Introduction
D2 radical gastrectomy is currently considered to be
the standard procedure for the treatment of gastric cancer. Due to
the more extensive anatomic separation and resection compared with
conventional gastrectomy surgery, the incidence of
intraoperative-associated injury and post-operative complications
is significantly increased (1,2). The
overall morbidity and mortality rates of post-operative
complications have been reported as 38.4% and 2.7%, respectively
(1). Subsequent to gastrectomy,
patients are vulnerable to malnutrition from reduced oral intake,
which contributes to the increased risk of post-operative
complications and morbidity (3).
Therefore, the insertion of a feeding jejunostomy tube (J-tube) at
the time of gastroesophageal resection is recommended to optimize
post-operative nutritional status or facilitate adjuvant therapy
following surgery (4,5). The insertion procedure is routinely
performed. However, the benefit of feeding tube placement and
post-operative enteral nutrition continue to be debated due to the
possible complications caused by infection that can result from the
procedure (6,7). The present study describes a case in
which the patient developed the unusual complication of multiple
splenic abscesses secondary to the removal of the J-tube,
subsequent to total gastrectomy. Therefore the routine management
of J-tube placement at the time of total gastrectomy may be
questionable. To the best of our knowledge, this is the first
reported case of septicemia combined with splenic abscess
subsequent to total gastrectomy. The patient provided written
informed consent.
Case report
A 75-year-old male presented to the First Hospital
of Tsinghua University (Beijing, China) with chills and
hyperpyrexia, and was subsequently referred to the Department of
Respiratory Medicine. The patient had undergone a standard D2 total
gastrectomy and intra-operative insertion of a feeding J-tube due
to gastric adenocarcinoma at Peking University People's Hospital
(Beijing, China) six weeks prior. The patient had suffered from
mild pneumonia on post-operative day three and had recovered
quickly subsequent to antibiotic therapy. The patient was
discharged on post-operative day 10 and initiated full oral feeding
despite the presence of the J-tube. The post-discharge course was
uneventful. The J-tube was removed on post-operative day 40 and
then the patient presented a sudden hyperpyrexia accompanied by
chills and a mild cough on the following day. The patient possessed
a background of coronary heart disease and unstable angina pectoris
of a 40-year duration, type 2 diabetes mellitus of a 20-year
duration, and had experienced a mild cerebral infarction 10 years
previously. On the day of admission, a physical examination
revealed a body temperature of 39.3°C, blood pressure of 160/80
mmHg and heart rate of 100 beats/min. The abdominal jejunostomy
fistula appeared to be clean and scabbed. The results of other
examinations appeared to be normal, with the exception of being
underweight and demonstrating a body mass index of 17.3 (normal
range, 18.5–23.9). The laboratory data revealed a white blood cell
(WBC) count of 7.3×109/l (normal range,
4.0–10.0×109/l), containing 79.6% neutrophils (normal
range, 53.0–75.0%), a serum sodium level of 118 mmol/l (normal
range, 133–146 mmol/l), a serum potassium level of 4.2 mmol/l
(normal range, 3.3–5.1 mmol/l), a serum albumin level of 27.3 g/l
(normal range, 35.0–52.0 g/l) and a blood glucose level of 16.5
mmol/l (normal range, 3.9–6.1 mmol/l). The patient was treated with
once-daily intravenous injection of 500 mg levofloxacin. The
following day, the patient developed rigor and a body temperature
of 39.8°C, resulting in treatment with intravenous injections of 1
g imipenem/cilastatin sodium three times daily. After two days, the
body temperature returned to normal and Klebsiella
pneumoniae was detected in blood culture. The patient then
chose to be transferred to the Third Central Hospital of Baoding
City (Baoding, China) and was only treated with the
imipenem/cilastatin sodium regimen for an additional five days.
On the seventh day after termination of the
treatment regimen, the patient presented again with hyperpyrexia,
possessing a body temperature of 39.5°C. Subsequent to four days of
treatment with intravenous injections of 4.5 g
piperacillin/tazobactam twice daily at the local hospital, the
condition of the patient demonstrated no improvement. Therefore,
the patient was again admitted to the Department of Respiratory
Medicine of the First Hospital of Tsinghua University. The second
set of laboratory data revealed a WBC count of 9.0×109/l
(normal range, 4.0–10.0×109/l), containing 67.7%
neutrophils (normal range, 53.0–75.0%), a serum albumin level of
26.9 g/l (normal range, 35.0–52.0 g/l) and a procalcitonin level of
3.6 ng/ml (normal range, 0–0.1 ng/ml). Based on these findings, the
patient was diagnosed with septicemia and insufficient antibiotic
treatment. The imipenem/cilastatin regimen was initiated again. The
following day, the body temperature returned to normal.
Nevertheless, on the fourth day after the second admission, the
patient experienced rigor again. An abdominal computed tomography
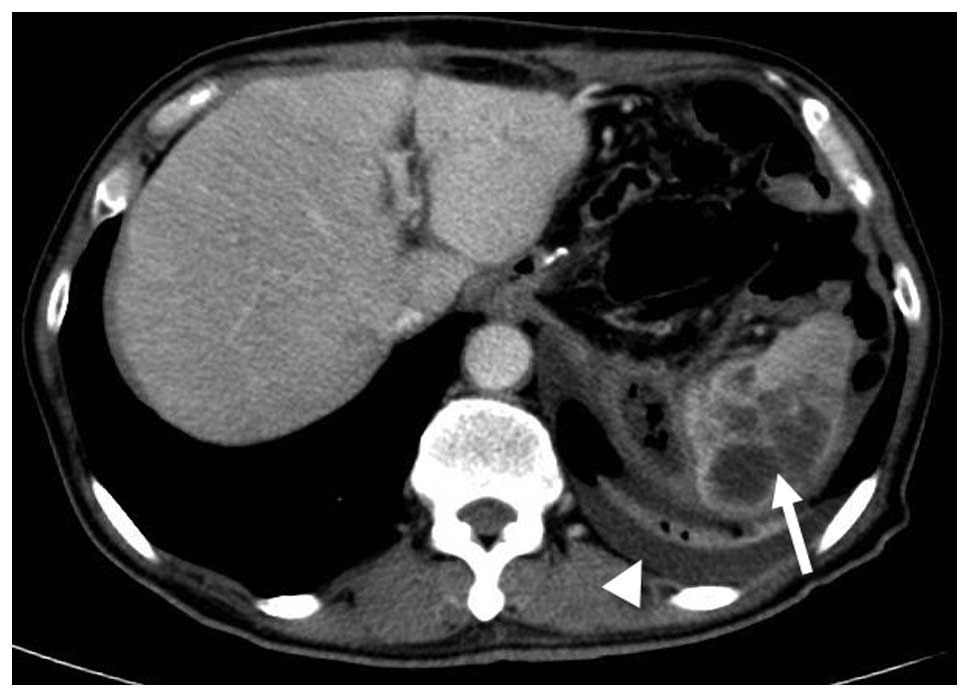
(CT) scan was then performed and multiple non-homogeneous
low-density lesions with ring enhancement located within the spleen
and left pleural effusion were revealed (Fig. 1). The patient was diagnosed with
multiple splenic abscesses and was transferred to the Department of
General Surgery immediately. On the following day, an
ultrasonography (US)-guided percutaneous aspiration was performed
and 40 ml of pus was obtained. A percutaneous drainage catheter was
then inserted. The pus culture also indicated the presence of K.
pneumonia. On the sixth day after transference, a
laparoscopic-assisted splenotomy was performed and another catheter
was inserted due to inadequate drainage. A combination of 4.5 g
piperacillin and tazobactam was also administered twice daily for
three days. The patient's body temperature rapidly returned to
normal. The catheter was removed one week later. The abscess cavity
was revealed to have decreased in size by US and the patient was
discharged on post-splenotomy day 14. The condition of the patient
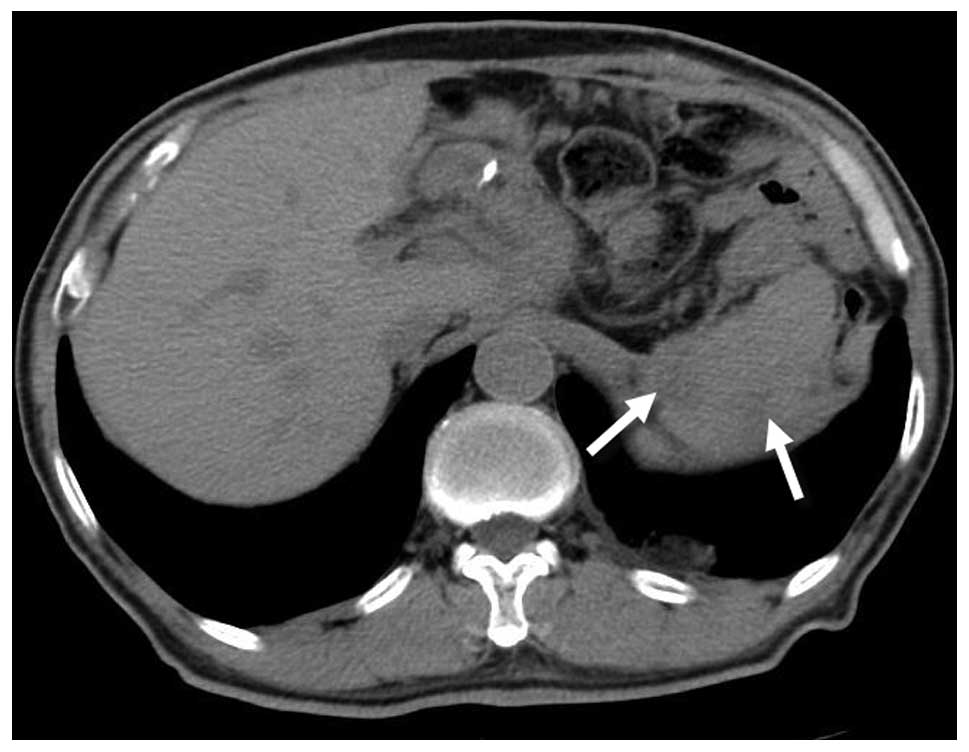
was stable at the eight-month follow-up, and only two small
low-density lesions were revealed within the spleen by a
non-enhanced CT scan (Fig. 2). Due to
this severe complication, no adjuvant chemotherapy was
administered.
Discussion
Prior to 1970, the diagnosis of splenic abscess was
one of exclusion, and a delay prior to treatment was frequent
(8). Recently, the diagnosis of
splenic abscesses appears to be increasing in frequency, and one
contributing factor may be the increasing number of patients who
are immunocompromised and or suffer from cancer (9–11). The
predisposing conditions for splenic abscesses include metastatic
hematogenous infection, trauma, infection in an adjacent site, and
immunodeficiency (12,13), particularly in immunocompromised
patients with acquired immune deficiency syndrome (10), those with diabetes or patients using
immunosuppressive agents (11,14). With
the development of medical technology, novel predisposing factors
have emerged for splenic abscesses. Splenic infarction and the
subsequent splenic abscess can be induced by laparoscopic Nissen
fundoplication (15) or laparoscopic
sleeve gastrectomy (16). Splenic
abscesses can also be encountered when endoscopic injection of
Histoacryl is established as an effective therapy for bleeding
gastric varices (17). The most
probable cause of the formation of these post-operative abscesses
may be the insufficient perfusion and ischemia of the pole of the
spleen due to the ligation of the short gastric vessels (SGVs)
during the procedures (15,16).
Common clinical manifestations of splenic abscesses
exhibited by patients include chills, fever, upper left-side
abdominal pain, and leukocytosis (9,11,13,18). Other
symptoms include nausea and vomiting or a left pleural effusion
(9,13,18).
However, due to the nonspecific nature of the symptoms and signs
developed from splenic abscess, the diagnosis of abscess has
frequently been delayed in the past and the incidence of morbidity
and mortality was occasionally high (9). Notably, the mortality rate in
undiagnosed or untreated cases has been reported as 100% (12,14). From
the culture of the abscess, a wide range of aerobic microbes has
been obtained, including Staphylococcus, Escherichia
coli, and Salmonella. Other reported microbes include
fungi and anaerobic organisms (12).
However, the gram-negative bacilli have become the predominant
microbes associated with splenic abscess due to geographical
variations and population differences (9,11). The
modern imaging modalities are sensitive tools for the diagnosis of
splenic abscess. The sensitivity of CT for the diagnosis of splenic
abscess can reach 96%, and that of US can reach 82% (12).
Splenectomy has long been considered the standard
treatment for bacterial splenic abscess (9,12–14). Chang et al (19) suggested that early surgical
intervention should be encouraged in patients with risk factors
such as multiple splenic abscesses, gram-negative bacillus
infection and high acute physiology and chronic health evaluation
II scores. However, the strategy for surgery remains debatable in
certain patients due to the consideration of complications
(18). Although laparoscopic
splenectomy has been revealed to be a feasible and safe procedure
(20), laparoscopic-assisted
splenotomy may be preferred for splenic abscess patients who are at
risk of requiring technically demanding procedures, particularly
the post-operative occurrences of splenic abscesses with
unavoidable fibrous adhesions, congestive splenomegaly, and
perisplenitis.
In the present study, the main septicemia symptoms,
including rigor, fever and positive culture in blood, were
immediately present subsequent to the removal of the J-tube on
post-gastrectomy day 40. It was hypothesized that there is a
time-dependent association between the removal of the J-tube and
the onset of symptoms. It was hypothesized that the infection began
in the sinus tract, perhaps as a result of a lack of healing due to
the presence of diabetes and malnutrition, and enteric bacterium
entered in the vessel through a tiny breakage. Due to the presence
of septicemia and inappropriate antibiotic therapy, the enteric
bacterium spread to the spleen and produced the metastatic abscess.
In addition, the collateral circulation in the spleen may be
damaged due to the division of the SGVs during the total
gastrectomy, which was proposed to promote the complication in the
present patient. During the progression of the disease, the
diagnosis of splenic abscess was neglected partly due to the
absence of the classic triad of fever, leukocytosis and left-upper
quadrant abdominal pain. In consideration of possible
post-operative dense fibrous adhesions and the intense inflammatory
process around the congestive spleen, the laparoscopic assisted
splenotomy and catheter drainage were performed and splenectomy was
avoided. Patel et al (7)
suggested that the routine use of J-tubes subsequent to subtotal
gastrectomy was not justified due to the increased post-operative
complications. The present study concluded that the routine
placement of the J-tube at the time of resection for total
gastrectomy requires reassessment due to the serious complications
that arise in certain patients.
In conclusion, the routine feeding jejunostomy at
the time of total gastrectomy may be of no clinical benefit or
inadvisable for certain patients. The unusual complication of
splenic abscess subsequent to gastrectomy should be considered in
patients in spite of the absence of classic manifestations. To
reduce the risk of complications associated with a repeat surgical
procedure on a post-operative patient, laparoscopic assisted
splenotomy may remain a selective indication in certain patients
with multiple abscesses.
References
|
1
|
Gil-Rendo A, Hernandez-Lizoain JL,
Martinez-Regueira F, et al: Risk factors related to operative
morbidity in patients undergoing gastrectomy for gastric cancer.
Clin Transl Oncol. 8:354–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Songun I, Putter H, Kranenbarg EM, Sasako
M and van de Velde CJ: Surgical treatment of gastric cancer:
15–year follow-up results of the randomized nationwide Dutch D1D2
trial. Lancet Oncol. 11:439–449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
No authors listed: Perioperative total
parenteral nutrition in surgical patients. The Veterans Affairs
Total Parenteral Nutrition Cooperative Study Group. N Engl J Med.
325:525–532. 1991.PubMed/NCBI
|
|
4
|
Chin KF, Townsend S, Wong W and Miller GV:
A prospective cohort study of feeding needle catheter jejunostomy
in an upper gastrointestinal surgical unit. Clin Nutr. 23:691–696.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llaguna OH, Kim HJ, Deal AM, Calvo BF,
Stitzenberg KB and Meyers MO: Utilization and morbidity associated
with placement of a feeding jejunostomy at the time of
gastroesophageal resection. J Gastrointest Surg. 15:1663–1669.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Srinathan SK, Hamin T, Walter S, Tan AL,
Unruh HW and Guyatt G: Jejunostomy tube feeding in patients
undergoing esophagectomy. Can J Surg. 56:409–414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel SH, Kooby DA, Staley CA III and
Maithel SK: An assessment of feeding jejunostomy tube placement at
the time of resection for gastric adenocarcinoma. J Surg Oncol.
107:728–734. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarr MG and Zuidema GD: Splenic abscess –
presentation, diagnosis, and treatment. Surgery. 92:480–485.
1982.PubMed/NCBI
|
|
9
|
Tung CC, Chen FC and Lo CJ: Splenic
abscess: an easily overlooked disease? Am Surg. 72:322–325.
2006.PubMed/NCBI
|
|
10
|
Llenas-Garcia J, Fernandez-Ruiz M, Caurcel
L, Enguita-Valls A, Vila-Santos J and Guerra-Vales JM: Splenic
abscess: a review of 22 cases in a single institution. Eur J Intern
Med. 20:537–539. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song FL, Lu LX, Li CX, Yu XZ and Li Y: A
retrospective analysis of 19 splenic abscess patients. Zhonghua Nei
Ke Za Zhi. 52:313–317. 2013.(In Chinese). PubMed/NCBI
|
|
12
|
Nelken N, Ignatius J, Skinner M and
Christensen N: Changing clinical spectrum of splenic abscess. A
multicenter study and review of the literature. Am J Surg.
154:27–34. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Green BT: Splenic abscess: report of six
cases and review of the literature. Am Surg. 67:80–85.
2001.PubMed/NCBI
|
|
14
|
Ooi LL and Leong SS: Splenic abscesses
from 1987 to 1995. Am J Surg. 174:87–93. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martinez DG, Sanchez AW and Garcia AP:
Splenic abscess after laparoscopic Nissen fundoplication: a
consequence of short gastric vessel division. Surg Laparosc Endosc
Percutan Tech. 18:82–85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stamou KM, Menenakos E, Gomatos IP,
Panousopoulos SG, Smparounis S, Leandros E and Zografos G: Clinical
implications of sleeve gastrectomy as a source of spleen infarction
or ischemia. Obes Surg. 21:1490–1493. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang CJ, Su CW and Hou MC: Abdominal pain
after endoscopic hemostasis of gastric tumor bleeding. Splenic
infarction with abscess formation. Gastroenterology. 137:e7–e8.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee WS, Choi ST and Kim KK: Splenic
abscess: a single institution study and review of the literature.
Yonsei Med J. 52:288–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang KC, Chuah SK, Changchien CS, et al:
Clinical characteristics and prognostic factors of splenic abscess:
a review of 67 cases in a single medical center of Taiwan. World J
Gastroenterol. 12:460–464. 2006.PubMed/NCBI
|
|
20
|
Feldman LS: Laparoscopic splenectomy:
standardized approach. World J Surg. 35:1487–1495. 2011. View Article : Google Scholar : PubMed/NCBI
|