Introduction
Myoepithelial tumors comprise a peculiar group of
lesions displaying heterogeneous morphological features, including
dual epithelial and myoid differentiation. As a relatively newly
recognized entity, cutaneous myoepithelioma is continually being
assigned novel gradings and classifications. To date, <40 cases
of cutaneous syncytial myoepithelioma have been reported (1,2). Recently,
a distinctive variant, designated as cutaneous syncytial
myoepithelioma, has been identified as part of this group (1). This particular entity has been reported
to occur predominantly on the extremities and trunk. Clinically,
these lesions present as a solitary papule or polypoid nodule.
Histologically, cutaneous syncytial myoepithelioma is characterized
by the well-circumscribed, solid, sheet-like growth of ovoid to
spindled or histiocytoid cells, with palely eosinophilic syncytial
cytoplasm (1). Due to the rarity of
cutaneous syncytial myoepithelioma, the incidence of this tumor
remains unclear. The current study describes a case of cutaneous
syncytial myoepithelioma arising in the thigh. Given the unusual
histological features, myoepithelioma must also be considered in
the differential diagnosis of a superficial dermal tumor. Written
informed consent was obtained from the patient.
Case report
In January 2014, a 50-year-old female patient
presented to Sijhih Cathay General Hospital (New Taipei City,
Taiwan) with a small, painless and papular lesion on the right
thigh, which had grown slowly over the previous 6 months.
Subsequently, a dermatologist performed a local excision of the
tumor. Histopathological examination revealed a well-circumscribed
nodule of 4 mm in maximum diameter in the superficial dermis. The
tumor was composed of ovoid to histiocytoid cells, of uniform size,
growing in solid sheets or a vaguely fascicular pattern, and
abutting the overlying epidermis (Fig.
1 and 2). The tumor cells exhibited an eosinophilic syncytial
cytoplasm (Fig. 3). Sparse mitotic
activity was observed, with 4 or fewer mitotic figures per 10
high-power microscopic fields (HPF). Chondromyxoid stroma, ductal
components or melanin pigment were not identified.
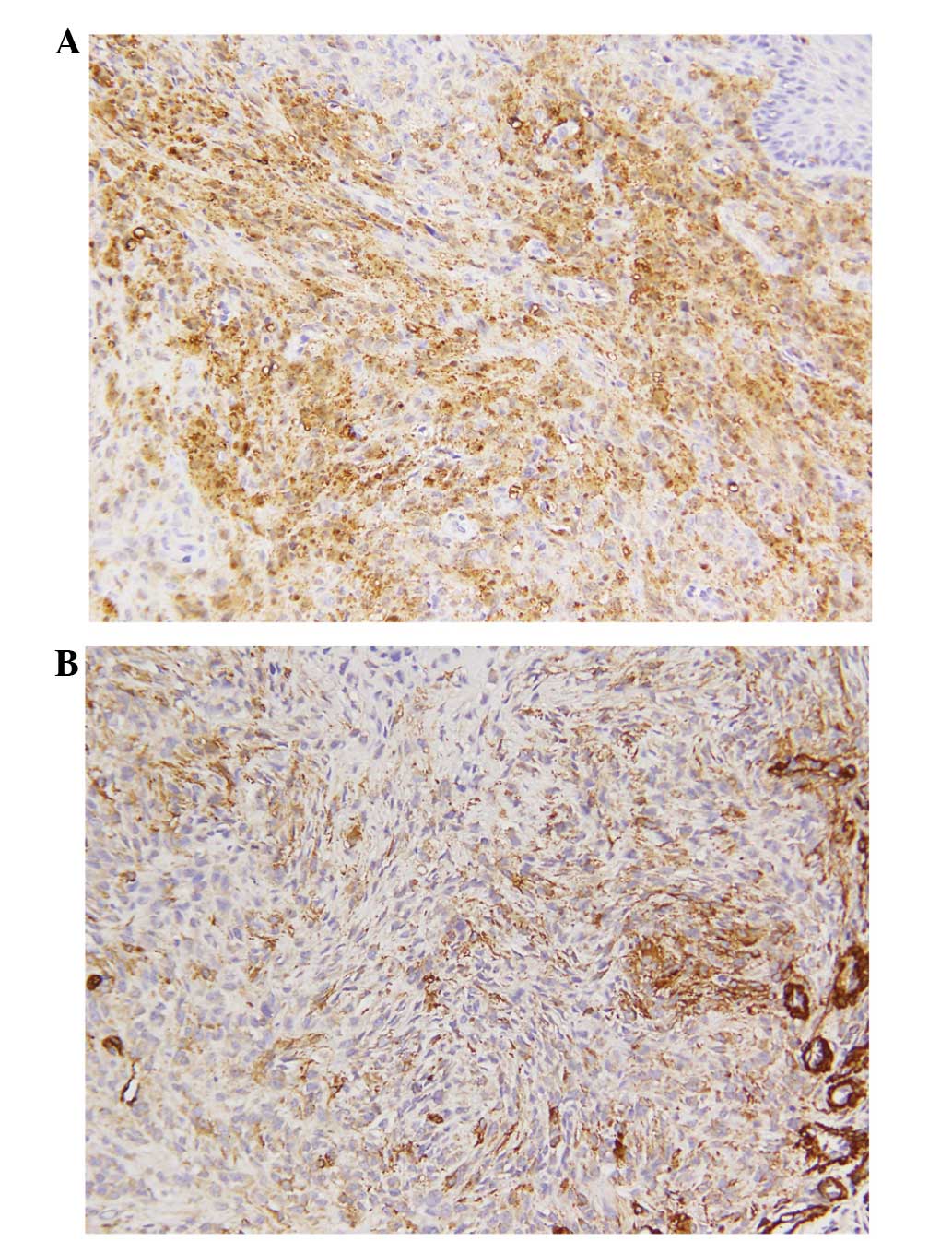
Immunohistochemically, the tumor cells were positive
for S-100 protein (Fig. 4A), focally
positive for smooth muscle actin (SMA; Fig. 4B) and epithelial membrane antigen
(EMA). Notable immunoreactivity was not observed for cluster of
differentiation (CD)68 and CD34. Subsequent molecular genetic tests
were performed, and a fluorescence in situ hybridization
assay of the tumor cells revealed Ewing sarcoma RNA-binding protein
1 (EWSR1) rearrangement. The results of immunohistochemical
analysis and cytogenetic aberration confirmed a diagnosis of
cutaneous syncytial myoepithelioma. Subsequently, the tumor was
excised with free margins. The patient received no further
treatment and follow-up examination was performed twice in the year
after surgery. At the time of writing, the patient was alive with
no evidence of local recurrence or distant metastasis.
Discussion
Neoplasms of myoepithelial cells are uncommon skin
tumors that consist of chondroid syringoma, cutaneous
myoepithelioma and their malignant counterparts. Cutaneous
myoepithelioma has only been identified recently, and in contrast
to chondroid syringoma, exhibits purely myoepithelial
differentiation without a ductal component (3). Clinically, cutaneous myoepithelioma
typically presents as a painless cutaneous nodule, presenting with
a wide anatomical distribution, with tumors commonly occurring in
the extremities (2–4). Development in younger patients is
relatively common. The majority of cutaneous myoepitheliomas behave
in a benign manner; whilst there appears to be a significant risk
for local recurrence, the tumors have a low metastatic potential
(2). In a previous study conducted in
Taiwan, 1 out of 3 patients developed local recurrence due to
incomplete excision (5).
Hornick and Fletcher (2) presented a case series of 14 patients
that initially described a morphologically and
immunohistochemically distinct subset of cutaneous myoepithelioma
exhibiting a solid syncytial growth pattern. The tumors were formed
from ovoid, spindled or histiocytoid cells with eosinophilic
cytoplasm, and exhibited immunophenotypical EMA and S-100
positivity, with infrequent keratin staining (2). In 2013, the clinicopathological
characteristics of cutaneous syncytial myoepithelioma were further
analyzed in a large study of 38 cases (1). A wide age range (2 months-74 years) was
noted, however, there appeared to be a predilection for the third
to fifth decades. Males were more frequently affected than females
(2.5:1). The tumor size ranged from 0.3–2.7 cm (median, 0.8 cm),
and the most common sites of tumor growth were the extremities,
although the trunk and the face were also affected. Grossly and
histopathologically, the tumors were predominantly located in the
dermis as polypoid or papular lesions (1–3). The tumor
cells are reported to be uniform in size, with the presence of
eosinophilic syncytial cytoplasm and vesicular nuclei. The majority
of tumors display no mitotic activity, and the highest mitotic
count observed is 4 per 10 HPF. Chondro-osseous differentiation is
infrequent (1,2). Immunohistochemically, the tumor cells
are positive for EMA and S-100 protein, whilst staining for glial
fibrillary acidic protein (GFAP), SMA and p63 may be positive, and
keratin staining is infrequent. The syncytial variant of cutaneous
myoepithelioma appears to behave in a benign manner, and the
standard treatment is complete excision with negative margins
(1,2).
The criteria for diagnosing a cutaneous myoepithelial carcinoma
have not been well established, however, tumors exhibiting
cytological atypia, a high mitotic rate and necrosis have been
shown to behave in a more aggressive fashion (2,6–8).
Similar to previously reported series, the present
case involved a well-circumscribed, dermal-based tumor with a
typically syncytial growth pattern. The differential diagnosis of
cutaneous myoepitheliomas is dependent on the predominant
histological pattern (5). Epithelioid
fibrous histiocytoma (EFH) may mimic cutaneous syncytial
myoepithelioma to a certain extent: The two tumor types frequently
present as a dermal nodule of epithelioid cells with an epidermal
collarette, however, EFH usually lacks the typical syncytial
architecture. Furthermore, EFH commonly contains scattered
binucleated cells and more intervening collagenous or variably
vascular stroma (3). Whilst the two
tumors may each exhibit EMA positivity, EFH is negative for S-100
protein, GFAP and p63 (9).
Early-stage juvenile xanthogranuloma (JXG), which
lacks the characteristic multinucleated giant cells and
lipidization, may also mimic cutaneous syncytial myoepithelioma
(3,10). JXG displays a marked predilection for
young children. Immunohistochemically, JXG exhibits CD163 and CD68
positivity, however, it lacks reactivity for EMA and S-100
protein.
Cutaneous syncytial myoepithelioma contains a
mixture of epithelioid, ovoid or histiocytoid cells, thus the
primary diagnostic consideration includes melanocytic tumors, such
as Spitz nevi (5). Spitz nevi show a
nested pattern with downward maturation, but lack the sheet-like
syncytial architecture of cutaneous myoepithelioma. Whilst the two
tumors are each positive for S-100 protein, Spitz nevi are also
positive for melanocytic markers, including human melanoma black
45, Melan A and microphthalmia-associated transcription factor.
Additionally, Spitz nevi are negative for EMA and GFAP.
Epithelioid sarcoma may also enter the differential
diagnosis of myoepithelioma. This lesion also commonly affects
young adults on the extremities, however, more morphological
uniformity is observed in epithelioid sarcoma compared with
myoepithelioma. In addition, whilst the two tumor types are each
positive for EMA, epithelioid sarcoma also exhibits positivity for
cytokeratin and CD34, and is generally negative for other typical
myoepithelial differentiation markers (S-100 protein, GFAP and
myogenic markers) (3,5).
EWSR1 gene rearrangement occurs in a subset of
cutaneous myoepithelial tumors (1,11),
providing a genetic association between myoepithelial tumors of the
skin and their counterparts in bone, soft tissue and visceral
locations (11). The presence of
EWSR1 gene rearrangement in the majority of cutaneous synovial
myoepithelioma supports the concept of its close association to
other subsets of myoepithelial tumors. However, Jo et al
(1) hypothesized that this
morphologically distinctive tumor type may be associated with a
novel fusion gene.
In summary, the current study presents a unique case
of cutaneous syncytial myoepithelioma. This distinct variant of
myoepithelioma must be included in the differential diagnosis of
superficial dermal tumors, and confirmatory immunohistochemical
study may be valuable in determining a diagnosis in problematic
cases. Wide excision with safe surgical margins and regular
follow-up are crucial for the management of cutaneous
myoepitheliomas.
References
|
1
|
Jo VY, Antonescu CR, Zhang L, Dal Cin P,
Hornick JL and Fletcher CD: Cutaneous syncytial myoepithelioma:
clinicopathologic characterization in a series of 38 cases. Am J
Surg Pathol. 37:710–718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hornick JL and Fletcher CD: Cutaneous
myoepithelioma: a clinicopathologic and immunohistochemical study
of 14 cases. Hum Pathol. 35:14–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gleason BC and Hornick JL: Myoepithelial
tumours of skin and soft tissue: an update. Diagn Histopathol.
14:552–562. 2008. View Article : Google Scholar
|
|
4
|
Lewin MR, Montgomery EA and Barrett TL:
New or unusual dermatopathology tumors: a review. J Cutan Pathol.
38:689–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu PH, Hsu HC, Chen CH, Shih IH, Yang CH
and Kuo TT: Myoepitheliomas of the Skin and Soft Tissue: A
Clinicopathologic Study of Three Cases. Dermatol Sin. 27:59–67.
2009.
|
|
6
|
Mentzel T, Requena L, Kaddu S, et al:
Cutaneous myoepithelial neoplasms: clinicopathologic and
immunohistochemical study of 20 cases suggesting a continuous
spectrum ranging from benign mixed tumor of the skin to cutaneous
myoepithelioma and myoepithelial carcinoma. J Cutan Pathol.
30:294–302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanahashi J, Kashima K, Daa T, et al: A
case of cutaneous myoepithelial carcinoma. J Cutan Pathol.
34:648–653. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Law RM, Viglione MP and Barrett TL:
Metastatic myoepithelial carcinoma in a child. J Cutan Pathol.
35:779–781. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doyle LA and Fletcher CD: EMA positivity
in epithelioid fibrous histiocytoma: a potential diagnostic
pitfall. J Cutan Pathol. 38:697–703. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Janssen D and Harms D: Juvenile
xanthogranuloma in childhood and adolescence: a clinicopathologic
study of 129 patients from the kiel pediatric tumor registry. Am J
Surg Pathol. 29:21–28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Flucke U, Palmedo G, Blankenhorn N,
Slootweg PJ, Kutzner H and Mentzel T: EWSR1 gene rearrangement
occurs in a subset of cutaneous myoepithelial tumors: a study of 18
cases. Mod Pathol. 24:1444–1450. 2011. View Article : Google Scholar : PubMed/NCBI
|