Introduction
Carcinoid tumors have been reported in a wide range
of organs, but they most commonly involve the gastrointestinal and
bronchopulmonary systems. Carcinoid tumors are rare in the testis
and comprise <1% of all testicular neoplasms (1). In a review of 13,715 carcinoid tumors,
9176 cases (66.9%) occurred in the gastrointestinal tract, whereas
113 cases (0.8%) occurred in the ovary, with only 8 cases (0.06%)
observed in the testicle (2). Unlike
ovarian carcinoid tumors, testicular carcinoid tumors are rarely
associated (in <3% of cases) with carcinoid syndrome (3).
Testicular carcinoid tumors can be divided into
three subgroups: primary pure testicular carcinoid tumors,
carcinoid tumors associated with teratoma (∼20% of cases), and
carcinoid metastasis to the testis (∼9% of cases). Cases have been
reported with patients ranging in age from ten to eighty-three
years, which is older than that reported with most primary germ
cell tumors (20–40 years) (1).
The first published case of a testicular carcinoid
tumor was observed as an element of a benign cystic teratoma in
1954 (4). Since then, around 150
cases of carcinoid tumors in the testis have been reported
(5–11). Data on ethnicity and geographical
distribution have revealed that the majority of carcinoid tumors
occurred in Europe or the United States. Our extensive literature
search yielded fewer than 30 cases from Asia (3,6,12). In this study, we investigated the
clinicopathological and immunophenotypical characteristics of
primary pure carcinoid tumors of the testis (pPCTT) in 11 Chinese
patients, and performed a comparison with the cases reported in the
literature.
Materials and methods
Clinical information
This study was approved by the ethics committee of
West China Hospital in Sichuan University, Chengdu, China. Between
January 1978 and January 2014, 688 male patients with testicular
neoplasms (including germ cell tumors, sex cord/gonadal stromal
tumors, lymphomas and leukemia) underwent surgical resections in
our medical center. Only 7 cases (1.02%) were pathologically
diagnosed as primary pure carcinoid tumors of the testis. In
addition, 6 pPCTT patients from other hospitals were sent to our
hospital for consultation and further evaluation. Two of the 13
patients were excluded following further evaluation due to
insufficient materials or incomplete clinical data. Finally, 11
male patients were enrolled in the study. All 11 patients underwent
unilateral radical orchiectomy.
The clinical features of the 11 patients including
clinical course and follow-up data were compiled. Furthermore, the
patients had staging evaluations that consisted of thorough
physical examinations by pre-operative imaging studies including
computed tomography (CT) scans of the chest, abdomen and pelvis,
duodenal fibroscopy and colonoscopy as well as somatostatin
receptor scintigraphy. In addition, the serum levels of three tumor
markers β human chorionic gonadotrophin (β-HCG), α-fetoprotein
(AFP) and lactate dehydrogenase (LDH) were also determined in the
patients.
Histological examination
Surgical resected specimens of testicular tumors
from the 11 patients were fixed with 10% formalin, routinely
embedded in paraffin, and the tissue sections were stained with
hematoxylin and eosin (H&E). The H&E sections were
independently diagnosed by two experienced pathologists.
Histological diagnosis of the tumors was based on the World Health
Organization criteria for the classification of carcinoid tumors in
the pancreas (13).
Immunophenotypical analysis
Primary antibodies used in this study were rabbit
polyclonal antibodies to adrenocorticotropin hormone (ACTH),
gastrin, glucagon, pancreatic polypeptide (PP) and somatostatin
(Maxim Biotech, San Francisco, CA, USA); chromogranin A (CgA; Zymed
Laboratories, San Francisco, CA, USA); CD117 (DakoCytomation,
Glostrup, Denmark); mouse monoclonal antibodies to inhibin-α, human
melanoma black 45, neuron-specific enolase (NSE), epithelial
membrane antigen, placental alkaline phosphatase (PLAP), thyroid
transcription factor 1 (TTF-1) and Syn (DakoCytomation); Ki67,
CDX-2 and S-100 (Maxim Biotech); a broad-spectrum cytokeratin (CK)
cocktail and CD56 (Zeta Corporation, Sierra Madre, CA, USA) and
melanoma antigen recognized by T cells 1 (Zymed Laboratories). The
Envision kit (DakoCytomation) was used for immunohistochemical
staining according to the manufacturer's instructions.
Results
Clinicopathological features
The clinical characteristics of the 11 male patients
with pPCTT are presented in Table I.
The median age of the patients was 47 years (range, 26–68 years).
The mean time from the initial presentation of symptoms to
diagnosis was 22.3 months. Seven patients (63.6%) noted a painless
mass in one testicle, while 4 patients (36.4%) experienced
testicular swelling and pain or discomfort. Case 5 had noted his
right testicle to be larger than the left since his childhood, but
did not pay close attention until he experienced a dull scrotal
pain for one month. Case 3 was identified as having a mass in his
right testicle during skin degerming prior to surgery for acute
appendicitis.
 | Table I.Clinical and pathological features of
11 male patients with primary pure carcinoid tumors of the
testis. |
Table I.
Clinical and pathological features of
11 male patients with primary pure carcinoid tumors of the
testis.
| Case no. | Age (years) | Clinical history | Durationa (months) | Location
(testicle) | Tumor size (cm) | Histological
grade | Mitosis (/10
HPF) | Follow-up
(years) |
|---|
| 1 | 48 | Painless mass |
4 | Right | 2.5 | Classical | ≤1 | Lost to
follow-up |
| 2 | 26 | Painless mass | 36 | Right | 5.2 | Classical | ≤1 | Lost to
follow-up |
| 3 | 31 | Painless mass | 24 | Right | 2.0 | Classical | ≤1 | Lost to
follow-up |
| 4 | 49 | Swelling and
pain | 120 | Right | 2.5 | Classical | ≤1 | 7 years,
diedb |
| 5 | 36 | Swelling and
pain |
1 | Right | 3.1 | Classical | ≤1 | 9 years, alive |
| 6 | 59 | Swelling and
discomfort |
0.25 | Right | 4.0 | Classical | ≤1 | 5 years, alive |
| 7 | 66 | Painless mass | 24 | Right | 6.0 | Classical | ≤1 | 4 years, alive |
| 8 | 68 | Swelling and
discomfort |
0.5 | Right | 3.2 | Atypical | 2–4 | 3 years, alive |
| 9 | 41 | Painless mass | 12 | Left | 3.6 | Atypical | 2–3 | 3 years, alive |
| 10 | 30 | Painless mass | 12 | Right | 2.5 | Atypical | 2–4 | 3 years, alive |
| 11 | 61 | Painless mass | 12 | Left | 5.0 | Atypical | 2–5 | 5 years, alive |
Among the 11 patients, 9 (81.8%) had the tumor in
the right testicle, while 2 (18.2%) had the tumor in the left
testicle, as confirmed by ultrasound scan (Fig. 1). Case 1 had right inguinal
lymphadenopathy. For all 11 patients, no visible metastasis was
observed in the liver, retroperitoneum or other organs by the
imaging studies. None of the patients had associated carcinoid
syndrome. Furthermore, the serum levels of the three tumor markers
(β-HCG, AFP and LDH) were in the normal range. Finally, one (9%)
patient was classified as pT2 and 10 (91%) as pT1. All were N0M0
and without carcinoid syndrome at initial diagnosis according to
pathological tumor-node-metastasis staging.
 | Figure 1.Hematoxylin and eosin-stained
histological images of pure carcinoid tumors and surrounding
testicular parenchyma. (A) Carcinoid tumor with a mixed insular,
acinar, rosetted, solid and trabecular pattern separated by
delicate fibroconnective stroma (magnification, ×20). (B) Diffuse
solid growth pattern (right bottom) with a desmoplastic stromal
reaction and mixed with acinar and trabecular patterns from case 10
(magnification, ×10). (C) Neoplastic cells demonstrating follicular
or pseudoglandular patterns filled with condensed secretory fluid
stimulating the psammoma body (magnification, ×50). (D) Neoplastic
cells demonstrating follicular or pseudoglandular patterns filled
with eosinophilic secretory fluid, and erythrocytes resembling
colloid goiter (magnification, ×20). (E) Neoplastic cells are
relatively homogeneous, medium in size, have oval to round nuclei,
with prominent chromatin clumping along the nuclear envelope,
inconspicuous nucleoli and a scant-to-moderate amount of
eosinophilic granular cytoplasm (magnification, ×40). (F) Focal
nuclear pleomorphism and apoptosis are present in the neoplastic
cells (magnification, ×40). (G) Diffuse solid growth pattern with a
mucoid stromal reaction from case 11 (magnification, ×20). (H)
Pathological mitotic figures are observed in an atypical carcinoid
tumor (magnification, ×40). |
Pathological findings
The gross tumor size ranged from 2.0 to 6.0 cm
(mean, 3.6 cm) in diameter in the largest dimension (Table I). In all 11 cases the tumors were
well demarcated from the surrounding parenchyma but not
encapsulated. They were nodular tumors with solid, homogeneous,
yellow-tan cut surfaces. The residual testicular parenchyma
surrounding the tumors was compressed and atrophic. Case 1 had
slightly enlarged lymph nodes in the right inguinal region, which
demonstrated reactive hypertension in the biopsy specimens.
Histologically, the carcinoid tumors demonstrated
multiple growth patterns at low-power magnification. The most
common type was a mixed insular, acinar, rosetted, solid and
trabecular pattern separated by delicate fibroconnective stroma
(Fig. 1A and B). Occasionally, the
neoplastic cells were arranged in follicular or pseudoglandular
patterns filled with condensed secretory fluid, stimulating the
psammoma body (Fig. 1C), and
erythrocytes resembled colloid goiter (Fig. 1D). At higher power magnification, the
neoplastic cells were relatively homogeneous, medium in size, had
oval to round nuclei with prominent chromatin clumping along the
nuclear envelope, inconspicuous nucleoli and a scant-to-moderate
amount of eosinophilic granular cytoplasm. Focal nuclear
pleomorphism was noted, occasionally with giant cells (Fig. 1E and F). Two cases (cases 10 and 11)
demonstrated a diffuse solid growth pattern with a desmoplastic or
mucoid stromal reaction in certain areas (Fig. 1B right bottom and G).
In the 11 patients, 7 cases (cases 1 to 7) were
diagnosed as classical carcinoid tumors with ≤1 mitotic figure per
10 high-power fields (HPF), while the other 4 cases (cases 8 to 11)
were identified as atypical carcinoid tumors with 2–5 mitoses per
10 HPF (Table I, Fig. 2H). Focal inflammatory cells (mainly
lymphocytes) were only observed in 3 cases. Necrosis and vascular
invasion by tumor cells were not observed in any of the 11 cases.
Neither teratomatous elements nor intratubular germ cell neoplasia
were observed in any cases. The tunica albuginea was thickened by
fibrosis, while the epididymis and spermatic cords did not
significantly change.
Immunohistochemical features
All neoplastic cells were strong and diffusely
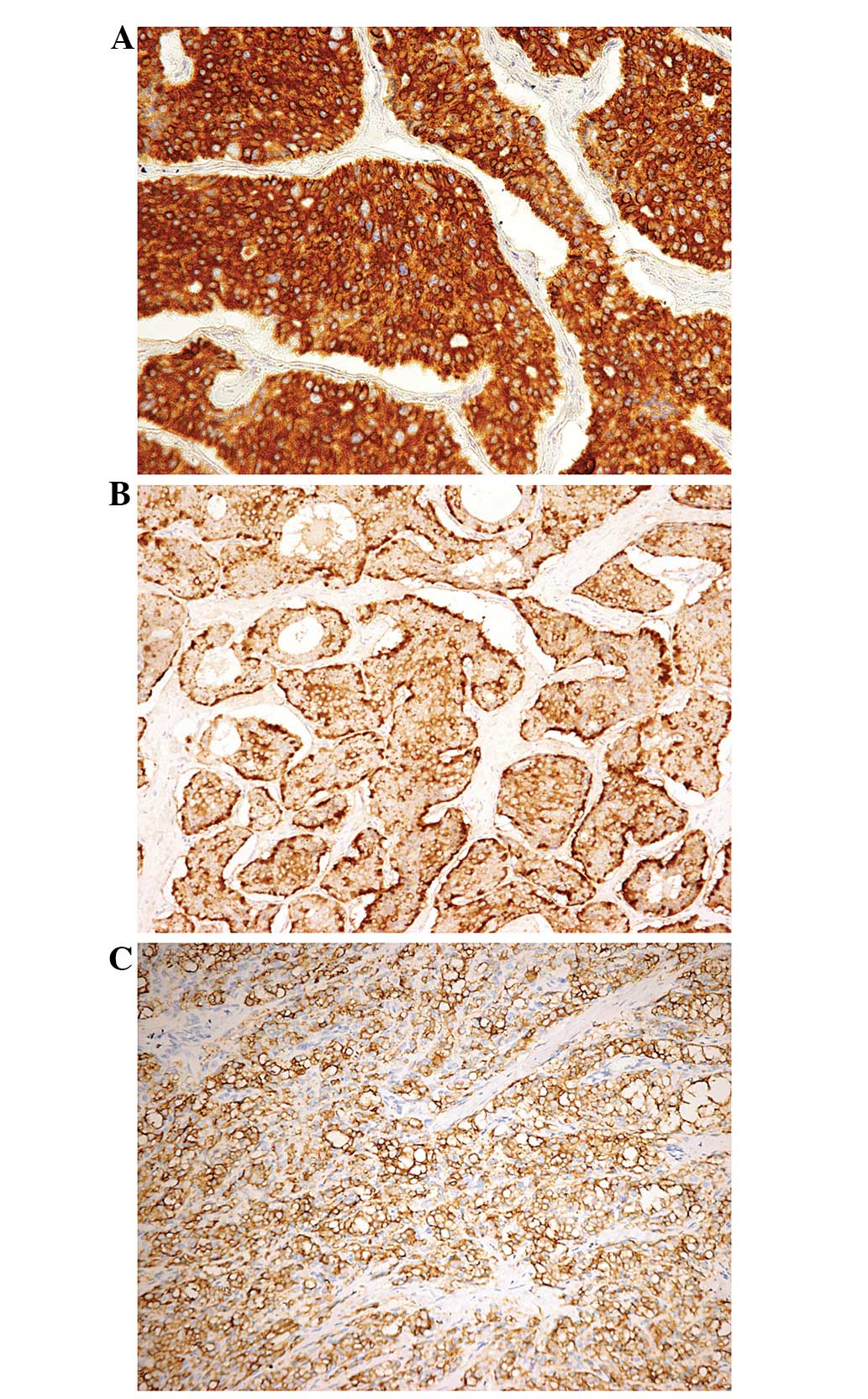
positive for NSE staining. CK antigen (Fig. 2A) was expressed in 9 cases (81.8%),
while CgA (Fig. 2B) and Syn (Fig. 2C) antigens were expressed in 8 (72.7%)
and 10 cases (90.9%), respectively. In 9 of the 11 cases (81.8%),
the neoplastic cells were positive for CD56. Ki67 labeling was
performed in 10 cases, which revealed that ≤2% of the tumor cells
were typical carcinoid and 3–7% of the tumor cells were atypical
carcinoid. None of the tumors reacted with CD117, PLAP, AFP, ACTH,
gastrin, glucagon, PP or somatostatin. CDX-2 and TTF-1 were
negative in all cases. An additional five markers (HCG, α-inhibin,
calretinin, MART1 and HBME1) were used for 2 patients (cases 10 and
11), in whom the tumors demonstrated negative staining for these
five markers.
Follow-up results
In addition to radical orchiectomy, 9 patients
(81.7%) received a combined modality of treatment (radiotherapy,
chemotherapy or both). Follow-up data were available for 8 patients
(72.7%). The mean follow-up time was 4.9 years, ranging from 3 to 9
years (Table I). Seven patients were
alive during the follow-up without recurrence or metastases, while
one patient had a history of hypertension and succumbed to cerebral
hemorrhage 7 years after the surgery.
Discussion
Testicular carcinoid tumors are rare, and only
limited studies have been carried out in China. The incidences of
primary testicular carcinoids in North America and Japan are lower
than 1 and 0.2%, respectively (6,8). At the
West China Hospital, 688 male patients with testicular neoplasms
underwent surgical resections between 1978 and 2013. Primary
testicular carcinoid tumors accounted for 1.02% of these, which is
similar to the reported incidence in North America.
Clinically, our cases presented with symptoms
similar to those described in the literature. Stroosma and Delaere
(1) reviewed 62 cases of testicular
carcinoids reported from 1930 to 2006 all over the world. The most
common presenting symptom was a painless scrotal mass in 35/62 of
patients (79%), which is slightly higher than the 7/11 (63.6%)
noted in our series. Since the symptoms are not specific and onset
is insidious, a testicular carcinoid tumor is often not diagnosed
early and not recognized until the physical examination prior to
surgery for other diseases. In our 11 cases the longest duration
from the initial presentation of symptoms to diagnosis was 10
years. The peak age was older than that observed with most primary
germ cell tumors.
Carcinoid syndrome tumors are associated with
1.1–3.1% of patients with primary testicular carcinoid tumors
(3). Carcinoid syndrome is more
common in carcinoid tumors with metastasis (3,14,15). It has been reported that 50% of
carcinoid tumor patients with metastases had carcinoid syndrome,
compared with 5.6% of patients without metastases (16). It is unclear whether the presence of
carcinoid syndrome is a feature associated with a malignant course
in the testis (17). The absence of
hormone immunoreactivity in our series may explain the lack of
carcinoid syndrome in the majority of testicular carcinoid tumor
cases. Our case study confirms that carcinoid syndrome is uncommon
in primary testicular carcinoid tumors without metastasis.
Although the rate of metastasis from an
extra-testicular source is low, occasionally a gastrointestinal
primary carcinoid may metastasize to the testicle. Therefore,
during the differential diagnosis of testicular carcinoid,
metastasis or gastrointestinal primary carcinoid must be
considered. There are significant implications for survival, as
metastatic carcinoids are usually part of a widely disseminated
disease with a poorer clinical course (5). One case of testicular metastasis was
reported 10 years after resection of an appendiceal carcinoid
(18). It is of limited value for
site-specific markers to distinguish metastatic from primary pPCTT.
Nuclear CDX-2 immunoreactivity was mainly observed in midgut
neuroendocrine tumors, in which the majority of cells demonstrated
strong positivity (19,20). TTF-1 was identified in respiratory
epithelial cells, but has also been reported in other sites
including the breast, and in poorly differentiated neuroendocrine
carcinoma of the cervix (21).
However, none of the cases in our series demonstrated nuclear
expression of CDX-2 or TTF-1. Therefore, extensive medical
examinations, including a 24-h urinary 5-HIAA test, chest X-ray, CT
scan of the abdomen and pelvis, PET-CT scan and possibly a
gastrointestinal contrast study should be performed to exclude the
possibility of metastasis from a carcinoid tumor in another organ
(11). However, the metastatic tumors
are generally multiple nodules in the bilateral testis and the
persistence of carcinoid syndrome following orchiectomy.
Furthermore, primary carcinoids of the testis are usually
associated with other teratomatous elements, and occasionally with
seminoma (22). Therefore, thorough
gross examination and sufficient sampling are strongly recommended
to exclude other components.
It is still possible to misdiagnose a testicular
carcinoid tumor, particularly an atypical carcinoid tumor. For
example, six consultant cases in our series of 11 patients with
pPCTT from other hospitals were initially diagnosed as Sertoli cell
tumors, paraganglioma, granulosa cell tumor or embryonal carcinoma.
Sertoli cell tumors arranged in a hollow or solid tubular formation
may demonstrate a neuroendocrine-like pattern, but the cytoplasm is
clear or lightly vacuolated. Sertoli cell tumors are positive for
α-inhibin but negative for neuroendocrine markers. The insular and
trabecular patterns of granulosa cell tumors may be mistaken for a
carcinoid tumor. The presence of nuclear grooves and the absence of
neuroendocrine markers distinguish the granulosa cell tumor from a
carcinoid tumor. Paraganglioma arranged in an organoid nesting
pattern may resemble a carcinoid tumor. However, this is extremely
rare in the testis, and lacks CK staining which is frequently
positive in carcinoids. Undifferentiated carcinomas and poorly
differentiated adenocarcinomas may resemble atypical carcinoids.
These carcinomas have abundant mitotic figures and have frequently
already extended beyond the testis at presentation. Notably, case
11 in our study was initially misinterpreted as Sertoli cell tumors
which were negative for α-inhibin, calretinin, HBME1, CD30, PLAP,
CgA and Syn, but positive for CK. We recommended CD56 and NSE
staining for further evaluation, and the neoplastic cells were
strongly positive for CD56 and NSE markers. When CgA and Syn
markers are sparse or negative in the poorly differentiated tumors,
the positive reaction of CD56 and NSE may support the diagnosis of
a carcinoid tumor, although these markers have poor
specificity.
In general, primary testicular carcinoids have an
excellent prognosis, with certain cases only incidentally observed
at autopsy (8). Approximately 11% of
primary testicular carcinoid tumors exhibit malignant behavior
(3,15). Zavala-Pompa et al revealed that
larger tumors (7.3 vs. 2.9 cm) predicted increased metastatic
potential, while tumor necrosis and local tumor invasion were not
associated with adverse prognosis (17). In our study of 11 Chinese cases, the
tumor sizes ranged from 2 to 6 cm, and the majority (81.8%) of the
tumors were located in the right testicle. None of the patients had
recurrence or metastases at presentation. Our case study suggests
that the metastatic potential cannot be predicted by the
histological appearance and tumor size ≤6.0 cm.
Chemotherapy and radiotherapy are known to have
minimal benefits for metastatic disease (23). Radical orchiectomy with close
follow-up is the treatment of choice for organ-confined carcinoid
(5,7).
The longest reported interval between the presentation and
diagnosis was 43 years for a case of uveal metastasis following
resection of a bronchogenic carcinoid tumor (24). In our series of 11 patients, follow-up
data were available for 8 patients (72.7%) for 3–9 years. Although
none of the 8 patients experienced recurrence or metastases, at
least during the follow-up, the follow-up time may not be long
enough for the natural course of carcinoid tumors. Follow-up should
include physical examination and a 24-h urinary 5-HIAA test every 3
months for 1 year and then annually (25).
In conclusion, localized pPCTT is a rare disease
with an indolent clinical course. When a testicular carcinoid tumor
is identified, a multimodal approach should be taken to exclude an
extra-testicular primary source, particularly when the testicular
tumor is large. A tumor size ≤6.0 cm and the histological
appearance had little relation with metastatic behavior. Follow-up
must be extremely close due to the potential for delayed
metastases.
Acknowledgements
The authors would like to thank Dr Ying Wu for
editorial assistance in the preparation of the manuscript. This
study was supported by the Ministry of Education PhD Program Fund
(20120181110090).
References
|
1
|
Stroosma OB and Delaere KP: Carcinoid
tumours of the testis. BJU Int. 101:1101–1105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Modlin IM, Lye KD and Kidd M: A 5-decade
analysis of 13,715 carcinoid tumors. Cancer. 97:934–959. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hayashi T, Iida S, Taguchi J, et al:
Primary carcinoid of the testis associated with carcinoid syndrome.
Int J Urol. 8:522–524. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simon HB, McDonald JR and Culp OS:
Argentaffin tumor (carcinoid) occurring in a benign cystic teratoma
of the testicle. J Urol. 72:892–894. 1954.PubMed/NCBI
|
|
5
|
Wolf M, Wunderlich H, Hindermann W, et al:
Primary carcinoid tumor of the testicle without metastases in
combination with testicular atrophy and testosterone deficiency.
Int Urol Nephrol. 38:625–628. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kato N, Motoyama T, Kameda N, et al:
Primary carcinoid tumor of the testis: Immunohistochemical,
ultrastructural and FISH analysis with review of the literature.
Pathol Int. 53:680–685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reyes A, Moran CA, Suster S, et al:
Neuroendocrine carcinomas (carcinoid tumor) of the testis. A
clinicopathologic and immunohistochemical study of ten cases. Am J
Clin Pathol. 120:182–187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang WP, Guo C, Berney DM, et al: Primary
carcinoid tumors of the testis: a clinicopathologic study of 29
cases. Am J Surg Pathol. 34:519–524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ulbright TM and Young RH: Carcinoid tumor
of the testis. Am J Clin Pathol. 121:297–298. 2004.PubMed/NCBI
|
|
10
|
Talerman A, Gratama S, Miranda S, et al:
Primary carcinoid tumor of the testis: case report, ultrastructure
and review of the literature. Cancer. 42:2696–2706. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neely D and Gray S: Primary carcinoid
tumour of the testis. Ulster Med J. 80:79–81. 2011.PubMed/NCBI
|
|
12
|
Kim HJ, Cho MY, Park YN, et al: Primary
carcinoid tumor of the testis: immunohistochemical, ultrastructural
and DNA flow cytometric study of two cases. J Korean Med Sci.
14:57–62. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oberg K, Akerstrom G, Rindi G, et al:
Neuroendocrine gastroenteropancreatic tumours: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol 21 Suppl. 5:v223–v227. 2010. View Article : Google Scholar
|
|
14
|
Son HY, Ra SW, Jeong JO, et al: Primary
carcinoid tumor of the bilateral testis associated with carcinoid
syndrome. Int J Urol. 11:1041–1043. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaufman JJ and Waisman J: Primary
carcinoid tumor of testis with metastasis. Urology. 25:534–536.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thomas JC and Jones JS: Primary carcinoid
tumor of the testis found at the time of elective sterilization. J
Androl. 25:338–339. 2004.PubMed/NCBI
|
|
17
|
Zavala-Pompa A, Ro JY, el-Naggar A, et al:
Primary carcinoid tumor of testis. Immunohistochemical,
ultrastructural and DNA flow cytometric study of three cases with a
review of the literature. Cancer. 72:1726–1732. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fucs M, Romero FR, de Castewm Germanos, et
al: Testicular metastasis 10 years after resection of appendiceal
carcinoid. Urology. 65:5912005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin X, Saad RS, Luckasevic TM, et al:
Diagnostic value of CDX-2 and TTF-1 expressions in separating
metastatic neuroendocrine neoplasms of unknown origin. Appl
Immunohistochem Mol Morphol. 15:407–414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan ES, Alexander J, Swanson PE, et al:
PDX-1, CDX-2, TTF-1 and CK7: a reliable immunohistochemical panel
for pancreatic neuroendocrine neoplasms. Am J Surg Pathol.
36:737–743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCluggage WG, Kennedy K and Busam KJ: An
immunohistochemical study of cervical neuroendocrine carcinomas:
Neoplasms that are commonly TTF1 positive and which may express
CK20 and P63. Am J Surg Pathol. 34:525–532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hodzic J, Golka K and Schulze H: Primary
testicular carcinoid. Med Sci Monit. 10:CS46–CS48. 2004.PubMed/NCBI
|
|
23
|
Modlin IM, Latich I, Kidd M, et al:
Therapeutic options for gastrointestinal carcinoids. Clin
Gastroenterol Hepatol. 4:526–547. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaiser ED, See RF, Rechdouni AK, et al:
Uveal metastasis 43 years after resection of bronchogenic
carcinoid. Br J Ophthalmol. 86:1191–1192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sutherland RS, Wettlaufer JN and Miller
GJ: Primary carcinoid tumor of the testicle: a case report and
management schema. J Urol. 148:880–882. 1992.PubMed/NCBI
|