Introduction
Facial nerve schwannomas are rare benign tumors,
which originate along the facial nerve. On imaging, those
presenting as an enhancing cerebellopontine angle mass may be
difficult to distinguish from vestibular schwannoma (also termed
acoustic neuroma) and meningiomas. The geniculate ganglion is
located in the temporal bone and contains cell bodies associated
with facial nerve-specialized taste and general somatic sensory
fibers. In the absence of pathological conditions, the geniculate
ganglion is not usually observed under intravenous gadolinium-based
enhancement.
Common clinical presentations of vestibular
schwannoma include ipsilateral sensorineural hearing loss/deafness,
disturbed sense of balance and altered gait, vertigo, nausea and
vomiting, as well as a sensation of pressure in the ears (1). It is generally hypothesized that as
the tumor increases in size, it compresses the brainstem and other
cranial nerves. The facial nerves are rarely involved and, thus,
facial paralysis is an uncommon symptom. However, Mackle et
al (2) reported that 3.7% of
vestibular schwannoma patients do not present with the typical
audiovestibular symptoms that are associated with vestibular
schwannoma. Written informed consent was obtained from the
patient.
Case report
In December 2012, a 36-year-old male was referred to
Shuang Ho Hospital (Taipei, Taiwan) presenting with the sudden
onset of right facial palsy three days previously. The patient had
experienced right facial palsy two years previously and recovered
completely following treatment at another hospital (Far Eastern
Memorial Hospital, Taipei, Taiwan). The patient also experienced
the sudden onset of right-sided tinnitus without noticeable hearing
impairment or dizziness. On physical examination, a House-Brackmann
(HB) grade III facial palsy was observed (3). No spontaneous or provoked nystagmus
were identified during a head shaking maneuver. Furthermore, the
tympanic membranes and nasopharynx were unremarkable.
Pure tone audiometry did not reveal any hearing
impairment and the patient’s speech recognition was 100%
bilaterally. In addition, the auditory brainstem response appeared
to be normal. Vestibular evoked myogenic potentials were
symmetrical on either side. The caloric test revealed 38% right
canal paresis, while facial electroneurography demonstrated 80%
degeneration on the right side. The patient did not experience
facial paresthesia or altered sensations of taste. Brain magnetic
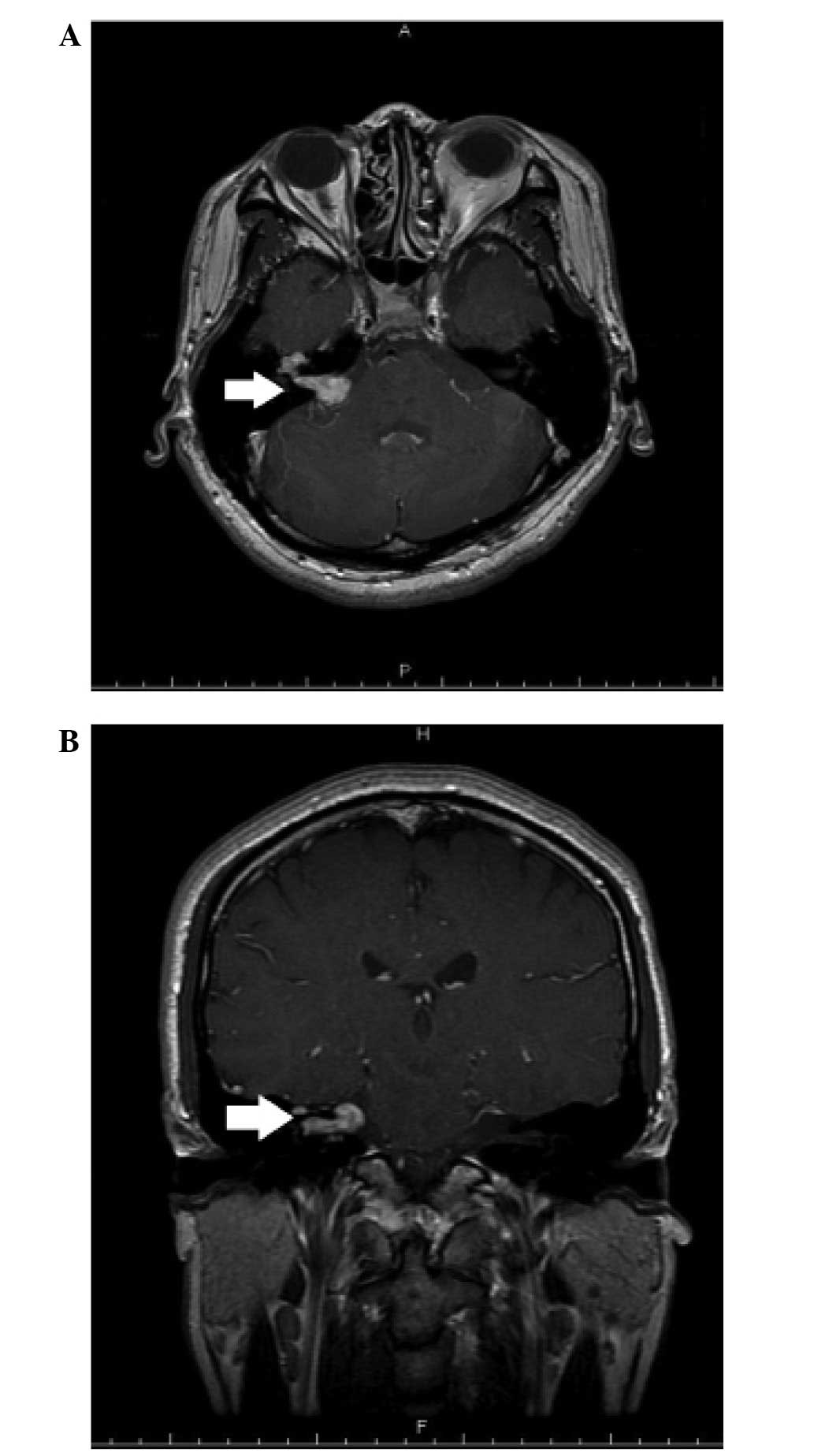
resonance imaging (MRI) demonstrated a mass (maximal diameter, 1.2
cm) in the right internal auditory canal (Fig. 1). No enhancement was observed at the
labyrinthine portion of the facial nerve or at the geniculate
ganglion.
Following steroidal therapy with prednisolone (1
mg/kg per day) for two weeks, the facial weakness improved to HB
grade I. Considering the patient’s hearing status and that the
tumor remained intracanalicular without any immediate risk of
compressing other vital structures, conservative treatment
(watchful waiting) with an MRI follow-up was performed.
At the one-year follow-up the tumor had enlarged and
extended along the facial nerve to the peri-geniculate area
(Fig. 2). Right-sided tinnitus was
observed, however, the patient’s hearing remained normal. Gamma
Knife radiosurgery using a margin dose of 13 Gy was performed. Six
months following radiosurgery, follow-up MRI imaging revealed that
the tumor was stable without progression. In addition, the
patient’s facial nerve function and hearing remained intact.
Discussion
Facial nerve tumors are rare tumors of the temporal
bone, which may involve any aspect of the facial nerve. However,
facial nerve tumors predominantly present in the peri-geniculate
area and the tympanic segment. Skip lesions or multiple segments of
involvement are occasionally identified. Typical symptoms include
the slow progression of facial nerve paresis or paralysis, as well
as hearing loss, tinnitus, pain and vestibular symptoms.
Furthermore, an ear canal mass may be present (9). Facial twitching followed by
progressive paresis is also a common symptom of this type of tumor.
Facial nerve tumors account for 5% of all cases of facial
paralysis, worldwide and, therefore, must be considered in all
cases of facial palsy (11).
Facial nerve schwannoma are the most common type of
tumor involving the facial nerve. In a series analyzing 600
temporal bones, the incidence of intratemporal facial schwannoma
was 0.8% (12). Perez et al
(13) observed growth in four out
of 13 facial schwannomas that were managed via expectant treatment,
with a mean growth rate of 1.4 mm/year among the tumors that grew
(although if all 13 tumors were considered, the mean growth rate
was 0.42 mm/year). Facial nerve tumors are rarely considered during
the differential diagnoses of enhancing cerebellopontine angle
lesions. In the present case, the diagnosis was based on MRI
enhancement of the geniculate ganglion, a structure that is located
along the facial nerve within the temporal bone.
The management of facial nerve tumors has evolved
from the performance of microsurgical excision with facial nerve
repair (MER) to an increased adoption of more conservative
techniques, which are regarded as facial nerve preservation
approaches (14). These include
observation with the watchful waiting approach, fallopian canal
decompression and stereotactic radiosurgery. All of which are
considered to be important treatment options for the management of
facial nerve tumors (15). There is
controversy regarding the resection of facial nerve tumors of any
size when the facial nerve function is normal or near normal (HB
grade I–II). In cases where tumors are determined to have a high
probability of being facial nerve schwannoma, via imaging or
electrophysiological analysis, preservation of the best possible
facial nerve function must be prioritized regardless of the tumor
size. An exception to this principle includes cases where the tumor
is causing compressive symptoms, such as brainstem compression or
labyrinthine erosion.
Stereotactic radiosurgery is an emerging treatment
modality. Although it has been widely used for treating acoustic
neuroma, there are few reports regarding its use in facial nerve
tumors, however, the number is increasing (2). For facial nerve tumors, the advantages
of stereotactic radiation include the avoidance of surgery,
potential growth arrest of the tumor and possible preservation of
facial nerve function. The selection of treatment is based on the
intent to preserve facial nerve functions at the best possible
level for the longest duration. If patients exhibit a progressively
worsening clinical and radiological condition (HB grade II–IV and
radiological evidence of growth or impending complications)
treatments including, fallopian canal decompression, radiation or
MER are considered to be treatment options that are dependent on
the patient’s situation. Irradiation is an alternative for patients
with early facial dysfunction (HB grade II–III) and a documented
worsening of the clinical and radiological condition. It has a
relatively small, although notable risk of worsening the auditory
and facial function, which must be considered and discussed with
the patient prior to determining the treatment plans (10).
The changes in facial function and tumor sizes of
patients with facial nerve tumors, following stereotactic
radiosurgery from all published studies identified during the
literature search are presented in Table I. Approximately 4% of patients
experienced a decline in facial nerve function and 7% exhibited
tumor enlargement. The current treatment modality relies on
observations with serial MRI for cases with no or limited facial
nerve dysfunction.
 | Table ISummary of variations in patient HB
grade and tumor size following stereotactic radiation treatment for
facial nerve tumors, obtained from the literature. |
Table I
Summary of variations in patient HB
grade and tumor size following stereotactic radiation treatment for
facial nerve tumors, obtained from the literature.
| Study [year,
(ref)] | Patients, n | Variation |
|---|
|
|---|
| HB grade | Tumor size |
|---|
| Litre et al
[2007, (4)] | 11 | 3 improved | 4 decreased |
| 8 unchanged | 6 stable |
| | 1 increased |
| Kida et al
[2007, (5)] | 14 | 5 improved | 8 decreased |
| 8 unchanged | 6 stable |
| 1 worsened | |
| Nishioka et al
[2009, (6)] | 4 | 4 unchanged | 2 decreased |
| | 2 stable |
| Madhok et al
[2009, (7)] | 6 | 1 improved | 3 decreased |
| 5 unchanged | 3 stable |
| Hillman et al
[2008, (8)] | 2 | 1 improved | 2 stable |
| 1 unchanged | |
| Wilkinson et
al [2011, (9)] | 6 | 1 improved | 3 decrease |
| 5 unchanged | 1 stable |
| | 2 increased |
| Channer et al
[2012, (10)] | 3 | 1 improved | 3 stable |
| 1 unchanged, 1
worsened | |
The deterioration of facial nerve function or
hearing, rapid tumor growth, or impending labyrinthine erosion or
other complications require the consideration of conducting more
aggressive treatment strategies. Irradiation or fallopian canal
decompression of tumors is often employed for facial nerve function
with an HB grade between II and IV. Microsurgical excision and
facial nerve repair is generally delayed until the facial nerve
function deteriorates to HB grades IV–VI. This is due to the
consideration that postoperative facial nerve paralysis is expected
to last for six to 18 months, which may be followed by an
improvement to an HB grade III at best. In conclusion, the present
case identified that enhancement of the geniculate ganglion is an
important characteristic to identify when evaluating the MRI
appearance of cerebellopontine angle masses. In addition, a more
conservative treatment modality may allow a greater number of
patients to experience improved long-term facial nerve function in
the management of facial nerve tumors.
References
|
1
|
Kirazli T, Oner K, Bilgen C, Ovul I and
Midilli R: Facial nerve neuroma: clinical, diagnostic, and surgical
features. Skull Base. 14:115–120. 2004.
|
|
2
|
Mackle T, Rawluk D and Walsh RM: Atypical
clinical presentations of vestibular schwannomas. Otol Neurotol.
28:526–528. 2007.
|
|
3
|
House JW and Brackmann DE: Facial nerve
grading system. Otolaryngol Head Neck Surg. 93:146–147. 1985.
|
|
4
|
Litre CF, Gourg GP, Tamura M, et al: Gamma
knife surgery for facial nerve schwannomas. Neurosurgery.
60:853–859. 2007.
|
|
5
|
Kida Y, Yoshimoto M and Hasegawa T:
Radiosurgery for facial schwannoma. J Neurosurg. 106:24–29.
2007.
|
|
6
|
Nishioka K, Abo D, Aoyama H, et al:
Stereotactic radiotherapy for intracranial nonacoustic schwannomas
including facial nerve schwannoma. Int J Radiat Oncol Biol Phys.
75:1415–1419. 2009.
|
|
7
|
Madhok R, Kondziolka D, Flickinger JC and
Lunsford LD: Gamma knife radiosurgery for facial schwannomas.
Neurosurgery. 64:2009.
|
|
8
|
Hillman TA, Chen DA and Fuhrer R: An
alternative treatment for facial nerve tumors: short-term results
of radiotherapy. Ear Nose Throat J. 87:574–577. 2008.
|
|
9
|
Wilkinson EP, Hoa M, Slattery WH III, et
al: Evolution in the management of facial nerve schwannoma.
Laryngoscope. 121:2065–2074. 2011.
|
|
10
|
Channer GA, Herman B, Telischi FF, Zeitler
D and Angeli SI: Management outcomes of facial nerve tumors:
comparative outcomes with observation, CyberKnife, and surgical
management. Otolaryngol Head Neck Surg. 147:525–530. 2012.
|
|
11
|
O’Donoghue GM, Brackmann DE, House JW and
Jackler RK: Neuromas of the facial nerve. Am J Otol. 10:49–54.
1989.
|
|
12
|
Saito H and Baxter A: Undiagnosed
intratemporal facial nerve neurilemomas. Arch Otolaryngol.
95:415–419. 1972.
|
|
13
|
Perez R, Chen JM and Nedzelski JM:
Intratemporal facial nerve schwannoma: a management dilemma. Otol
Neurotol. 26:121–126. 2005.
|
|
14
|
Minovi A, Vosschulte R, Hofmann E, Draf W
and Bockmuhl U: Facial nerve neuroma: surgical concept and
functional results. Skull Base. 14:195–200; discussion 200–191,
2004.
|
|
15
|
Shirazi MA, Leonetti JP, Marzo SJ and
Anderson DE: Surgical management of facial neuromas: lessons
learned. Otol Neurotol. 28:958–963. 2007.
|