Introduction
Myxedema is a thyroid dermopathy that occurs as a
component of exophthalmos, myxedema and osteoarthropathy (EMO)
syndrome or as an extrathyroidal complication of Graves' disease
(GD). The dermopathy is characterized by a localized skin
thickening that is confined in ~99% of cases to the area of the
shins and, in rare cases, to the upper limbs and eyelids (1). Myxedema is an uncommon manifestation of
autoimmune thyroid disease and occurs more often in patients with
hyperthyroidism, with 91% of cases arising in patients with
hyperthyroidism. Myxedema may develop in hypothyroidism subsequent
to therapy for GD (1), and appears
extremely rarely in Hashimoto's thyroiditis without thyrotoxicosis,
and even in euthyroid patients (1–3). In GD,
myxedema, together with ophthalmopathy, is one of the
extrathyroidal manifestations of the disease, in addition to
hyperthyroidism secondary to diffuse goiter (4). When myxedema occurs as part of a triad
of extrathyroidal manifestations that also includes exophthalmos
and osteoarthropathy, it constitutes EMO syndrome, which is
observed in <1% of patients with autoimmune thyroid disease
(5). The extrathyroidal symptoms of
EMO tend to develop chronologically with an onset of eye disease
followed by myxedema and then finally osteoarthropathy or acropachy
in cases of a long duration (6–8).
Clinically, myxedema presents with non-pitting
edema, nodules or plaques. In severe cases, the dermopathy may
progress to elephantiasis. The mean age at diagnosis of pretibial
myxedema is 53 years, with a female to male ratio of 4:1 (1).
Pathophysiologically, myxedema is characterized by
the accumulation of glycosaminoglycans in the dermis and panniculus
adiposus that compresses the dermal lymphatics, resulting in dermal
edema and the clinical features of lymphedema (2,9).
The thyrotropin receptor (TSH-R) has been nominated
as a common target antigen in Graves' ophthalmopathy (GO) and
pretibial myxedema (PTM) (10–12). In
previous studies, the presence of TSH-R immunoreactivity has been
also reported to be involved in the muscles and adipose tissues of
patients with GO and in the dermis of patients with PTM (6,10).
Therefore, the antigen-antibody reaction is hypothesized to result
in the stimulation of fibroblasts, with a secondary release of
cytokines leading to lymphocyte attraction and production of
glycosaminoglycans, eventually causing tissue swelling (2,10,11).
The cutaneous manifestations of hyperthyroidism
include warm, moist and pruritic skin. Ophthalmopathy in GD is
characterized by the swelling of rectus muscles, connective tissue
expansion in the posterior orbit that results in the protrusion of
the eyes, extraocular muscle dysfunction and impaired vision in
advanced cases, due to compressive neuropathy of the optic nerve
(13,14). Thyroid acropachy is a rare clinical
presentation of autoimmune thyroid disease and manifests with
clubbing and swelling of the digits due to osteoarthropathy and
periosteal reaction of the bones of the upper or lower extremities
(1,11).
The majority of patients with mild myxedema do not
require treatment, with 50% of cases concluding in spontaneous
remission. Moderate myxedema may require treatment in patients that
are symptomatic and experience pain, impaired motion of the joints
or disfigurement (2). The present
study reports the case of a patient with EMO syndrome that suffered
from extensive myxedema and was treated using radiotherapy as a
component of the therapeutic regimen. Written informed consent was
obtained from the patient.
Case report
A 48-year-old male patient was diagnosed with
thyrotoxicosis secondary to GD at his local clinic (Lengerich,
Germany). A complete check-up revealed elevated levels of thyroid
stimulating hormone receptor antibody (TSHR-Ab). The patient was
initially treated with a subtotal thyroidectomy in April 1987, and
then with radioiodine therapy in 1989 at the Department of Nuclear
Medicine, University Hospital of Muenster (Muenster, Germany). The
patient also demonstrated mild bilateral pretibial myxedema, which
disappeared within 17 months following radioiodine therapy.
Subsequently, the patient developed hypothyroidism, which was
compensated for through the daily administration of 250 µg
L-thyroxine. The dose of L-thyroxine was then gradually reduced
over the period of one year to 175 µg.
Whilst the patient was receiving 175 µg L-thyroxine
daily, an euthyreotic condition was identified, with the following
thyroidal values being obtained: TSH 1.23 µunits/ml (normal range,
0.35–4.50 µunits/ml); free triiodothyronine, 3.10 pg/ml (normal
range, 2.0–4.50 pg/ml); free thyroxine, 1.33 ng/dl (normal range,
0.70–1.65 ng/ml); thyroglobulin Ab, 10.1 units/ml (normal level,
<1.0 units/ml); TSHR-Ab, 69.6 units/l (normal level, <3.0
units/l); and thyroid peroxidase antibody, 14.4 units/ml (normal
level, <2.0 units/ml).
In 1993, the patient developed exophthalmos, which
was successfully treated by the administration of conventional
radiotherapy at a total dose of 36 Gy. Laser-based patient
positioning was used to ensure precise positioning prior to each
radiation session (15). The patient
experienced mild transient conjunctivitis and periorbital erythema
subsequent to the treatment.
In 2000, a myxedema began to develop on the right
hand, and then progressed to involve the lower right leg. Two years
later, the left hand and then the lower left leg were also
affected. The patient was treated using a variety of medications,
consisting of the administration of topical and systemic
corticosteroids, cyclophosphamide, hydroxychloroquin and
everolimus, as a trial to improve his skin condition by decreasing
the fibroblast production of hyaluronic acid. Compression bandaging
and regular lymphatic drainage were also administered. Subsequent
to these therapies, no sufficient improvement had been observed and
the patient was therefore referred to the Department of Radiation
Oncology at the University Hospital of Muenster in 2005.
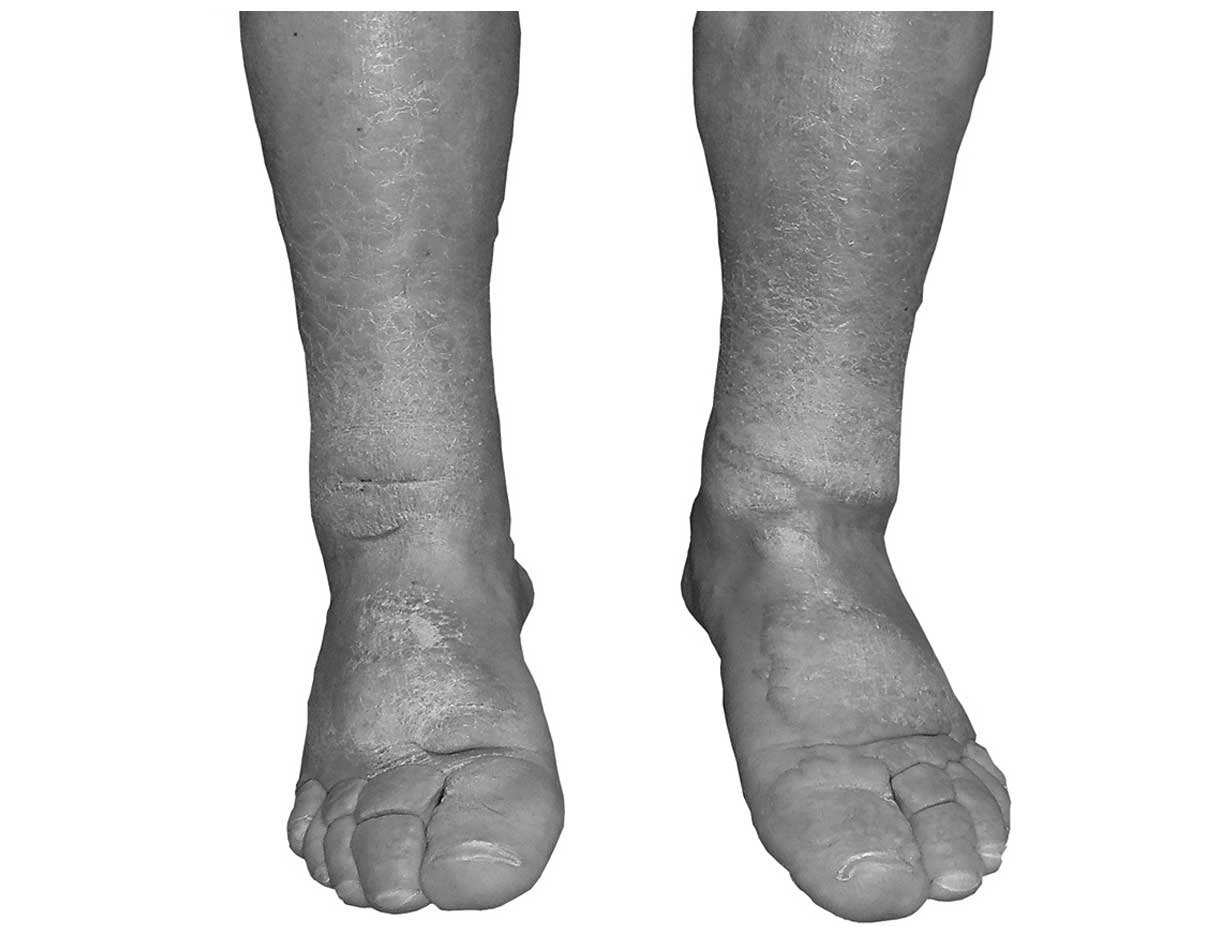
The clinical examination performed prior to
radiotherapy confirmed the presence of massive pretibial myxedema
in the form of non-pitting edema and skin plaques, combined with
areas of extensive fibrosis (Fig. 1).
Nodular tumors on the dorsal aspect of the hands and feet were
clinically apparent and resulted in a severe cosmetic impairment,
as well as discomfort due to continuous pressure from footwear and
impaired motion of the ankles. At the time of the diagnosis of
myxedema, the patient was non-thyrotoxic. The histological
examination of the myxedematous pretibial and hand lesions revealed
a typical deposition of mucin accompanied by fibrosis of
collagenous fibers and interstitial mucopolysaccharides.
Perivascular infiltration of lymphocytes was also noted. The
epidermis was found to be irregularly acanthotic with compact
hyperkeratosis.
As the patient had developed thyroid acropathy over
the course of the disease, with clubbing of the fingers and toes
being demonstrated, and had experienced a periosteal reaction in
the metacarpal bones and osteoarthropathy, as revealed by an X-ray
of the hands performed in 2002, the triad of exophthalmos, myxedema
and osteoarthropathy was fulfilled and a diagnosis of EMO syndrome
was established. Table I reports the
details of the radiation doses administered to the patient and the
clinical outcomes.
 | Table I.Different radiation treatments
delivered to the patient according to site of lesions. |
Table I.
Different radiation treatments
delivered to the patient according to site of lesions.
| Location of radiation
administration | Radiation dose,
Gy | Daily dose, Gy | Weekly fractions | Type of
radiation | Energy | Date of radiation,
month and year | Acute toxicity | Follow-up |
|---|
| Orbital | 36 (18+18) | 3 | 2 | Electrons +
photons | 15 MeV | July 1991 | Radiodermatitis I°,
conjuctivitis | Complete clinical
response to date |
| Lower left leg and
foot | 5 | 1 | 3 | Cobalt-60 |
| February 2005 | None | Regression of
myxedema |
| Right lower leg and
right foot | 20 | 2 | 5 | Cobalt-60 |
| February 2005 | None | Persistant
myxedema |
| Right lower right leg
and foot re-radiation due to persistent myxedema following 20
Gy | 16 | 2 | 5 | Cobalt-60 |
| May 2005 | Lower leg edema | Regression of
myxedema with local control for eight years |
| Left lower leg and
foot re-radiation | 30 | 2 | 5 | Photons | 6 MV | January 2009 | Radiodermatitis
II° | Local control for
four years |
| Back of left
hand | 26 | 2 | 5 | Electrons | 6 MeV | August 2012 | Hand edema,
radiodermatitis I° | Slow progressive
lymphedema |
| Graft donor site,
left thigh | 19, 80 | 1, 8 | 5 | Electrons | 6 MeV | October 2012 | None | Local control to
date |
In February 2005, radiation therapy was commenced to
treat the lower left leg and foot at a total dose of 5 Gy.
Simultaneously, radiotherapy of the lower right leg and foot was
commenced, with the administration of ≤20 Gy, due to severe skin
myxedema. Three months later, an additional 16 Gy was administered
to the lower right leg due to the persistence of myxedema. The
radiotherapy was well tolerated, with the exception of skin edema
and slight erythema occurring on the right side a few days
subsequent to the completion of radiotherapy. However, this
resolved spontaneously after three weeks.
In November 2008, due to skin disfigurement, the
patient was treated with surgical shave excision on the lower legs,
which was performed by dermatological surgeons. Subsequent to the
surgery on the left leg, adjuvant radiotherapy was commenced up to
a total dose of 30 Gy (Fig. 2). Since
2009, the present patient has received 400 mg pentoxifylline daily
as maintenance therapy to reduce the extent of fibrotic lesions
induced by radiation therapy (17).
In February 2010, due to the presence of severe
thyroid myxedema on the back of the left hand, the skin was
resected and the patient developed myxedematous recurrence after
seven months. In June 2012, the Departments of Dermatology and
Radiation Oncology jointly decided to perform wide skin excision
and skin transplantation on the back of the left hand and then to
perform radiation treatment at a dose of 26 Gy for consolidation.
Slight skin edema and erythema were noted. Five weeks later, the
graft donor site exhibited myxedematous skin changes, four months
subsequent to skin transplantation, in the form of skin elevation
and swelling. These symptoms were also treated with radiotherapy at
a dose of 19.8 Gy.
Subsequent to the completion of radiotherapy, the
skin erythema had clearly improved. The patient was highly
satisfied with the results of treatment. Nearly eight years
subsequent to irradiation, no recurrence of myxedema was observed
on the lower right leg. On the lower left leg no recurrence was
observed approximately four years subsequent to the completion of
radiotherapy. At the final follow-up examination six months prior
to writing, slight lower leg swelling was observed on each side,
which was considered to be recurrent myxedema. At present, this
recurrence continues to be treated locally with compression
bandaging. Additional lesions on the left hand and thigh due to
skin transplantation were also stable subsequent to the completion
of radiation treatments. The left hand demonstrates the presence of
slowly progressing lymphedema, nearly six months subsequent to
radiotherapy, which is also currently being treated with
compression bandaging.
Discussion
The mechanism of myxedema is not completely clear,
and it is assumed that the presence of the TSHR-Ab stimulates
fibroblasts, leading to glycosaminoglycans production and resulting
in the engorgement of the involved tissues (10,11). Mild
cases of myxedema or cases without cosmetic or local functional
limitation do not require topical treatment and are usually
followed up by endocrinologists. The administration of topical
corticosteroids coupled with occlusive or compressive dressings may
be used to treat symptomatic cases (2). The treatment of refractory cases is
usually suppressive in order to control symptoms without curative
intent. Other management options have been advocated. Shinohara
et al described success using intra-lesional octreotide for
>15 months (18). Felton et
al successfully treated a patient using a combination of
surgical and octreotide treatment, resulting in local control of
skin lesions lasting more than nine years (19). Jolles et al reported the
clinical improvement of pretibial myxedema in patients treated
using high dose intravenous immunoglobulin therapy (20).
To the best of our knowledge, the present study
reports the first case of extensive multilocular myxedema in a
patient with EMO syndrome that was treated using radiotherapy. The
skin lesions resulting from EMO syndrome in the present patient
resulted in severe cosmetic and local functional impairment. The
adjuvant radiotherapy administered subsequent to skin excision
produced a significantly improved long-term local control of the
skin lesions and improved ankle movements on each side. In addition
to the radiation sessions, psychosocial support for the present
patient aided in the development of coping strategies for the
medical condition (21).
In the present patient, no exophthalmos was observed
for 20 years subsequent to the administration of orbital
conventional radiotherapy using two opposing lateral fields. In
addition, radiotherapy has been used successfully for >50 years
for the treatment of GO. At present, orbital radiation is performed
using intensity-modulated radiotherapy to decrease incidence of
complications. Low dose orbital radiotherapy of 16–20 Gy over two
to three weeks was revealed to be effective in the treatment of GO
(22,23).
In summary, the routine use of radiation therapy for
EMO syndrome is not recommended, in particular for the treatment of
young patients with minimal cutaneous changes. It was also
hypothesized that radiotherapy, in combination with surgical
intervention, should be alternative modalities for those patients
who exhibit recurrent or refractory myxedematous lesions with
severe cosmetic disfigurement.
The present study reports the case of a patient that
experienced EMO syndrome in addition to extensive myxedema, which
demonstrated a good long-term clinical response to radiotherapy in
combination with surgical procedures. The current study reveals
that radiation therapy may be considered as an adjuvant therapy for
patients with refractory myxedema in order to prevent or delay the
recurrence of myxedema subsequent to surgical excision. Management
of EMO syndrome requires discussion within a multidisciplinary team
to determine individualized treatment options for each clinical
presentation of the syndrome.
Acknowledgements
The authors would like to thank Susan Nieschlag for
the language editing performed on the original manuscript.
References
|
1
|
Schwartz KM, Fatourechi V, Ahmed DD and
Pond GR: Dermopathy of Graves' disease (pretibial myxedema):
Long-term outcome. J Clin Endocrinol Metab. 87:438–446. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fatourechi V: Pretibial myxedema:
Pathophysiology and treatment options. Am J Clin Dermatol.
6:295–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen JJ and Ladenson PW: Euthyroid
pretibial myxedema. Am J Med. 82:318–320. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brent GA: Clinical practice. Graves'
disease. N Engl J Med. 358:2594–2605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson CK and Miller OF III: Triad of
exophthalmos, pretibial myxedema, and acropachy in a patient with
Graves' disease. J Am Acad Dermatol. 48:970–972. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bahn RS, Dutton CM, Heufelder AE and
Sarkar G: A genomic point mutation in the extracellular domain of
the thyrotropin receptor in patients with Graves' ophthalmopathy. J
Clin Endocrinol Metab. 78:256–260. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saito S, Sakurada T, Yamamoto M, Yamaguchi
T, Yoshida K, Sasai Y and Yoshinaga K: Exophthalmus-myxoedema
circumscriptum praetibiale-osteoarthropathia hypertrophicans
(E.M.O.) syndrome in Graves' disease: A review of eight cases
reported in Japan. Tohoku J Exp Med. 115:155–165. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Senel E and Güleç AT: Euthyroid pretibial
myxedema and EMO syndrome. Acta Dermatovenerol Alp Pannonica
Adriat. 18:21–23. 2009.PubMed/NCBI
|
|
9
|
Rongioletti F, Donati P, Amantea A,
Ferrara G, Montinari M, Santoro F and Parodi A: Obesity-associated
lymphoedematous mucinosis. J Cutan Pathol. 36:1089–1094. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Daumerie C, Ludgate M, Costagliola S and
Many MC: Evidence for thyrotropin receptor immunoreactivity in
pretibial connective tissue from patients with thyroid-associated
dermopathy. Eur J Endocrinol. 146:35–38. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fatourechi V, Ahmed DD and Schwartz KM:
Thyroid acropachy: Report of 40 patients treated at a single
institution in a 26-year period. J Clin Endocrinol Metab.
87:5435–5441. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stadlmayr W, Spitzweg C, Bichlmair AM and
Heufelder AE: TSH receptor transcripts and TSH receptor-like
immunoreactivity in orbital and pretibial fibroblasts of patients
with Graves' ophthalmopathy and pretibial myxedema. Thyroid.
7:3–12. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prabhakar BS, Bahn RS and Smith TJ:
Current perspective on the pathogenesis of Graves' disease and
ophthalmopathy. Endocr Rev. 24:802–835. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bahn RS: Clinical review 157:
Pathophysiology of Graves' ophthalmopathy: the cycle of disease. J
Clin Endocrinol Metab. 88:1939–1946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stieler F, Wenz F, Scherrer D, Bernhardt M
and Lohr F: Clinical evaluation of a commercial surface-imaging
system for patient positioning in radiotherapy. Strahlenther Onkol.
188:1080–1084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdfAccessed.
August 30–2013
|
|
17
|
Delanian S, Porcher R, Rudant J and Lefaix
JL: Kinetics of response to long-term treatment combining
pentoxifylline and tocopherol in patients with superficial
radiation-induced fibrosis. J Clin Oncol. 23:8570–8579. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shinohara M, Hamasaki Y and Katayama I:
Refractory pretibial myxoedema with response to intralesional
insulin-like growth factor 1 antagonist (octreotide):
Downregulation of hyaluronic acid production by the lesional
fibroblasts. Br J Dermatol. 143:1083–1086. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Felton J, Derrick EK and Price ML:
Successful combined surgical and octreotide treatment of severe
pretibial myxoedema reviewed after 9 years. Br J Dermatol.
148:825–826. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jolles S and Hughes J: Use of IGIV in the
treatment of atopic dermatitis, urticaria, scleromyxedema, pyoderma
gangrenosum, psoriasis, and pretibial myxedema. Int
Immunopharmacol. 6:579–591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schiel RO, Herzog W, Hof H, Debus J,
Friederich HC, Brechtel A, Rummel J, Freytag P and Hartmann M:
[Effect of systematic information about psychosocial support
services during outpatient radiotherapy. A controlled trial].
Strahlenther Onkol. 189:579–585. 2013.[(In German)]. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nguyen NP, Krafft SP, Vos P, et al:
Feasibility of tomotherapy for Graves' ophthalmopathy: Dosimetry
comparison with conventional radiotherapy. Strahlenther Onkol.
187:568–574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Staar S, Eich H and Seegenschmiedt M:
Graves' OrbitopathyRadiotherapy for Non-Malignant Disorders.
Seegenschmiedt M and Makoski H-B: Brady L, Springer Berlin
Heidelberg; Trott, K-R: pp. 469–486. 2008, View Article : Google Scholar
|