Introduction
Lung cancer is one of the most common tumors
globally; it is highly malignant and has a high rate of distant
metastasis (1). In total, ~50% of
these patients already have distant metastasis when diagnosed, with
the most common metastasis sites being the lungs, liver, bone,
brain and adrenal glands (1).
Cutaneous metastasis of lung cancer is rare, its pathogenesis is by
either lymphovascular invasion or hematogenous metastasis (2). The histology of cutaneous metastsis
most commonly reveals adenocarcinoma, then squamous/small-cell
carcinoma, followed by large-cell carcinoma (3). Common treatment modalities include
surgery, chemotherapy and radiotherapy. Currently, the prognosis
for patients with cutaneous metastasis of lung cancer is poor.
Intestinal metastases from lung cancer are rare and the diagnosis
is often late, with clinical symptoms of bowel occlusion and
intestinal bleeding (4). In certain
cases the clinical manifestations of the metastases have been
observed prior to those of the primitive tumour (5). However, in the presence of bowel
occlusion and intestinal bleeding of uncertain origin, obtaining a
clinical history is particularly important and diagnostic
procedures must be performed to rule out a secondary pathology
(6). Until now, simultaneous
cutaneous and intestinal metastases have never been reported. The
present study reports such a case that was recently admitted to The
Affiliated Hospital of Shandong Academy of Medical Sciences (Jinan,
China). Written informed consent was obtained from the patient.
Case report
A 62-year-old female was admitted to The Affiliated
Hospital of Shandong Academy of Medical Sciences in August 2013
with multiple lumps in the right thigh, armpit and scalp that had
been present for one month. A number of these lumps had ulcerated
two weeks prior to the visit. Three lumps were observed on the
scalp, among which the top lump was the largest. This lump was a
hard, 3×2 cm protrusion, which was recessed and ulcerated at the
center, with a clear embankment-like boundary. The other two bulges
looked like craters, with clear boundaries and no ulceration or
exudation. No tenderness was reported. In addition, a furuncle-like
lump was found on the right thigh, which was swollen and ulcerated,
with mild tenderness. A purple, protruding 2×2-cm lump could also
be observed in the right armpit, with furuncle-like embossing of
the top and clear boundaries. The lump was of moderate texture,
with a certain degree of tenderness (Fig. 1). Upon physical examination, chest
auscultation revealed clear breathing sounds for the left lung,
while those of the right lung were comparatively lower. There was
no rhonchus or moist rale and other parameters were normal. Chest
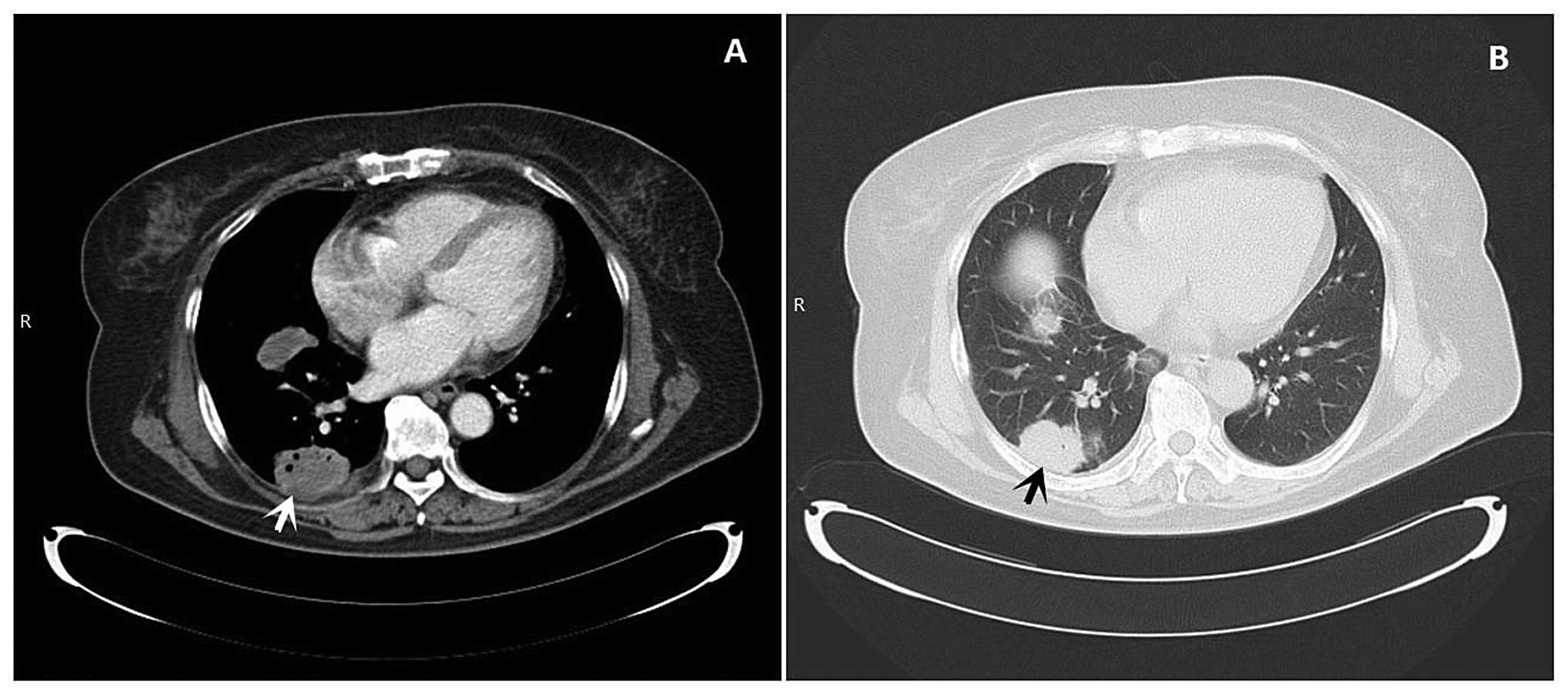
computed tomography (CT) showed nodules and masses in the right
lung, with multiple enlarged lymph nodes in the mediastinum and
right hilum (Fig. 2). This
suggested a diagnosis of primary right lung cancer with
intrapulmonary and lymphatic metastases in the mediastinum and
right hilum. Resection of the tumors on the scalp, right thigh and
armpit was performed due to the ulcerated cutaneous nature of the
tumors. Pathological examination showed moderately-differentiated
adenocarcinoma, which was considered to be metastatic cancer. A
percutaneous biopsy of the right lung tumor showed
moderately-differentiated adenocarcinoma. During the
hospitalization period, the patient experienced increased stool
frequency without obvious cause, which included tenesmus with blood
and pus, but no abdominal pain, nausea or vomiting. A digital
rectal examination revealed blood and a 4×3-cm lump at the rear of
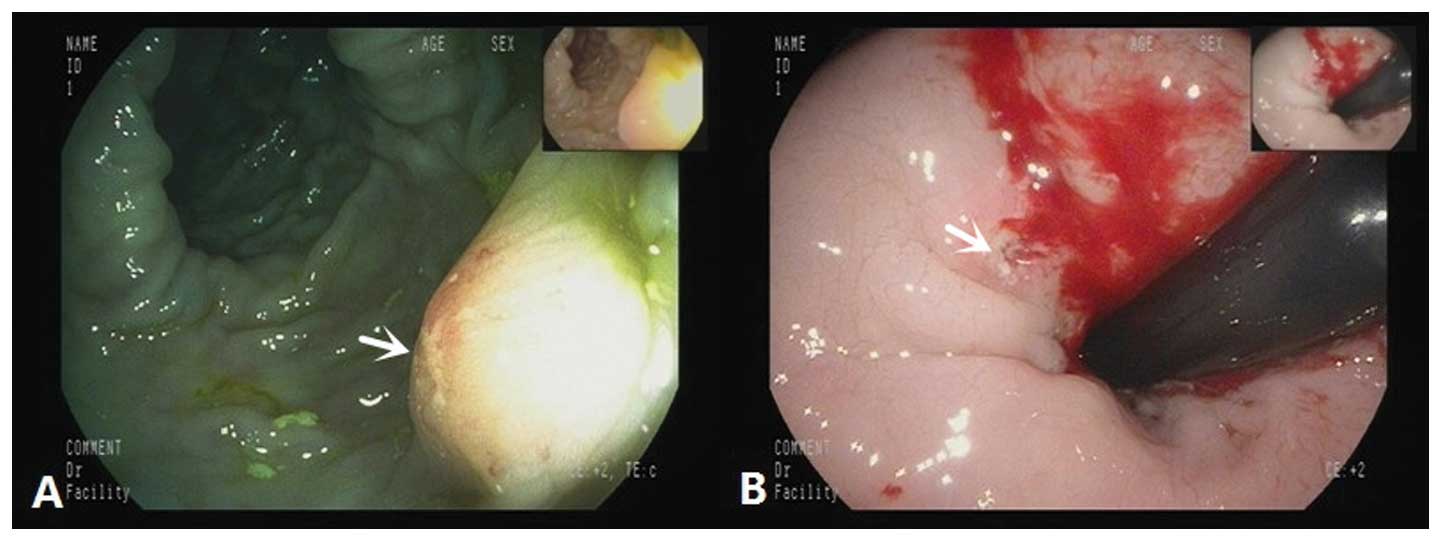
the perineal area, which was compressing the rectum. Colonoscopy
showed a 0.3×0.3-cm lump on the inside of the transverse colon,
which exhibited a rough mucosal membrane on the top, with clear
boundaries. An ulcer with a diameter of ~1 cm, a recessed center, a
peripheral bulge and a hard texture was observed on the rectum
(Fig. 3). Biopsies were taken from
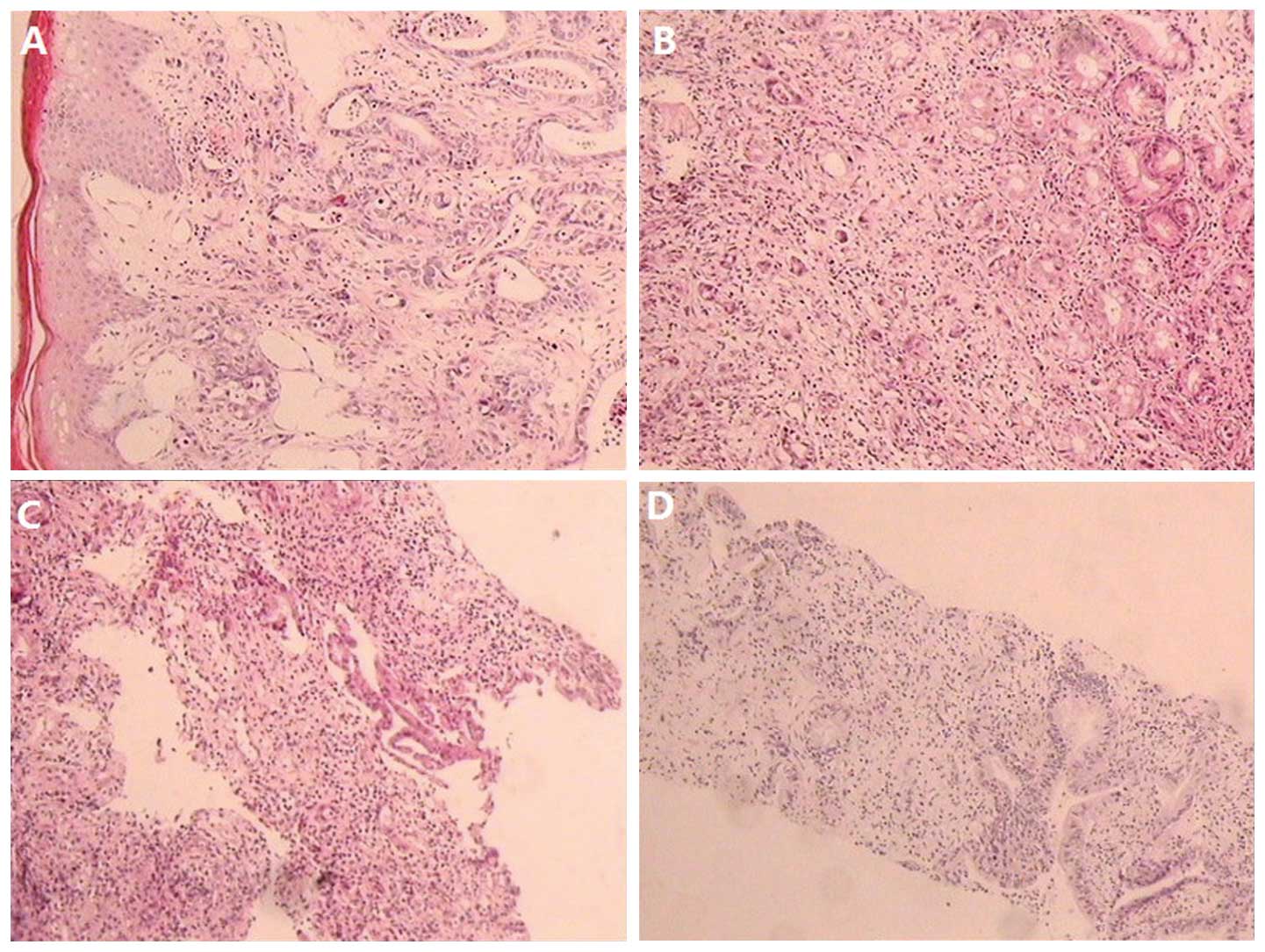
the two sites, which were both subsequently diagnosed as
moderately-differentiated adenocarcinoma. A similar pathology as
that shown on light microscopy (Fig.
4) and similar immunohistochemistry results (Table I) indicated that the tumors in the
intestines, scalp and thigh were all metastases of the primary lung
cancer. The patient is currently undergoing systemic chemotherapy
with intravenous gemcitabine (1.4 g, days 1 and 8) and cisplatin
(40 mg, days 1–3) every two weeks. At the time of writing, the
patient had undergone eight weeks of a six month treatment.
However, as the patient exhibits multiple cutaneous metastases and
intestinal metastasis, the expected surival time is poor.
 | Table IImmunohistochemistry results of
primary tumors and corresponding metastases. |
Table I
Immunohistochemistry results of
primary tumors and corresponding metastases.
| Primary and
metastatic sites | Immunohistochemistry
result |
|---|
| Primary lung
cancer | CK7(+); CDX2
spotty(+); CK20(−); TTF-1(−); vimentin(−) |
| Cutaneous
metastasis | CK7(+); CK19(+);
CA19-9 spotty(+); CDX2 scattered(+); CK20(−); TTF-1(−);
GCDFP-15(−); ER(−); PR(−) |
| Rectal
metastasis | CK7(+); CK19(+); CDX2
spotty(+); CK20(−); TTF-1(−) |
| Transverse colon
metastasis | CK7(+); CK19(+); CDX2
spotty(+); CK20(−); TTF-1(−); villin(−) |
Discussion
More than half of all lung cancers have metastasized
when diagnosed. These metastases occur most often in the thoracic
lymph nodes (46–85%), pleura (14–46%), brain (14–45%), adrenal
gland (36–64%), bone (21–41%), contralateral lung (13–43%) and
kidney (13–43%), and a small percentage of metastases are in the
abdominal lymph nodes, spleen, pancreas, heart, pericardium and
other regions (1,7). Cases of lung cancer with cutaneous and
intestinal metastases are rare.
Cutaneous metastasis is caused by primary
cancer-derived cells that grow in the skin (8). According to the published literature,
the incidence of cutaneous metastasis is 2.9–5.3% in general
(9), and 1–12% for lung cancer
(10). Clinically, cutaneous
metastasis often manifests as single or multiple nodules, possibly
at multiple sites (usually close to the primary tumor), which
differ in size (often 0.5–10 cm), and are round or oval, hard,
relatively immobilized, normally colored, purple or bright red, and
ulcerated or cauliflower-like, with bleeding in certain cases
(8,11). Certain cases may also manifest as
erysipelas-like cancer, vascular dilation or bullous-like lesions,
papules, plaques or scarring (12).
For the majority of cases, cutaneous metastasis occurs during the
progression of primary tumor following the initial diagnosis, but
for a few cases, cutaneous metastasis is found prior to the primary
tumor or simultaneously with the latter (7,10).
Cutaneous metastasis of the breast and oral cancers often result
from hematogenous and lymphatic metastasis, where the latter
pathway is pivotal, while for other tumors, hematogenous metastasis
is usually the primary cause. Metastasis through the lymphatic
pathway may explain why cutaneous metastasis occurs proximal to the
primary tumor (13). Lung
cancer-derived cutaneous metastasis cannot be differentiated from
cutaneous metastases of other sources based on the gross specimen.
Lung cancer-derived cutaneous metastases are most commonly from
lung adenocarcinoma, followed by squamous cell carcinoma, small
cell lung cancer and large cell lung cancer (14). Lung adenocarcinoma is usually
derived from bronchial epithelial goblet cells, usually the
borderline type, and is often asymptomatic in the early stages and
not diagnosed till the later stages when metastasis or compression
symptoms occur. The prognosis of cutaneous metastasis of lung
cancer is poor, and despite the use of chemoradiotherapy, the
median survival time is only 3–6 months (11).
Intestinal metastasis of lung cancer is even rarer
than cutaneous metastasis, with an incidence of ~0.19% according to
the published literature (15).
Intestinal metastasis causes abdominal symptoms such as abdominal
pain, bloating, bowel dysfunction and bleeding. CT or even positron
emission tomography-CT examinations rarely detect small metastases,
and false-negative results are common. Patients may initially
present with intestinal symptoms rather than typical signs of
primary lung cancer due to a lack of specific symptoms. The
intestinal metastasis diagnosed by colonoscopy may be misdiagnosed
as the primary tumor, therefore a biopsy is required for an
accurate diagnosis (16). The path
of the intestinal metastasis of lung cancer is currently unclear,
although it is generally considered to be lymphatic or
hematogenous. However, from previous clinical experience we propose
the following two possibilities: i) Metastasis may have occurred
through the paravertebral venous system to the intestinal mucosa;
or ii) since the patient had a long-term cough with sputum, the
cancer cells may have be coughed up with the sputum and swallowed
into the digestive tract, where they adhered to the intestines and
became established as metastasis. As the patient in the current
report exhibited no obvious cough, intestinal metastasis was
potentially the result of lymphatic and hematogenous metastaiss.
There is currently no solid evidence to support these hypotheses,
so relevant basic research is required. Intestinal metastasis
indicates an advanced grade of lung cancer, which leaves palliative
treatment and supportive care as the only treatment options
(17). The median survival time is
only 4–8 weeks after the diagnosis of intestinal metastasis of lung
cancer (18).
The patient in the present study exhibited no
obvious cough or any abnormal lung-related signs or symptoms, and
originally presented with multiple cutaneous lumps whose
characteristics were similar to that reported by the literature.
The primary cancer was identified during the CT examination, and
intestinal metastasis was detected by digital rectal examination,
colonoscopy and biopsy due to rectal irritation. In summary, the
patient exhibited multiple cutaneous and intestinal metastases,
possibly the additive result of lymphatic and hematogenous
metastasis. The diagnosis of this case was quick, and the patient
is currently undergoing systemic chemotherapy with gemcitabine plus
cisplatin. As the patient already exhibits multiple cutaneous
metastases and intestinal metastasis, the expected survival time
may not be long. The efficacy and prognosis of the treatment
requires further observation and analysis.
In conclusion, lung cancer is highly malignant and
prone to distant metastases, however, cutaneous and intestinal
metastases are rare. The present patient originally presented with
cutaneous metastases, and the primary tumor and intestinal
metastasis was only found during the examination, after which
systemic chemotherapy was administered. For suspected cutaneous and
intestinal lesions, a comprehensive analysis and examination should
be performed, including a timely pathological biopsy according to
the characteristics of the cutaneous lesion and a digital rectal
examination. A colonoscopy plus biopsy should be routinely used for
intestinal lesions to obtain an accurate diagnosis, so that the
correct treatment can be applied quickly and patient survival can
be prolonged.
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alkhayat H and Hong CH: Cutaneous
metastases from non-small cell lung cancer. J Cutan Med Surg.
10:304–307. 2006.
|
|
3
|
Song Z, Lin B, Shao L and Zhang Y:
Cutaneous metastasis as a initial presentation in advanced
non-small cell lung cancer and its poor survival prognosis. J
Cancer Res Clin Oncol. 138:1613–1617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nishizawa Y, Kobayashi A, Saito N, et al:
Surgical management of small bowel metastases from primary
carcinoma of the lung. Surg Today. 42:233–237. 2012. View Article : Google Scholar
|
|
5
|
Berger A, Cellier C, Daniel C, et al:
Small bowel metastases from primary carcinoma of the lung: clinical
findings and outcome. Am J Gastroenterol. 94:1884–1887. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cipollone G, Santarelli G, Quitadamo S, et
al: Small bowel metastases from lung cancer. Chir Ital. 56:639–648.
2004.(In Italian). PubMed/NCBI
|
|
7
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riahi RR and Cohen PR: Clinical
manifestations of cutaneous metastases. Am J Clin Dermatol.
13:103–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krathen RA, Orengo IF and Rosen T:
Cutaneous metastasis: a meta-analysis of data. South Med J.
96:164–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mollet TW, Garcia CA and Koester G: Skin
metastases from lung cancer. Dermatol Online J. 15:12009.PubMed/NCBI
|
|
11
|
Triller Vadnal K, Triller N, Pozek I, et
al: Skin metastases of lung cancer. Acta Dermatovenerol Alp
Pannonica Adriat. 17:125–128. 2008.PubMed/NCBI
|
|
12
|
Inamadar AC, Palit A, Athanikar SB, et al:
Inflammatory cutaneous metastasis as a presenting feature of
bronchogenic carcinoma. Indian J Dermatol Venereol Leprol.
69:347–349. 2003.
|
|
13
|
Pathak S, Joshi SR, Jaison J and Kendre D:
Cutaneous metastasis from carcinoma of lung. Indian Dermatol Online
J. 4:185–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sha D, Wang C and Wang W: Skin metastasis
of lung cancer: A clinical analysis and review of literature.
Central China Medical Journal. 33:57–59. 2009.
|
|
15
|
Kim MS, Kook EH, Ahn SH, et al:
Gastrointestinal metastasis of lung cancer with special emphasis on
a long-term survivor after operation. J Cancer Res Clin Oncol.
135:297–301. 2009. View Article : Google Scholar
|
|
16
|
Yang CJ, Hwang JJ, Kang WY, et al:
Gastro-intestinal metastasis of primary lung carcinoma: clinical
presentations and outcome. Lung Cancer. 54:319–323. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McNeill PM, Wagman LD and Neifeld JP:
Small bowel metastases from primary carcinoma of the lung. Cancer.
59:1486–1489. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
John AK, Kotru A and Pearson HJ: Colonic
metastasis from bronchogenic carcinoma presenting as pancolitis. J
Postgrad Med. 48:199–200. 2002.PubMed/NCBI
|