Introduction
The accumulation of non-haemic iron in the brain,
particularly in the basal ganglia (BG), is a common process
initiated shortly following birth (1). During the first two decades of life,
the iron concentration in the BG increases rapidly and then the
accumulation slows down, and the concentration of iron in the BG
follows an approximately exponential association curve. Alterations
in the deposition of iron and other paramagnetic substances have
been associated with various diseases. Increased deposition of
paramagnetic substances in the brain has been observed in patients
with liver diseases (2),
psychiatric (3) and
neurodegenerative disorders (4,5).
Although iron is an essential element in human
nutrition, free iron (Fe2+) is closely associated with
the production of reactive oxygen species (ROS), which may induce
biological damage through oxidative stress. In the Fenton reaction,
Fe2+ reduces H2O2 and subsequently
produces a hydroxyl radical (•OH). Increased iron deposition in
ferritin molecules is therefore considered to be a protective
mechanism. The Fe2+ ions are trapped by ferritin,
oxidised to Fe3+ and safely stored inside the protein
molecule.
However, increased iron stores may be associated
with an elevated risk of cancer. The association between iron and
carcinogenesis has been recognised for a number of years (6). Increased body iron stores (indicated
by higher serum ferritin levels and elevated transferrin
concentrations) have been associated with malignant neoplasms
(7) and leukaemia (8). Animal experiments as early as 1959
revealed that repeated injections of iron induce malignant tumours
(9). Iron may be involved in the
initiation or promotion of colorectal, liver, kidney, lung, stomach
and other types of cancer (10).
In addition, increased concentrations of an
iron-storage protein, ferritin, have been detected in the
cerebrospinal fluid (CSF) of patients with glioblastoma (11). The authors ascertained the presence
of ferritin in tumour cells in one patient and hypothesised that
CSF ferritin was secreted by the tumour cells. The transfer of
ferritin from the serum through the blood-CSF barrier does not
occur on a large scale, due to the high molecular weight of
ferritin and its hydrodynamic radius.
Attempts have been made to reduce the impact of iron
redox activity during cancer treatment through the chelating of
surplus iron ions (12); however,
the alterations in Fe metabolism in cancer are not yet fully
understood. Changes may occur during iron absorption, iron
transport and iron storage (7,8,11,12).
Tumour cells are also known for the upregulation of transferrin
receptor expression on the cell surface (12).
Magnetic resonance imaging (MRI) is sensitive to the
presence of paramagnetic ions in tissues. Alterations in
paramagnetic or superparamagnetic ion concentrations manifest as a
hyperintense signal on T1-weighted images or as a
hypointense signal on T2-weighted images, as the ions
substantially shorten relaxation times. Iron in iron-storage
molecules substantially shortens T2 due to its magnetic
properties. The most common type of iron-storage molecule,
ferritin, which is soluble, also exerts a clear effect on the
T1 relaxation time, whereas insoluble pathological
hemosiderin markedly influences T2, but has no
significant effect on T1 (13). The changes in relaxation times may
be quantified by MR relaxometry (14).
In the present study, the deposition of paramagnetic
substances in patients with brain tumours was retrospectively
evaluated using T2 relaxometry.
Materials and methods
Patients
A total of 23 patients (mean age 46±12 years) were
examined at the Department of Diagnostic and Interventional
Radiology, Institute for Clinical and Experimental Medicine
(Vídeňská, Czech Republic). The patients were divided into two
groups. Group 1 consisted of 12 subjects (mean age, 46±13 years)
with an untreated tumour in the brain: Six subjects with high-grade
gliomas (HGG), four subjects with low-grade gliomas (LGG) and two
subjects with lymphomas (Lym). Group 2 consisted of 11 subjects
(mean age, 46±11 years) with tumour recurrence following treatment
(chemotherapy and radiotherapy and/or resection). The primary
tumour was HGG in six subjects and LGG in five subjects. All
patients involved in the study had the diagnosis verified by
histological methods. The control group consisted of 19 age-matched
healthy patients (mean age, 47±12 years). All patients were
informed with regard to the examination procedure and provided an
informed consent approved by the local ethical committee. Clinical
procedures were certified according to the ISO 9001:2008 norm.
MRI
A 3 Tesla clinical MR imager Magnetom Trio (Siemens,
Erlangen, Germany) equipped with a transmit/receive (Tx/Rx) head
coil was used. A standard imaging procedure consisting of native
T1- and T2-weighted images and
contrast-enhanced T1-weighted images (in the patient
group only) was supplemented with a Carr Purcell Meiboom Gill
sequence (32 echoes, echo-spacing echo time = 13.2 ms, repetition
time = 3,000 ms and slice thickness = 5 mm) for
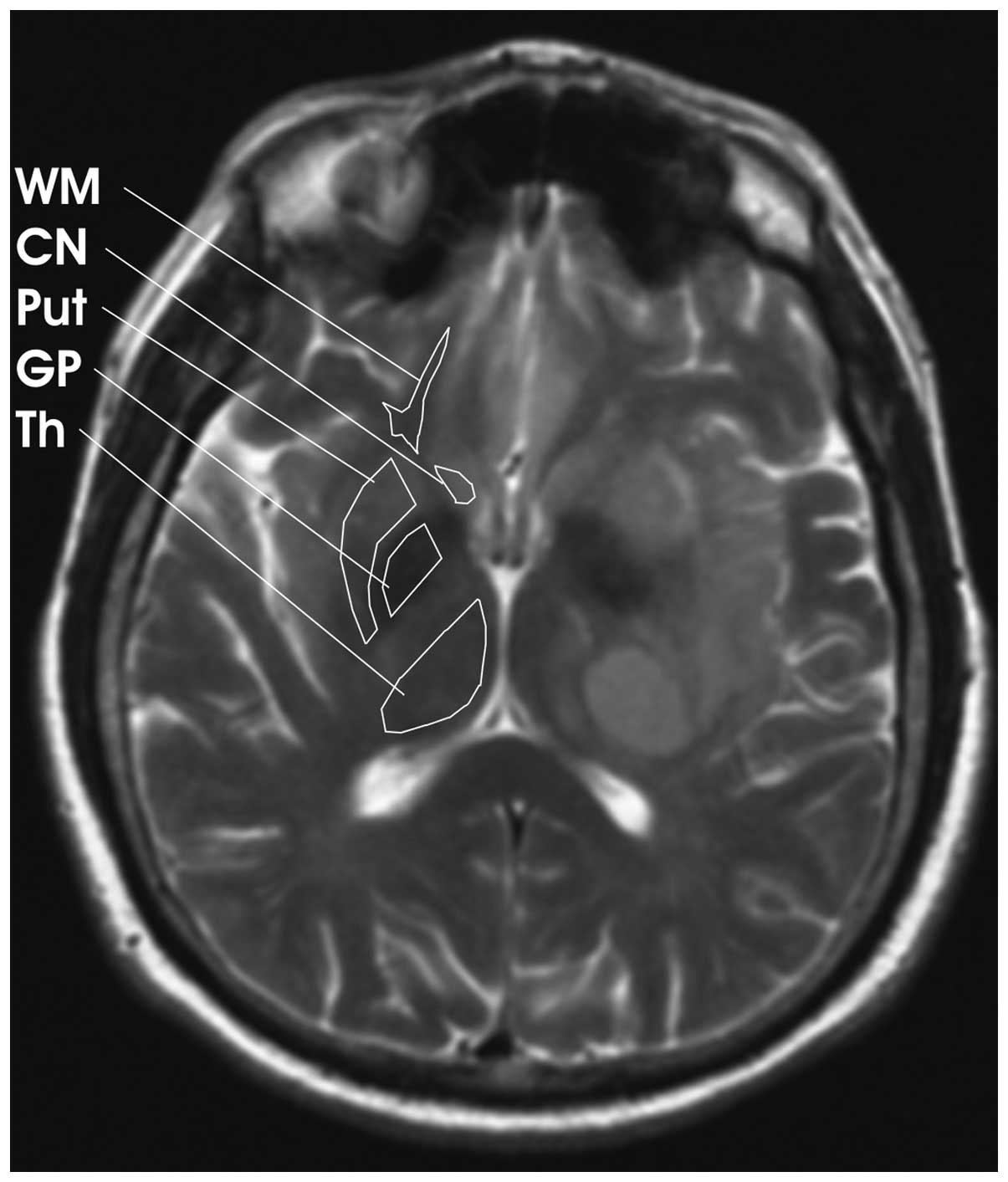
T2-mapping. A slice containing the BG was evaluated.
T2 maps were calculated using custom-made ViDi software
(Department of Diagnostic and Interventional Radiology, Institute
for Clinical and Experimental Medicine) utilising a three-parameter
fit (15). T2 values
were then obtained from the globus pallidus (GP), putamen (Put),
caudate nucleus (CN), thalamus (Th) and frontal white matter (WM)
in the two hemispheres. However, tumours are commonly accompanied
by extensive oedema, which may significantly prolong the
T2 relaxation time in BG. This influence in the
ipsilateral hemisphere cannot be separated from other changes that
affect T2 relaxation, thus, the T2 values
were substantially dispersed. Therefore, for further evaluation and
comparison, only values found in the contralateral hemisphere were
used (Fig. 1). Infiltration of the
tumours into the contralateral hemisphere was not observed in the
selected patients.
Statistical analysis
Student’s t-test was used for comparing the
relaxation times of the different patient groups and the controls.
P<0.05 was considered to indicate a statistically significant
difference. The equality of variances was analysed using an F-test.
For unequal variances, a variant of the t-test for unequal
variances was employed; this was the case for data obtained from
the WM.
Results
T2 relaxation times in
patients with untreated and recurrent, treated brain tumours
Significantly lower T2 relaxation times
were identified in the GP, Put, CN and Th of brain tumour patients
as compared with those of the controls (P<0.05, Table I). In the group of patients with
untreated brain tumours, the T2 in the GP, CN and Th was
significantly reduced, as compared with the controls (P<0.05).
However, the difference in the Put T2 values was not
statistically significant due to the high data dispersion. In the
patients with recurrent tumours who had received treatment,
significantly lower T2 values in the GP, Put, CN, Th and
WM were detected, as compared with the controls (P<0.05).
 | Table IT2 relaxation times in the
globus pallidus, putamen, caudate nucleus, thalamus and white
matter of patients (contralateral to the lesion) and controls. |
Table I
T2 relaxation times in the
globus pallidus, putamen, caudate nucleus, thalamus and white
matter of patients (contralateral to the lesion) and controls.
| T2
(ms) |
|---|
|
|
|---|
| Patient group | Globus pallidus | Putamen | Caudate nucleus | Thalamus | White matter |
|---|
| All patients | 51.4±2.2a | 63.2±4.1a | 73.4±4.8a | 70.8±2.7a | 69.2±4.6 |
| Patients with an
untreated tumour | 52.0±2.0a | 63.5±3.8 | 74.0±5.0a | 70.6±2.4a | 70.3±5.3 |
| Patients with a
recurrent tumour | 50.9±2.3a | 62.8±4.3a | 72.9±4.5a | 71.0±3.0a | 68.0±3.3a |
| Controls | 55.2±2.0 | 67.3±4.7 | 78.4±3.4 | 75.1±2.2 | 70.5±2.5 |
No statistically significant differences were
identified between patients with untreated tumours and those with
recurrent tumours in any of the examined structures. However, a
trend toward lower T2 values in the patients with
recurrent tumours was detected, which was verified by a paired
t-test between the average values in the given structures in the
two patient groups.
No difference in the WM T2 values between
the group of patients with untreated tumours and the control group
was observed; however, a significant difference in these values
between the group of patients with recurrent tumours and the
control group was identified (P<0.05).
T2 relaxation times in
patients with different types of brain tumour
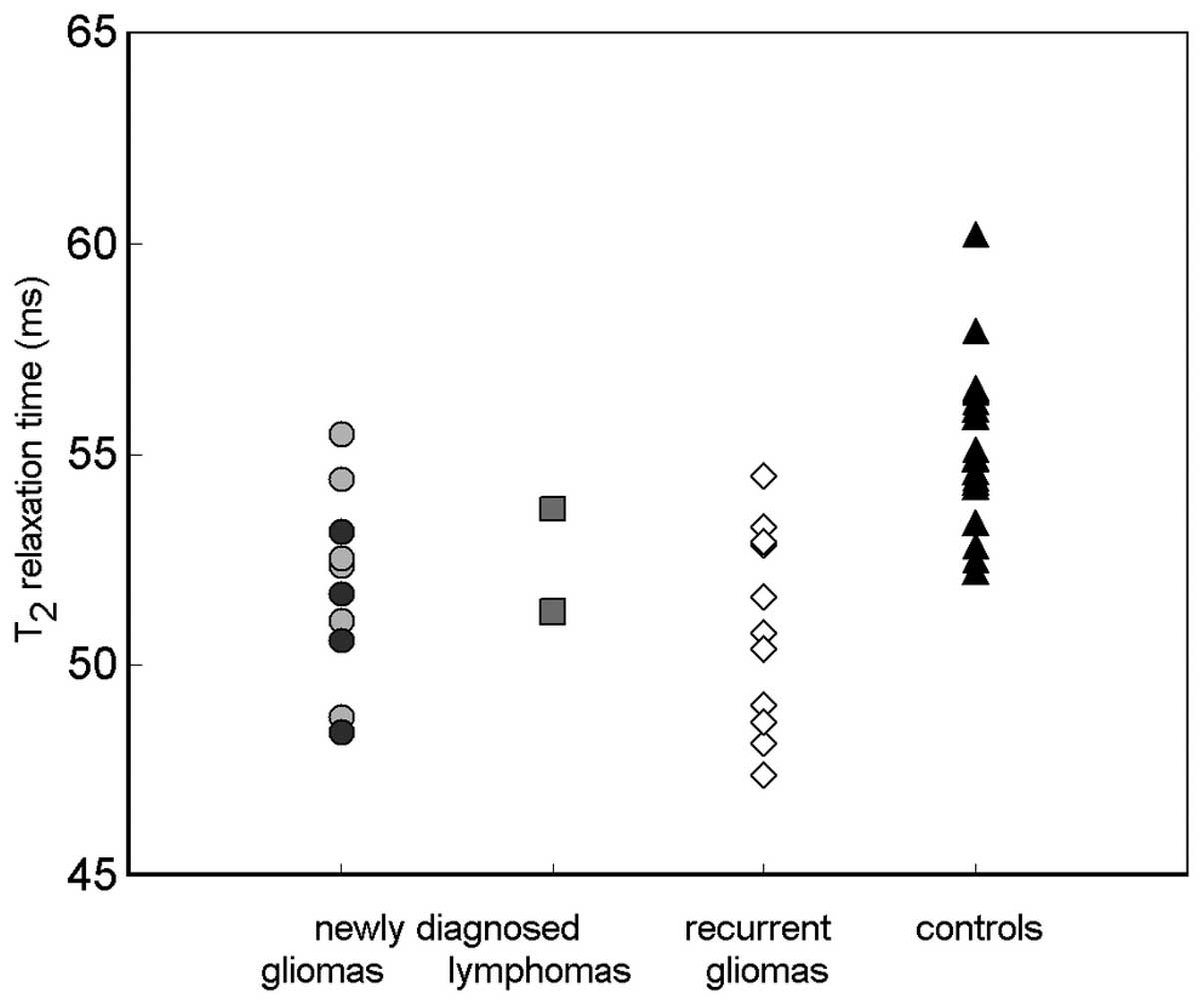
The graph in Fig. 2
shows the distribution of T2 values of GP in all
investigated groups. The two patients with Lym are presented
separately. No statistically significant differences were
identified between subjects with different grades of newly
diagnosed gliomas (HGG and LGG) or those with Lym. However, the
numbers of subjects in these subgroups were too low for meaningful
statistical analysis.
Discussion
MRI is an in vivo imaging method sensitive to
the paramagnetic ion content in tissues. In addition to
T2 mapping, other potentially more sensitive methods,
such as T2* mapping or susceptibility
weighted imaging (SWI), may be of interest and may be examined in a
prospective study. However, the length of the procedures in the
present study did not permit the addition of these sequences.
Although the T2 relaxation time reflects the tissue
structure and the water content, in addition to the presence of
paramagnetic ion, which renders interpretation more difficult, this
measurement is less sensitive to possible artefacts resulting from
external field heterogeneities than T2*
mapping or SWI, and is a robust and effective technique. In
addition, T2 mapping was included in our standard
imaging procedure and the majority of the subjects were evaluated
retrospectively, therefore alterations to the method were not
possible.
The reduction in the T2 relaxation time
in the BG observed in the present study may be caused by
paramagnetic ion accumulation. A similar effect (i.e. T2
shortening) has been observed in a range of psychiatric and
neurodegenerative diseases, and has been predominantly associated
with iron accumulation (3,16). The trend detected in the present
study towards lower T2 values in the patients with
recurrent tumours, as compared with the patients with newly
diagnosed lesions, may indicate the worsening of the patient’s
state with time and disease progression, or the possible further
damage to the tissue caused by radio/chemotherapy.
Although relaxometry is completely unspecific, the
element responsible for T2 shortening is hypothesised to
be iron, which is closely associated with cancer initiation
(6,10) and tumour growth (17). Iron, an essential element for human
life, may also be a carcinogenic agent (6), which has already been demonstrated in
hepatocellular carcinoma (18) and
breast cancer (19). Divalent iron
contributes to the formation of ROS, which may cause damage to the
DNA resulting in mutations and the initiation of cancer growth.
Iron is also required for cellular proliferation due to its role in
the active sites of a wide range of proteins involved in energy
metabolism, respiration and DNA synthesis.
The reduction in T2 (hypothetically
caused by an increased iron concentration) in the BG and Th
observed in the present study may be an indirect consequence of
tumour development rather than direct damage. Direct damage to the
BG tissue by the active tumour is improbable; reduced T2
values contralateral to the tumour were observed and no changes
were detected in the WM in the case of untreated tumours, although
the majority of the tumours had affected the WM. Lower
T2 values in the WM of patients with recurrent tumours
(not observed in the patients with newly diagnosed tumours) may be
a consequence of the damage to the tissue due to the therapy
undergone. The observed changes in the BG are possibly the result
of a general systemic effect of the tumours.
Although the concentration of the iron-storage
protein ferritin in CSF in patients with glioblastoma has been
found to be high, as compared with controls (11), the contribution of ferritin to
T2 shortening may be disregarded. The reported mean
concentration of ferritin in the CSF was 103 ng/ml (10.3 μg/100 g)
(11), which is negligible, as
compared with the concentration of non-haem iron in the BG, as
reported in another study (21.3 mg/100 g fresh weight in the GP),
the Th (4.76 mg/100 g fresh weight) or even the frontal WM (4.24
mg/100 g) (1).
The key issue is the transport of the putative iron
into the BG or Th. In the present study, no signal enhancement to
the contrast-enhanced MR images was observed in the BG; therefore,
the blood brain barrier was presumed to be intact. Thus, the direct
transport of ferritin molecules between the plasma and the tissue
is improbable. However, previously identified elevated ferritin
concentrations in the CSF indicate that the CSF may be an
alternative route for iron transportation to the brain tissue
(11).
As the tumour may be responsible for increased iron
concentrations in the plasma (17),
the transport may also be corrupted at the transferrin/transferrin
receptor level. An increase in the concentration of ferrous ions
inside the cell indicates the threat of oxidation stress induced by
Fe2+. To protect the tissue, the ferrous ions are
trapped and oxidised to ferric ions within the ferritin molecules
and stored. This accelerated protective process may result in a
substantially higher deposition of iron compounds in the BG or Th.
This hypothesis is in accordance with the known role of iron in
carcinogenesis and the finding that the maintenance of iron
homeostasis by chelation slows tumour growth (19).
Notably, in the present study, similarly reduced
T2 values were detected in the two patients with Lym and
the patients with gliomas (Fig. 2),
although the origin of Lym tumours is in the lymphatic tissue, in
contrast to gliomas, which are intrinsic to the brain tissue. This
finding corresponds to the hypothesis that increased iron
accumulation is a reaction to localised alterations in iron
homeostasis associated with tumour growth and angiogenesis rather
than a consequence of direct changes in the brain tissue.
The present study demonstrated that the reduction in
the T2 relaxation time in patients with brain tumours
was possibly caused by the deposition of paramagnetic ions in the
BG and Th. Non-specific changes in the T2 relaxation
times in the BG occurred in patients with untreated tumours and
those with recurrent tumours, regardless of tumour localisation. We
hypothesise that the changes in the BG and Th are caused by iron
deposition in ferritin molecules to eliminate excessive ferrous
ions from the tissue in order to provide protection from oxidative
stress.
Although the present study has no direct impact on
current diagnosis or tumour treatment practices, the study
contributes to the understanding of how brain tumours influence,
through homeostatic changes, even distant healthy tissues.
Acknowledgements
This study was supported by the Ministry of Health,
Czech Republic-Conceptual Development of Research Organisation
(Institute for Clinical and Experimental Medicine-IKEM; IN
00023001).
References
|
1
|
Hallgren B and Sourander P: The effect of
age on the non-haemin iron in the human brain. J Neurochem.
3:41–51. 1958. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vymazal J, Babis M, Brooks RA, Filip K,
Dezortova M, Hrncarkova H and Hajek M: T1 and T2 alterations in the
brains of patients with hepatic cirrhosis. AJNR Am J Neuroradiol.
17:333–336. 1996.PubMed/NCBI
|
|
3
|
Wolozin B and Golts N: Iron and
Parkinson’s disease. Neuroscientist. 8:22–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Correia S, Hubbard E, Hassenstab J, et al:
Basal ganglia MR relaxometry in obsessive-compulsive disorder: T2
depends upon age of symptom onset. Brain Imaging Behav. 4:35–45.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hájek M, Adamovičová M, Herynek V, Škoch
A, Jírů F, Krepelová A and Dezortová M: MR relaxometry and 1H MR
spectroscopy for the determination of iron and metabolite
concentrations in PKAN patients. Eur Radiol. 15:1060–1068. 2005.
View Article : Google Scholar
|
|
6
|
Toyokuni S: Iron-induced carcinogenesis:
The role of redox regulation. Free Radic Biol Med. 20:553–566.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evans AE, D’Angio GJ, Propert K, Anderson
J and Hann HW: Prognostic factor in neuroblastoma. Cancer.
59:1853–1859. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Potaznik D, Groshen S, Miller D, Bagin R,
Bhalla R, Schwartz M and de Sousa M: Association of serum iron,
serum transferrin saturation and serum ferritin with survival in
acute lymphocytic-leukemia. Am J Pediat Hematol Oncol. 9:350–355.
1987. View Article : Google Scholar
|
|
9
|
Richmond HG: Induction of sarcoma in the
rat by iron-dextran complex. Br Med J. 1:947–949. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang X: Iron overload and its association
with cancer risk in humans: evidence for iron as a carcinogenic
metal. Mutat Res. 533:153–171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato Y, Honda Y, Asoh T, Oizumi K, Ohshima
Y and Honda E: Cerebrospinal fluid ferritin in glioblastoma:
evidence for tumor synthesis. J Neurooncol. 40:47–50. 1998.
View Article : Google Scholar
|
|
12
|
Richardson DR, Kalinowski DS, Lau S,
Jansson PJ and Lovejoy DB: Cancer cell iron metabolism and the
development of potent iron chelators as anti-tumour agents. Biochim
Biophys Acta. 1790:702–717. 2009. View Article : Google Scholar
|
|
13
|
Vymazal J, Urgosík D and Bulte JW:
Differentiation between hemosiderin- and ferritin-bound brain iron
using nuclear magnetic resonance and magnetic resonance imaging.
Cell Mol Biol (Noisy-le-grand). 46:835–842. 2000.
|
|
14
|
Schenker C, Meier D, Wichmann W, Boesiger
P and Valavanis A: Age distribution and iron dependency of the T2
relaxation time in the globus pallidus and putamen. Neuroradiology.
35:119–124. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herynek V, Wagnerová D, Hejlová I,
Dezortová M and Hájek M: Changes in the brain during long-term
follow-up after liver transplantation. J Magn Reson Imaging.
35:1332–1337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dusek P, Jankovic J and Le W: Iron
dysregulation in movement disorders. Neurobiol Dis. 46:1–18. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steegmann-Olmedillas JL: The role of iron
in tumour cell proliferation. Clin Transl Oncol. 13:71–76. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deugnier Y: Iron and liver cancer.
Alcohol. 30:145–150. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jian J, Yang Q, Dai J, Eckard J, Axelrod
D, Smith J and Huang X: Effects of iron deficiency and iron
overload on angiogenesis and oxidative stress - a potential dual
role for iron in breast cancer. Free Radic Biol Med. 50:841–847.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Richardson DR: Iron chelators as
therapeutic agents for the treatment of cancer. Crit Rev Oncol
Hematol. 42:267–281. 2002. View Article : Google Scholar : PubMed/NCBI
|