|
Mårtensson A, Oberg A, Jung A, et al:
Beta-catenin expression in relation to genetic instability and
prognosis in colorectal cancer. Oncol Rep. 17:447–452. 2007.
|
|
21
|
Chen Z, He X, Jia M, et al: Beta-catenin
overexpression in the nucleus predicts progress disease and
unfavourable survival in colorectal cancer: a meta-analysis. PloS
One. 8:e638542013.
|
|
22
|
Hörkkö TT, Klintrup K, Mäkinen JM, et al:
Budding invasive margin and prognosis in colorectal cancer - no
direct association with beta-catenin expression. Eur J Cancer.
42:964–971. 2006.
|
|
23
|
Roca F, Mauro LV, Morandi A, et al:
Prognostic value of E-cadherin, beta-catenin, MMPs (7 and 9), and
TIMPs (1 and 2) in patients with colorectal carcinoma. J Surg
Oncol. 93:151–160. 2006.
|
|
24
|
Lee SJ, Choi SY, Kim WJ, et al: Combined
aberrant expression of E-cadherin and S100A4, but not beta-catenin
is associated with disease-free survival and overall survival in
colorectal cancer patients. Diagn Pathol. 8:992013.
|
|
25
|
Wangefjord S, Brändstedt J, Lindquist KE,
et al: Associations of beta-catenin alterations and MSI screening
status with expression of key cell cycle regulating proteins and
survival from colorectal cancer. Diagn Pathol. 8:102013.
|