Introduction
As the fifth most common cause of cancer-related
mortality, ovarian cancer accounts for >50% of all mortalities
associated with gynecological cancer (1). With an incidence of 35–78%, nodal
metastases occur frequently, particularly in advanced-stage ovarian
tumors (stages III-IV) (2,3).
Primary debulking surgery (PDS) is the current
standard treatment for advanced ovarian cancer, followed by
post-surgical chemotherapy (4). An
improved prognosis may be expected in cases where optimal debulking
(residual disease, <1 cm) can be achieved (5). Neoadjuvant chemotherapy (NAC) has been
recognized as an alternative treatment to PDS for patients with a
poor performance status or apparently unresectable bulky tumors
(6). NAC is expected to become a
standard treatment or one of the effective treatment options for
advanced ovarian cancer (7) as
other phase III studies (8,9) begin to produce similar positive
results. Radiotherapy may be an effective treatment modality, even
in the setting of otherwise chemotherapy refractory disease
(10). Tactics for the
consolidation of a complete response following chemotherapy remain
of great interest, but future studies are required to determine
which consolidation treatment is optimal for advanced ovarian
cancer (11).
A previous study has indicated that the majority of
females with advanced ovarian cancer, in whom tumor control was
achieved, will go on to develop recurrent disease (12). Combined positron emission
tomography/computed tomography (PET/CT) is particularly useful for
differentiating between ovarian cancer and benign disease, and for
locating distant metastases. Borderline tumors may be interpreted
as benign on PET/CT (13). In the
present study, PET/CT technology was used to guide the use of the
radiotherapy. The study was approved by the Ethics Committee of the
People’s Liberation Army (PLA) 323 Hospital (Xi’an, China) and the
patient provided written informed consent.
Case report
This study presents the case of a 68-year-old female
who underwent biological intensity-modulated radiotherapy (BIMRT)
and neoadjuvant chemotherapy for multiple peritoneal metastases of
ovarian cancer [International Federation of Gynecology and
Obstetrics (FIGO) stage IIIc] (14)
on February 2, 2012, at the PLA 323 Hospital. The patient presented
with urination and defecation difficulties, and felt severe pain at
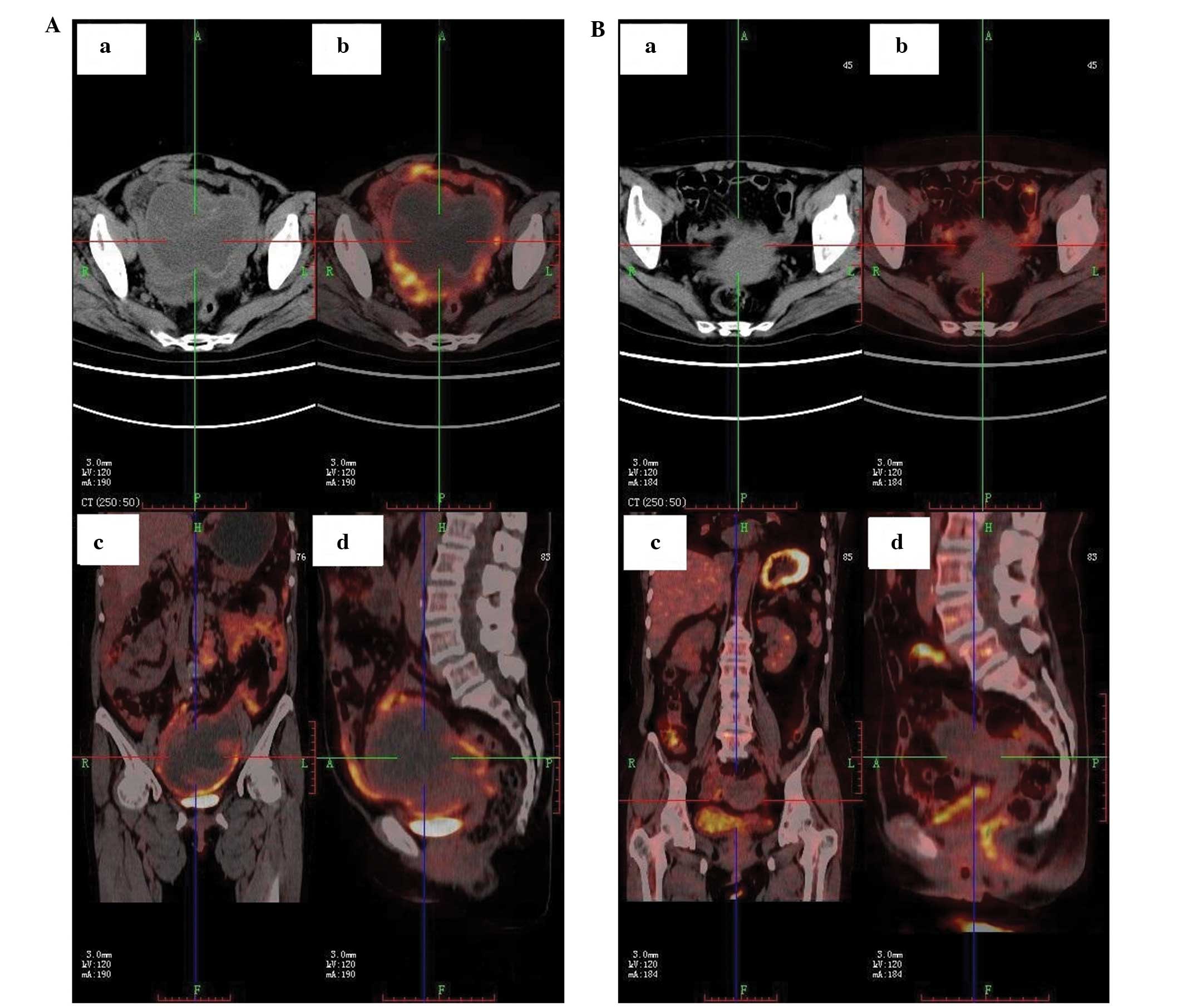
multiple abdominal sites. The PET/CT examination indicated an
ovarian cystic or solid package, frequent abdominal and
retroperitoneal lymph node enlargement and abdominal cavity
effusion (Fig. 1A and 2A). Transverse, coronary and sagittal
PET/CT scans of the patient for peritoneal metastases prior to
treatment revealed the presence of metastatic cancer (Fig. 3A).
Following BIMRT, two cycles of neoadjuvant
combination chemotherapy (180 mg Taxol and 100 mg cisplatin) were
administered. In the Department of Oncology, radiation treatment
was administered at a total dose of 48 Gy, as single 4.0-Gy doses
12 times. A total of 100 mg cisplatin was initially administered
via peritoneal perfusion, with two cycles of chemotherapy. One
chemotherapy cycle involved Taxol (180 mg/m2) dissolved
in 500 ml intravenous saline, administered intravenously over 3
hours, following a 1-hour interval, 100 mg/m2 cisplatin
was injected. In total, two cycles, each lasting 21 days were
completed. The severe pain previously experienced by the patient,
particularly the abdominal pain, was alleviated by symptomatic
treatment. The tumor shrank and the patient’s condition was
stabilized (Fig. 1B). Furthermore,
PET/CT images following treatment showed normalizing
18F-fluorodeoxyglucose uptake in the para-aortic lymph
nodes (Figs. 2B and 3B). Following radiotherapy treatment, the
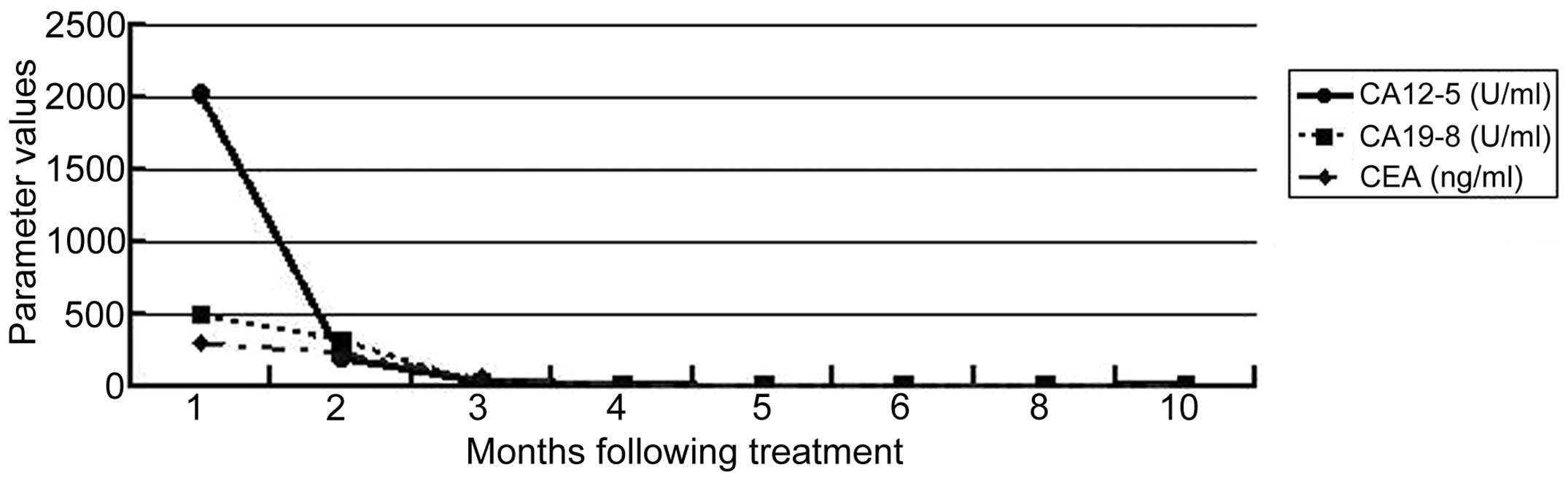
CA-125 tumor marker level declined sharply, while the CA19-9 and
CEA levels declined gradually. All the tumor marker levels
eventually returned to within the normal ranges (Fig. 4).
At the time of writing this manuscript, the patient
is well and without relapse.
Discussion
Ovarian cancer is one of the most commonly occurring
gynecological malignancies Patients with ovarian carcinoma have a
poor prognosis, as in the majority, the diagnosis is made at an
advanced stage. Recurrence occurs frequently, particularly in the
initial two years after first-line therapy. There have previously
been no studies on the use of modern radiotherapy techniques, such
as BIMRT, in ovarian cancer patients with multiple peritoneal
metastases in whom surgery is not suitable. The majority of studies
have reported the use of initial surgery or chemotherapy, followed
by subsequent radiotherapy, for the treatment of ovarian cancer
(15,16). However, the clinical outcome remains
unsatisfactory (17).
The current standard treatment of maximal
cytoreductive surgery and adjuvant combination carboplatin and
taxane chemotherapy is aggressive, however, the prognosis for
patients with an advanced disease stage remains poor. The median
time to recurrence is less than two years, and the predominance of
recurrence occurs intraperitoneally. The five-year survival rate
for FIGO stage IIIc disease is 20–25% (18–20).
According to the present results, it is reasonable to guide
treatment based on the BIMRT for ovarian cancer patients with
multiple peritoneal metastases in whom surgery is not suitable.
Rochet et al (21) showed
the clinical feasibility of intensity-modulated whole abdominal
radiotherapy in combination with modern chemotherapy and surgery.
The technique provides coverage of the entire peritoneal cavity,
including frequent sites of abdominal recurrence, such as the
diaphragm and liver capsule, and also the pelvic and para-aortic
lymph node regions. The treatment effectively spares the kidneys,
liver and bone marrow, and is subject to high compliance by
patients.
In conclusion, the present study indicates that when
adjuvant combination chemotherapy (Taxol and cisplatin) is
available, modern radiotherapy techniques, such as BIMRT, may be
considered as a beneficial treatment option for ovarian cancer
patients with multiple peritoneal metastases in whom surgery is not
suitable.
References
|
1
|
Bristow RE, Duska LR, Lambrou NC, et al: A
model for predicting surgical outcome in patients with advanced
ovarian carcinoma using computed tomography. Cancer. 89:1532–1540.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parazzini F, Valsecchi G, Bolis G, et al:
Pelvic and paraortic lymph nodal status in advanced ovarian cancer
and survival. Gynecol Oncol. 74:7–11. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pereira A, Magrina JF, Rey V, Cortes M and
Magtibay PM: Pelvic and aortic lymph node metastasis in epithelial
ovarian cancer. Gynecol Oncol. 105:604–608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du Bois A and Pfisterer J: Future options
for first-line therapy of advanced ovarian cancer. Int J Gynecol
Cancer. 15(Suppl 1): 42–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winter WE III, Maxwell GL, Tian C, et al:
Prognostic factors for stage III epithelial ovarian cancer: a
Gynecologic Oncology Group Study. J Clin Oncol. 25:3621–3627. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Onda T: Neoadjuvant chemotherapy for
ovarian cancer. Gan To Kagaku Ryoho. 39:882–886. 2012.PubMed/NCBI
|
|
7
|
Muggia FM, Braly PS, Brady MF, et al:
Phase III randomized study of cisplatin versus paclitaxel versus
cisplatin and paclitaxel in patients with suboptimal stage III or
IV ovarian cancer: a gynecologic oncology group study. J Clin
Oncol. 18:106–115. 2000.PubMed/NCBI
|
|
8
|
Mouratidou D, Gennatas C, Michalaki V, et
al: A phase III randomized study comparing paclitaxel and cisplatin
versus cyclophosphamide and cisplatin in patients with advanced
ovarian cancer. Anticancer Res. 27:681–685. 2007.PubMed/NCBI
|
|
9
|
Markman M, Bundy BN, Alberts DS, et al:
Phase III trial of standard-dose intravenous cisplatin plus
paclitaxel versus moderately high-dose carboplatin followed by
intravenous paclitaxel and intraperitoneal cisplatin in
small-volume stage III ovarian carcinoma: an intergroup study of
the Gynecologic Oncology Group, Southwestern Oncology Group, and
Eastern Cooperative Oncology Group. J Clin Oncol. 4:1001–1007.
2001.
|
|
10
|
Kumar A, Gilks CB, Mar C, Santos J and
Tinker AV: Patterns of spread of clear cell ovarian cancer: Case
report and case series. Gynecol Oncol Rep. 6:25–27. 2013.
View Article : Google Scholar
|
|
11
|
Petit T, Velten M, d’Hombres A, et al:
Long-term survival of 106 stage III ovarian cancer patients with
minimal residual disease after second-look laparotomy and
consolidation radiotherapy. Gynecologic Oncology. 104:104–108.
2007. View Article : Google Scholar
|
|
12
|
Marsden DE, Friedlander M and Hacker NF:
Current management of epithelial ovarian carcinoma: a review. Semin
Surg Oncol. 19:11–19. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Risum S, Høgdall C, Loft A, et al: The
diagnostic value of PET/CT for primary ovarian cancer - a
prospective study. Gynecol Oncol. 105:145–149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
FIGO-International Federation of
Gynaecologists and Obstetricians Cancer Committee. Annual Report on
the Results of Treatment in Gynaecological Cancer. FIGO; Stockholm:
1979
|
|
15
|
Ozols RF, Bundy BN, Greer BE, et al:
Gynecologic Oncology Group: Phase III trial of carboplatin and
paclitaxel compared with cisplatin and paclitaxel in patients with
optimally resected stage III ovarian cancer: a Gynecologic Oncology
Group study. J Clin Oncol. 21:3194–3200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bristow RE, Tomacruz RS, Armstrong DK, et
al: Survival effect of maximal cytoreductive surgery for advanced
ovarian carcinoma during the platinum era: a meta-analysis. J Clin
Oncol. 20:1248–1259. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gadducci A, Cosio S, Conte PF and
Genazzani AR: Consolidation and maintenance treatments for patients
with advanced epithelial ovarian cancer in complete response after
first-line chemotherapy: a review of the literature. Critl Rev
Oncol Hematol. 55:153–166. 2005. View Article : Google Scholar
|
|
18
|
Morrison J, Haldar K, Kehoe S and Lawrie
TA: Chemotherapy versus surgery for initial treatment in advanced
ovarian epithelial cancer. Cochrane Database Syst Rev.
8:CD0053432012.PubMed/NCBI
|
|
19
|
Onda T and Yoshikawa H: Neoadjuvant
chemotherapy for advanced ovarian cancer: overview of outcomes and
unanswered questions. Expert Rev Anticancer Ther. 11:1053–1067.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lambert HE, Gregory WM, Nelstrop AE and
Rustin GJ: Long-term survival in 463 women treated with platinum
analogs for advanced epithelial carcinoma of the ovary: Life
expectancy compared to women of an age-matched normal population.
Int J Gynecol Cancer. 14:772–778. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rochet N, Sterzing F, Jensen AD, Dinkel J,
Herfarth KK, Schubert K, et al: Intensity-modulated whole abdominal
radiotherapy after surgery and carboplatin/taxane chemotherapy for
advanced ovarian cancer: phase I study. Int J Radiat Oncol Biol
Phys. 76:1382–1389. 2010. View Article : Google Scholar
|