Introduction
Renal cell carcinoma (RCC) is the most common type
of kidney parenchymal cancer (1).
Malignant tumors of the renal pelvis constitute only 5% of urinary
tract neoplasms and ~90% of pelvic cancer cases are transitional
cell carcinoma (TCC) (2). Although
the majority of malignancies originating in the renal pelvis are
TCC, RCC has a tendency to grow into the renal pelvis. The imaging
findings of RCC and TCC in the renal pelvis are nonspecific;
therefore, preoperative differentiation between RCC and TCC is
important in order to identify the type of surgical treatment
required: Nephrectomy or ureteronephrectomy (3–5). The
present study presents a rare case of an RCC growing into the renal
pelvis, in which TCC of the renal pelvis was not able to be
distinguished using contrast enhanced computed tomography (CT) or
CT urography (CTU). Due to the uncertainty of diagnosis, the
present study emphasizes the role of ureteroscopy and biopsy in
accurate preoperative diagnosis and the theoretical importance of
the present case. Written informed consent was obtained from the
patient.
Case report
In April 2013, a 51-year-old male presented to
Peking University Shenzhen Hospital (Shenzhen, China) with the
chief complaint of a three-year history of repeated dull pain in
the right flank and painless gross hematuria for six months. The
patient had undergone two previous surgical procedures of
extracorporeal shock wave lithotripsy for renal calculus in 1997
and 2009. Physical examination revealed percussion pain over the
right kidney region and laboratory analysis identified a marginal
increase in alanine aminotransferase (59.8 U/l; normal range,
5.0–40.0 U/l) and creatine (118.5 μmol/l; normal range, 62–115
μmol/l) levels. In addition, an abundance of red blood cells (2,103
cells/μl; normal range, 0–12 cells/μl) was detected upon
urinalysis, while urine cytology identified the presence of
inflammatory cells but no atypical cells. Intravenous pyelography
(IVP) was performed, demonstrating an obstruction to the right
renal pelvis and, therefore, no visualization of the later ureter
(Fig. 1). A right kidney tumor was
suspected based on the results of a dynamic CT scan, which revealed
a marginally-contrasted space-occupying lesion in the right kidney.
Furthermore, ureteroscopy verified the presence of a solid
intraluminal mass (0.6×0.8 cm), spreading into the upper urinary
tract in close proximity to the renal pelvis. The renal pelvic
masses exhibited wide bases with high central densities and a
brittle surface, which bled easily. A ureteroscopic biopsy was
performed prior to placing a double-J stent in the right ureter,
which was histologically and cytogenetically analyzed to determine
a diagnosis of clear-cell RCC. No apparent metastases to the lung,
liver, adrenal glands or lymph nodes were observed.
Based on the aforementioned results, a diagnosis of
a clear-cell RCC of the right kidney was proposed; however, TCC was
not be completely excluded. Therefore, to further define the renal
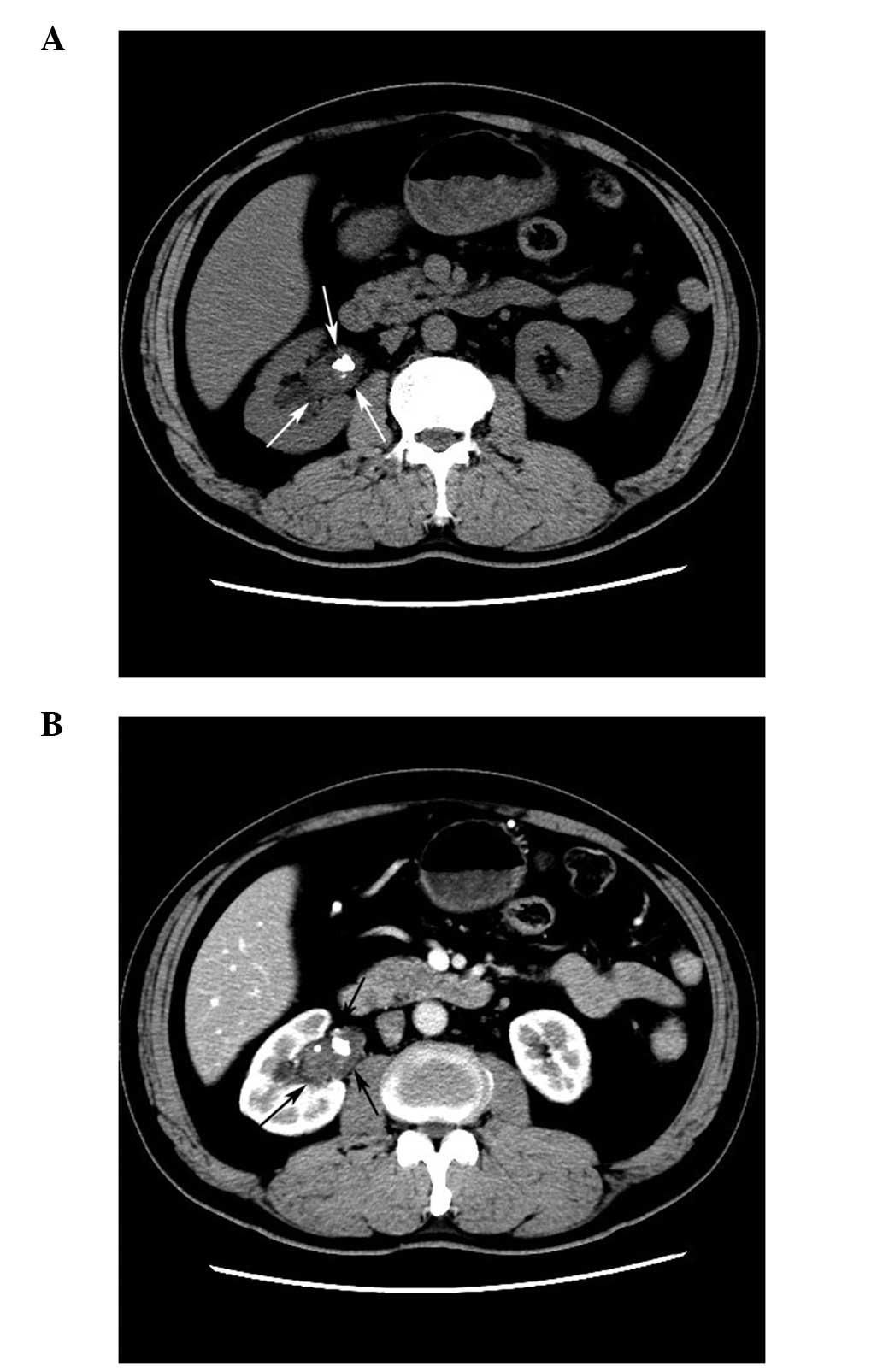
pelvic mass, CTU was performed, revealing a 2.0×1.6×3.4 cm
papillary right renal pelvic mass without renal parenchymal
(Fig. 2A). In the early phase, the
tumor presented contrast enhancement and was considered to
predominantly consist of TCC (Fig.
2B). Furthermore, the CT value of the tumor was 25 Hounsfield
units (HU) in the pre-contrast films and 56 HU in the post-contrast
films. However, due to the tentative diagnosis of clear-cell RCC
based on the ureteroscopic biopsy, the patient was subjected to
open right radical nephrectomy with retention of the ureteral
stump. During surgery, frozen section analysis revealed an
inflammatory polyp-covered transitional epithelium of mild
hyperplasia with blood clots and kidney stones.
Macroscopically, the localized tumor originated in
the renal calices of the right kidney lower pole, was attached to
the renal pelvis by its base and measured 4.0×1.0×0.5 cm.
Microscopically, the tumor was composed of atypical epithelioid
cells with a wide distribution, with interstitial edema and
infiltration of the focal lymphoid tissue; however, no invasion of
the ureteral wall was observed. Immunohistochemically, the tumor
exhibited diffuse positive staining for vimentin and P504S, while
it was only focally and weakly positive for cytokeratin (CK) 7,
cluster of differentiation 10 and Ki-67. In addition,
immunostaining for CK20, uroplakin, kidney-specific cadherin and
prostate-specific antigen was negative. These findings were in
accordance with a diagnosis of RCC; however, it remains unclear
whether the tumor was a clear-cell or papillary RCC as the patient
declined to undergo further assessment. No malignancy was observed
in the local renal pelvic or ureteral mucosa.
The patient was discharged nine days after surgery,
following an uncomplicated postsurgical recovery. No evidence of
recurrence or residual disease was detected on CT scans during the
nine-month follow-up period.
Discussion
The present study reported the challenging diagnosis
of a marginally-enhanced renal pelvic mass that was identified
using dynamic CT/CTU. Histological analysis revealed a clear-cell
RCC with renal pelvic invasion, which is a rare carcinoma reported
only in a small number of cases in the English literature (Table I) (3–9). Due
to inadequate preoperative diagnosis of TCC, nephroureterectomy has
been performed in a number of previous cases (3,5).
However, nephroureterectomy including cuff resection of the bladder
wall was performed in one case since frozen section analysis was
unable to exclude TCC (8). RCC with
renal pelvic extension was possibly mistaken for TCC only based on
CT findings or frozen section analysis. Therefore, the results of
various diagnostic approaches, particularly contrast enhanced
CT/CTU, ureteroscopy and biopsy, in the preoperative diagnosis of
such renal pelvic masses should be compared and assessed.
 | Table IReported cases of RCC growing into the
renal pelvis. |
Table I
Reported cases of RCC growing into the
renal pelvis.
| Case | Reference, year | Age
(years)/gender | CT description | Ureteroscopy or
cystoscopy/biopsy | Preoperative
diagnosis | Histological
diagnosis |
|---|
| 1 | Munechika et
al (3), 1990 | 22/M | Calcified tumor in
the lower pole of the right kidney, renal pelvis and proximal
ureter | Not performed | TCC | Mixed-type RCC |
| 2 | Chen et al
(6), 1996 | 62/F | Tumor in the right
kidney and renal pelvis | Not performed | Uncertain | RCC |
| 3 | Gulati et al
(7), 2007 | 67/M | Irregular kidney mass
and bladder mass | Cystoscopy revealed a
5-cm mass emanating from the left ureteral orifice/RCC | RCC | Clear-cell RCC |
| 4 | Fujita et al
(8), 2011 | 43/M | Enhanced tumor in the
left kidney, renal pelvis, ureter and bladder | Cystoscopy revealed
no obvious tumorous lesion/not performed | RCC | Clear-cell RCC |
| 5 | Kitazono et al
(4), 2011 | 64/M | Enhanced mass in the
left kidney upper pole, renal pelvis and ureter | Not performed | Uncertain | Clear-cell RCC |
| 6 | Kitazono et al
(4), 2011 | 77/F | Large mass in the
right kidney invaded the pelvicaliceal system | Ureteroscopy
confirmed pelvicaliceal involvement/not performed | Uncertain | Clear-cell RCC |
| 7 | Jeong and Kim
(5), 2012 | 58/F | Enhanced left renal
pelvic mass | Cystoscopy was
negative for TCC/not performed | TCC | Clear-cell RCC |
| 8 | Kakutani et al
(9), 2013 | 51/F | Contrasted tumor in
the left, kidney renal pelvis, ureter and bladder | Cystoscopy revealed a
tumor emanating from the left ureteral orifice/not performed | Renal pelvic
tumor | Clear-cell RCC |
| 9 | Present study,
2015 | 51/M | Enhanced right renal
pelvic mass | Ureteroscopy
confirmed a renal pelvic mass/clear cell RCC | Clear-cell RCC | RCC |
Dynamic CT/CTU has been increasingly performed as an
alternative to the traditional method (IVP) for the diagnosis of
upper tract lesions, due to its high diagnostic accuracy and
favorable comparisons with other imaging techniques (10). However, the principal limitation of
CTU is false-positive diagnosis; thus, a renal pelvic mass
diagnosed with CTU should be biopsied (guided by ureteroscopy) for
histopathological confirmation prior to proceeding with the
surgical procedure (10).
An enhanced mass, which may indicate a non-opaque
pyelolith, blood clot, lymphoma or, less commonly, RCC and
metastasis to the kidney with renal pelvic invasion, has been
identified in patients exhibiting painless hematuria using CT/CTU
(2,4). Dynamic CT images and HU values or
angiography with contrast injection may provide a comprehensive
view and vascular indications of the pelvic mass. By contrast,
three-dimensional CTU or magnetic resonance imaging aid in
delineating the precise location of the renal mass and its
association with the collecting system and renal vessels (2). However, occasionally the distinction
between TCC with renal invasion and RCC with renal pelvic extension
is difficult, since imaging findings of hypovascular RCC
demonstrate location, shape and enhancement that are
indistinguishable from TCC. Although significant differences in
multiphase CT attenuation have been reported between RCC and TCC,
the application of these results may be limited to facilitating the
differential diagnosis when the diagnosis is uncertain and avoiding
biopsy (11). In cases of uncertain
diagnosis, the present study advocates that appropriate
ureteroscopy and biopsy should be performed to provide crucial
histopathological information as the most important evidence. In
certain cases, ureteroscopy cannot be performed due to urethral
edema, stricture or obstruction; therefore, frozen section analysis
is recommended to determine any required changes to the surgical
strategy (4,5,9).
RCC with renal pelvic invasion is hypothesized to
occur due to the hollow structure of the renal pelvis. Invasion is
easier in the renal pelvis compared with the renal parenchyma when
localized RCC originates in the marginal parenchyma surrounding the
renal pelvis or when RCC invades the entire kidney. In the present
case, RCC did not invade the normal urothelium of the renal pelvis;
however, in a number of previous cases, the tumors had expanded
into the ureter and bladder (7,9).
Therefore, the present study provides fundamental evidence for the
following theory: Implantation and/or invasion of the urothelial
mucosa followed by intraluminal expansive growth results in the
pathogenesis of RCC with pelvic extension (9). Previous cases reporting RCC growth
along the urinary tract support the following mechanism of RCC
metastasis: Tumor cells may metastasize via intraluminal transit
down the urinary tract and invade the distal ureter or bladder
(7,9). Although these previous cases provide
evidence of a possible mechanism of RCC growth, they also challenge
the present tumor node metastasis (TNM) staging system. The case
reported in the present study indicates that RCC with invasion of
the urinary collecting system should be included in the present TNM
staging system. However, the inclusion of the urinary collecting
system invasion of RCC in the TNM staging system remains
controversial (12,13).
In conclusion, in this study, a rare case of RCC
growing into the renal pelvis was presented and the role of
ureteroscopy and biopsy in accurate preoperative diagnosis was
highlighted. The present study provided fundamental evidence for
the pathogenesis of RCC with pelvic extension and challenged the
present TNM staging system of RCC. However, whether the invasion of
the urinary collecting system is an independent prognostic factor
in RCC remains controversial and thus, further studies are
required.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81101922), the
Medical Scientific Research Foundation of Guangdong Province of
China (nos. A2012584 and A2013606), the Science and Technology
Development Fund Project of Shenzhen (no. JCYJ20130402114702124),
the Shenzhen Science and Technology Project (no. 201302050) and
funds from Guangdong Key Medical Subject.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rha SE, Byun JY, Jung SE, et al: The renal
sinus: pathologic spectrum and multimodality imaging approach.
Radiographics. (Suppl 1)24:S117–S131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Munechika H, Kushihashi T, Gokan T,
Hashimoto T, Higaki Y and Ogawa Y: A renal cell carcinoma extending
into the renal pelvis simulating transitional cell carcinoma. Urol
Radiol. 12:11–14. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitazono MT, Coakley FV, Naeger DM, Yeh
BM, Joe BN and Qayyum A: CT of unusual renal masses invading the
pelvicaliceal system: potential mimics of upper tract transitional
cell carcinoma. Clin Imaging. 35:77–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeong YB and Kim HJ: Is it transitional
cell carcinoma or renal cell carcinoma on computed tomography
image? Urology. 79:e42–e43. 2012. View Article : Google Scholar
|
|
6
|
Chen WC, Lee YH and Huang JK: Renal cell
carcinoma with renal pelvic extension simulating transitional cell
carcinoma: a case report. Zhonghua Yi Xue Za Zhi (Taipei).
58:147–150. 1996.
|
|
7
|
Gulati M, Gore JL, Pantuck AJ, Kim Y,
Barajas L and Rajfer J: Ureteral tumor thrombus from renal cell
carcinoma extending into bladder. Urol Oncol. 25:393–395. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujita O, Wada K, Yamasaki T, Manabe D,
Takeda K and Nakamura S: Renal cell carcinoma with a tumor thrombus
in the ureter: a case report. BMC Urol. 11:162011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kakutani S, Kume H, Hirano Y, Wakita T and
Homma Y: Renal cell carcinoma with intraluminal spread of the
entire upper urinary tract. Case Rep Med.
2013:3713872013.PubMed/NCBI
|
|
10
|
Cowan NC: CT urography for hematuria. Nat
Rev Urol. 9:218–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bata P, Tarnoki DL, Tarnoki AD, et al:
Transitional cell and clear cell renal carcinoma: differentiation
of distinct histological types with multiphase CT. Acta Radiol.
55:1112–1119. 2014. View Article : Google Scholar
|
|
12
|
Verhoest G, Avakian R, Bensalah K, et al:
Urinary collecting system invasion is an independent prognostic
factor of organ confined renal cell carcinoma. J Urol. 182:854–859.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Waalkes S, Merseburger AS, Herrmann TR, et
al: Urinary collecting system invasion is no independent prognostic
factor in renal cell carcinoma. World J Urol. 28:283–288. 2010.
View Article : Google Scholar : PubMed/NCBI
|