Introduction
Angiogenesis is necessary for tumor growth,
invasiveness and development of metastasis (1–4).
Angiogenesis is a complex process that is tightly regulated by pro-
and anti-angiogenic growth factors (5). At present, it is clear that the role
of angiogenic factors include VEGF, aFGF, bFGF, angiogenin,
PD-ECGF, TGF and TNF (6–9). The predominant regulator of tumor
angiogenesis is vascular endothelial growth factor (VEGF) (10–12),
which plays a vital role during normal embryonic angiogenesis,
endothelial cell proliferation and also in the physiological and
pathological angiogenesis. Furthermore, an essential role for VEGF
in tumor angiogenesis has been demonstrated in an animal model
(13). VEGF has received attention
as a target for therapeutic angiogenesis (14).
VEGF-targeted therapies were generally neutralizing
VEGF antibody and dominant-negative VEGF receptors (15). A previous study has described
antiangiogenic therapy with VEGF-Trap that may be a new and perhaps
more effective approach to block tumor-associated VEGF, and act as
a soluble decoy receptor and a high-affinity anti-VEGF agent for
VEGF. VEGF-Trap consists of portions of the extracellular domains
of both VEGFR-1 and VEGFR-2 fused to an Fc segment of human IgG
(16). VEGF-Trap, an antiangiogenic
agent, can prevent VEGF to bind to its receptor, in order to block
activities of VEGF. Suppressing vascularization and tumor growth
in vivo by VEGF-Trap, tumors appears to be stunted and
almost completely avascular. The high affinity fusion proteins have
been reported to cause regression of coopted vessels, therefore
suppressing tumor growth by inhibiting tumor induced angiogenesis
(17). A recently study
demonstrated that VEGF-Trap treatment was efficacious in both
initial and advanced phases of tumor development by significantly
prolonging survival (18). In order
to obtain long-term tumor-free survival by VEGF-Trap therapy, the
agent inhibiting tumor neovasculature needs to be systemically
maintained at stable therapeutic levels. Administration of purified
VEGF-Trap needs multiple administrations and may be limited by
their short half-life with associated discomfort and cost (4.0
mg/kg intravenously injection every two weeks in clinical trails).
Thus, production of VEGF-Trap may overcome these limitations after
gene transfer.
There are some requirements for vectors used in
antiangiogenic gene therapy, for example, no or low toxicity or
immunity, and the sustained expression. Therefore, adeno-associated
virus (AAV) is a very attractive candidate for creating viral
vectors for gene therapy (19–21).
Adeno-associated virus (AAV) as vectors are non-pathogenic and less
immunogenic compared with other gene therapy vectors (22). To date, AAV vectors have been used
for many clinical trials for treatment of certain tumors (23). The present study evaluated
recombinant AAV (rAAV) encoding secretable forms of VEGF-Trap. We
show that a single intravenous administration of adeno-associated
virus-mediated gene therapy expressing VEGF-Trap resulted in
persistent expression of VEGF-Trap, reduction of the concentration
of VEGF and other antigenic factors, such as aFGF, bFGF, angiogenin
and PD-ECGF, inhibition of tumor growth and prevention of lung
metastases formation in 4T1 metastatic breast cancer model.
Materials and methods
Cell lines
The murine breast carcinoma 4T1 cells were purchased
from the American Type Culture Collection (ATCC; Rockville, MD,
USA). These cells were cultured in RPMI-1640 supplemented with 10%
fetal bovine serum, 2 mM glutamine, and antibiotics (100 U/ml
penicillin and 100 μg/ml streptomycin). Cells were grown at 37°C in
a humidified 5% CO2 atmosphere.
Western blot analysis of AAV2-VEGF-Trap
in vitro
AAV-VEGF-Trap and AAV-Null virus were transfected
into HEK293 cells (ATCC; Rockville). After 48 h, the media of
tranfected 293 cells were collected, concentrated and
electrophoresed in 12% SDS-PAGE. Then, proteins were transferred at
100 V for 1 h to a polyvinylidene difluoride (PVDF) membrane
(Bio-Rad, Richmond, CA, USA). The membranes were blocked with 5%
milk in TBST at 4°C overnight, then incubated with either human
VEGFR1/Flt-1 antibody (R&D Systems, USA) or human VEGFR2/KDR
antibody (R&D Systems) for 1 h at 37°C. Membranes were washed
three times in TBST and HRP-conjugate rabbit anti-goat
immunoglobulins (R&D Systems) were incubated. The protein bands
were visualized via an enhanced chemiluminescence detection system
(Pierce, Rockford, IL, USA).
Breast carcinoma model
Female Balb/c mice, 6 weeks of age, were purchased
from Beijing Hua Fu Kang Biological Technology Co., Ltd. All
animals were housed under pathogen-free conditions and fed
autoclaved pellets and water. Mice were inoculated s.c. with
5×105 4T1 tumor cells. After small s.c. tumors became
about 3 mm (about 1 week after implantation), treatment with the
AAV2-VEGF-Trap was initiated. The mice were divided into three
groups after inoculated s.c. with 4T1 tumor cells and each group
had six animals. Seven days after inoculation, AAV2-VEGF-Trap or
AAV2-Null in 50 μl of PBS was administered intravenously. The first
group was injected with normal saline (NS). The second group of
animals was treated with 6×109 viral genomes (vg)
AAV2-VEGF-Trap, and the third group with 6×109 vg of
AAV2-Null. Tumor size was measured every 3 days with a digital
caliper for two-dimensional length and width, and tumor volume was
calculated as (length × width2)/2.
Two groups of normal female Balb/c mice, 6 weeks of
age, were intravenously administered with AAV2-VEGF-Trap
(6×109 vg) or AAV2-Null (6×109 vg). The sera
were collected every week for 13 weeks to measure the levels of
VEGF Trap by ELISA.
Expression of VEGF-Trap by ELISA
Levels of VEGF-Trap were measured by ELISA. Briefly,
blood was collected through the tail vein on days 0, 1, 3, 7, 21
and 28 after AAV2-VEGF-Trap administration in the tumor model and
the sera were collected every week for 13 weeks in two groups of
normal female Balb/c mice. ELISA plates were coated with 2 μg/ml
hVEGF165 in carbonate/bicarbonate buffer at 4°C overnight. Plates
were washed three times with PBST (0.1% Tween in PBS) and blocked
with 0.5% solution of non-fat dried milk in PBST for 1 h at 37°C.
Serum samples were diluted in 1:5 and added to the wells, then and
incubated for 1 h at 37°C. Later, plates were incubated with 1
μg/ml of human VEGFR1/Flt-1 biotinylated antibody (R&D Systems)
at 37°C of 1 h. After washed, the secondary antibody was
Streptavidin-HRP (R&D Systems) diluted 1:200. After five washes
with PBST, the substrate was developed with 3,3′,5,5′-tetramethyl
benzidine (TMB) (Sigma-Aldrich, USA), which was developed at RT for
20 min and stopped with 2 M H2SO4. Plates
were read by a Bio-Rad microplate reader at 450 nm.
Immunohistochemistry
The tissues were fixed in 4% paraformaldehyde and
embedded in paraffin. Lungs and tumors were cut into sections and
stained with hematoxylin and eosin. Frozen tumor tissues were
stained for blood vessels using a monoclonal rabbit anti-mouse
CD-31-phycoerythrin conjugate (BD, USA). Nuclei were stained with
4′,6-diamidino-2-phenylindole (DAPI; dilution 1:5000). Apoptosis
was determined by TUNEL staining, using DeadEnd™ Fluorometric TUNEL
System (Promega, USA). The sections were examined with an upright
fluorescence microscope. In addition, tissues simples of kidney,
liver, heart and brain were sectioned and stained with H&E to
measure the toxicity.
Analyzing angiogenesis-related proteins
levels in the serum
To examine the change of angiogenesis-related
protein levels, serum was collected and stored at −20°C. Mouse
Angiogenesis Array kit (R&D System), which can detect
expression levels of 53 mouse angiogenesis related proteins, was
used. Array data can be quantified on developed X-ray film by
scanning the film on transmission-mode and using image analysis
software (Quantity One) to analyze the array image file.
Statistical analysis
SPSS 17.0 was used for statistical analysis. Data
were expressed as mean ± SE. One-way analysis of variance or the
unpaired Student’s t-test were used for comparison between groups.
Differences were considered statistically significant at P<0.05.
Comparisons of image analysis measurement, tumor volume and
metastasis were performed.
Results
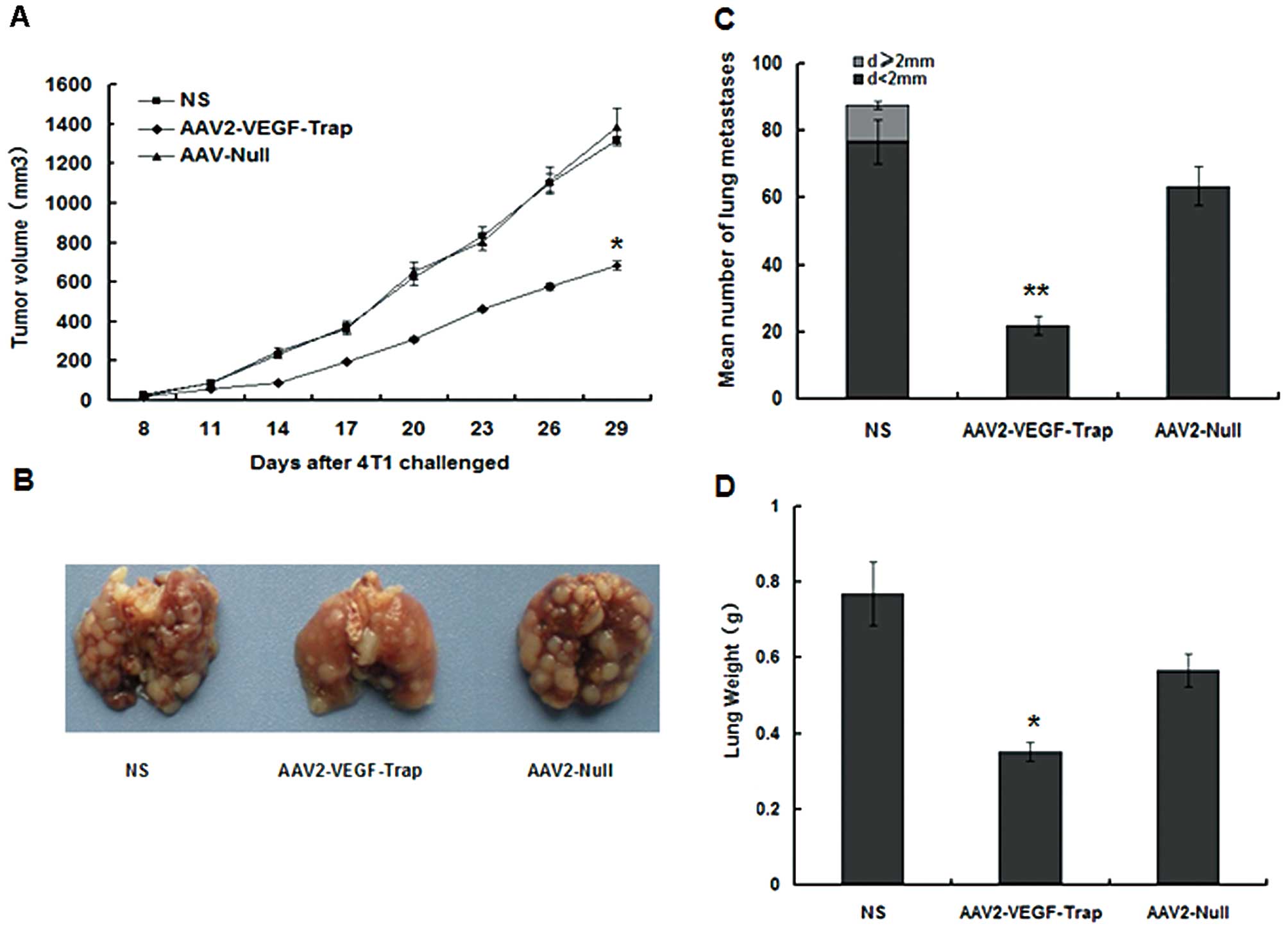
Inhibition of s.c. tumor growth and
spontaneous lung metastases. VEGF is necessary for the development
of neovasculatures at very early stages of tumorigenesis (24–26).
AAV2-VEGF-Trap was injected intravenously at early stage of tumor
about 3 mm in diameter. Although, every animal developed a tumor,
the growth rate of the AAV2-VEGF-Trap group was significantly
slower than that in the NS group and AAV2-Null group (Fig. 1A). The mice of all groups were
challenged with 5×105 4T1 breast tumor cells by s.c.
inoculation. 4T1 tumor cells can trigger spontaneous lung
metastases about 1.5 cm. Comparison of the respective metastatic
surface areas showed that AAV2-VEGF-Trap group significantly
reduced metastatic load, compared to the other groups (Fig. 1B and C).
However, pulmonary tumor deposits were strikingly
smaller in the AAV2-VEGF-Trap-treated lungs in comparison with the
controls (Fig. 2A). At the same
time, the lung weight of AAV-VEGF-Trap group was obviously less
than that of NS group and AAV2-Null group (Fig. 1D). These results indicated that
AAV2-VEGF-Trap can effectively inhibit the growth of primary breast
tumors and spontaneous pulmonary metastases in mice with
established 4T1 breast tumor.
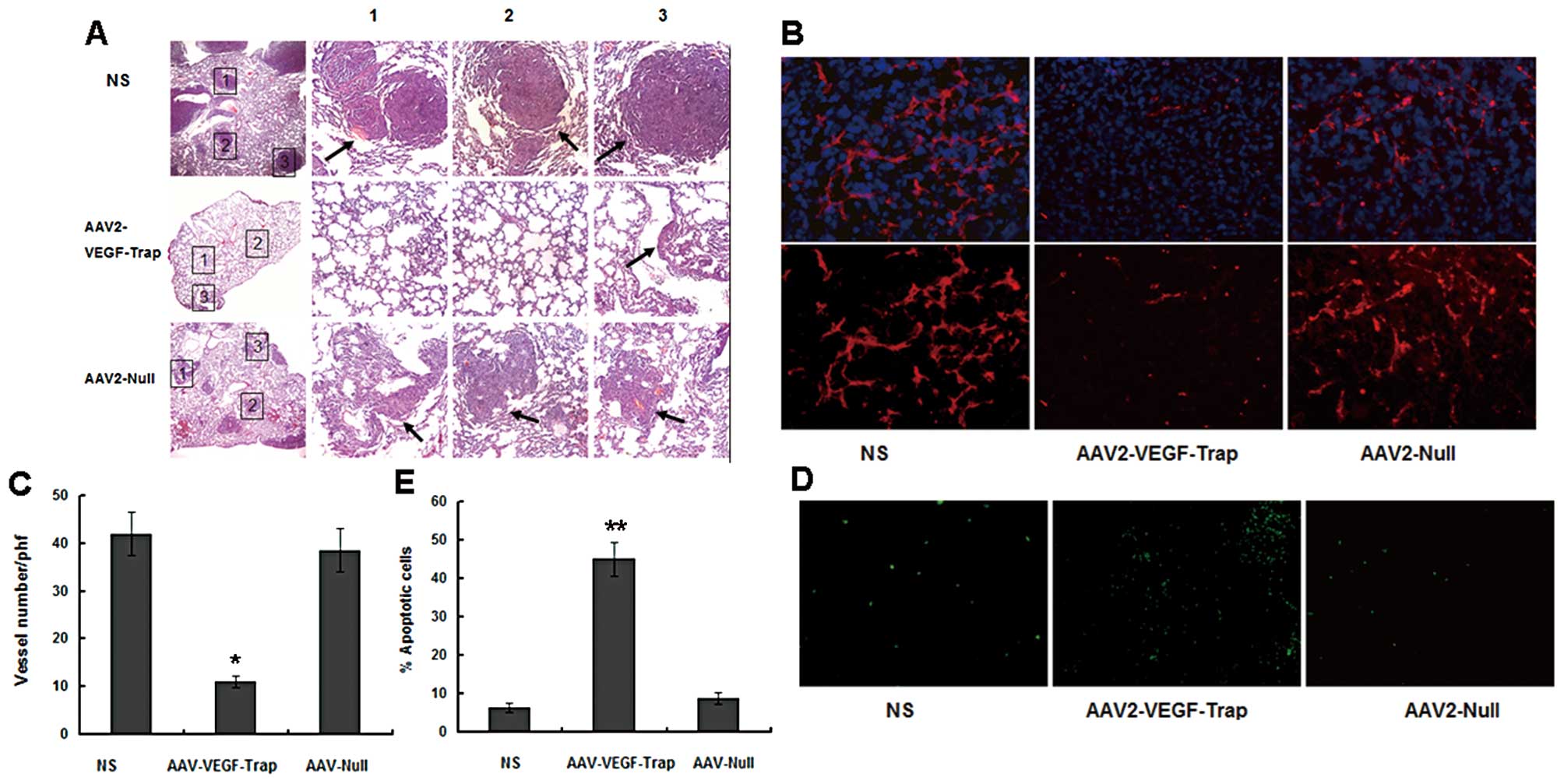
Inhibition of angiogenesis and promotion
of tumor apoptosis
To further investigate whether the decreased tumor
growth was associated with a reduction in the tumor angiogenesis,
frozen tumor sections were stained with anti-CD31 antibody
(Fig. 2B, lower) and counterstained
with DAPI (Fig. 2B, upper). The
AAV2-VEGF-Trap group exhibited apparent inhibition of angiogenesis
compared with the control groups (the NS group and AAV2-VEGF-Trap
group) (Fig. 2C). In order to
estimate apoptosis in tumor tissues, TUNEL staining was performed.
Apparently more apoptotic cells were observed in the tumor sections
of mice treated with AAV2-VEGF-Trap than that with AAV2-Null and NS
(Fig. 2D and E).
Expression of AAV2-VEGF-Trap in vitro and
long-term expression in vivo
The above studies indicated that AAV2-VEGF-Trap
could block the formation of neovascularization and inhibit tumor
growth, due to the expression of VEGF-Trap. In vitro,
western blot analysis of the cell supernatant revealed the
expression of VEGF-Trap following transfected with AAV2-VEGF-Trap
comparing with AAV2-Null (Fig. 3A).
Next, we monitored the expression of VEGF-Trap in the sera of mice
injected rAAV2-VEGF-Trap in the tumor model. The blood of the mice
was obtained on days 0, 1, 3, 7, 21 and 28 after intravenous
administration of 6×109 vg of AAV2-VEGF-Trap; and the
sera were used to analyze VEGF-Trap by ELISA. VEGF-Trap levels
remained at similar levels until the last evaluation at 28 days
(Fig. 3B). No VEGF-Trap was
detected in the sera from mice that received AAV2-Null and NS. To
evaluate the expression profile of AAV2-VEGF-Trap in normal mice
after intravenous administration of AAV2-VEGF-Trap, the blood was
obtained every week for 13 weeks. The expression of VEGF-Trap was
sustained for 13 weeks (Fig. 3C) by
adeno-associated virus mediated gene therapy. The result implied
that a single intravenous administration of AAV2-VEGF-Trap can
sustain the long-term expression of VEGF-Trap.
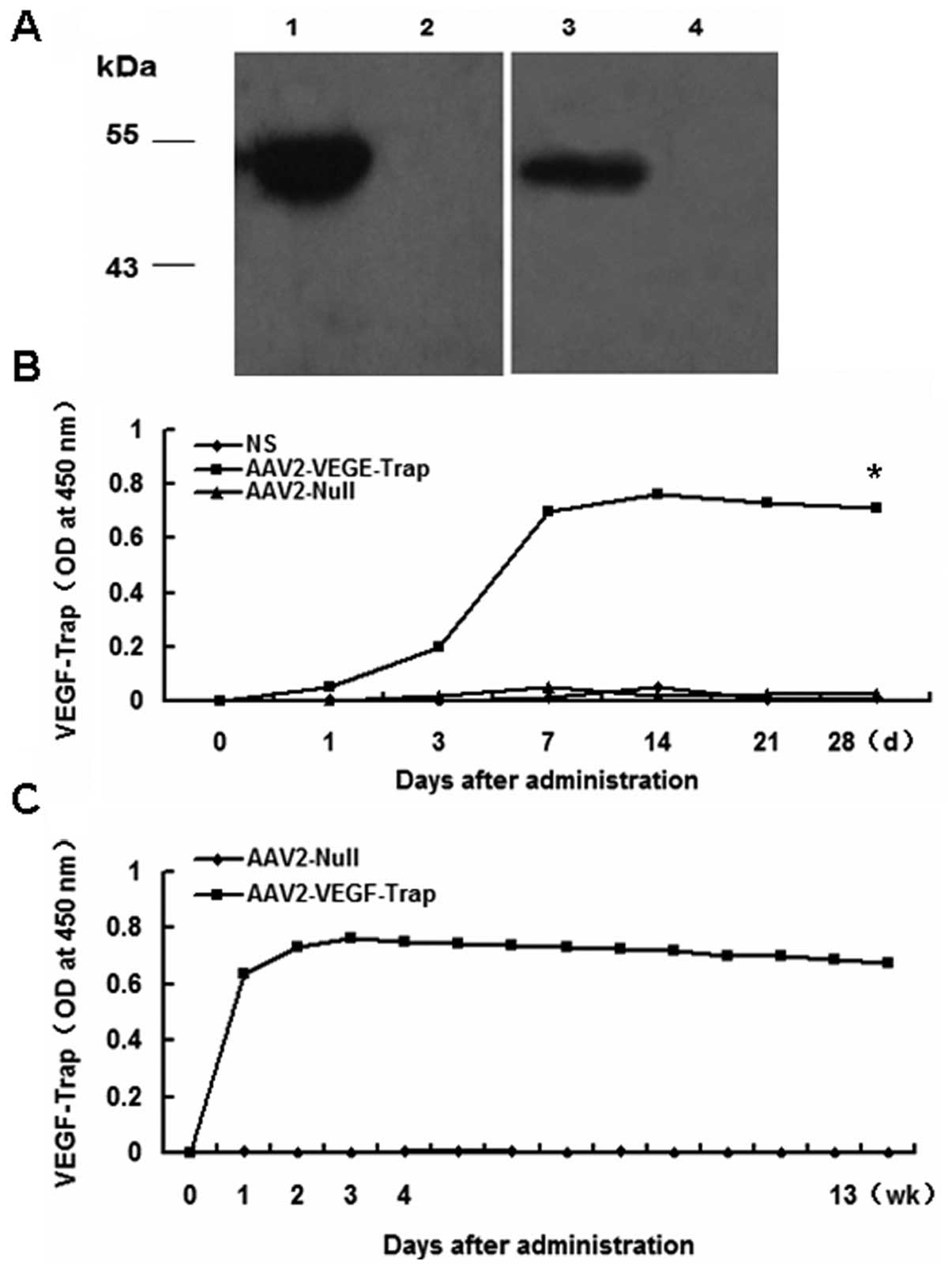 | Figure 3Expression of AAV2-VEGF-Trap. In
vitro, western blot analysis expression of VEGF-Trap from
HEK293 cells transfected AAV2-VEGF-trap and long-term expression of
AAV2-VEGF-Trap in serum. Blood was subsequently obtained on days 0,
1, 3, 7, 14, 21 and 28 in the mice with 4T1 tumor and obtained for
13 weeks in normal mice. Then, the sera were analyzed by ELISA. (A)
Western blot analysis of the expression of VEGF-Trap using
anti-hVEGFR1/flt-1 (lane 1, AAV2-VEGF-Trap; lane 2, AAV2-Null),
anti-hVEGFR2/KDR (lane 3, AAV2-VEGF-Trap; lane 4, AAV2-Null). (B)
Sustained expression of AAV2-VEGF-Trap for 28 days in the 4T1
model. (C) Sustained expression of AAV2-VEGF-Trap for 13 weeks in
normal mice injected AAV2-VEGF-Trap. (*P<0.05
AAV2-VEGF-Trap vs. AAV2-Null and NS). |
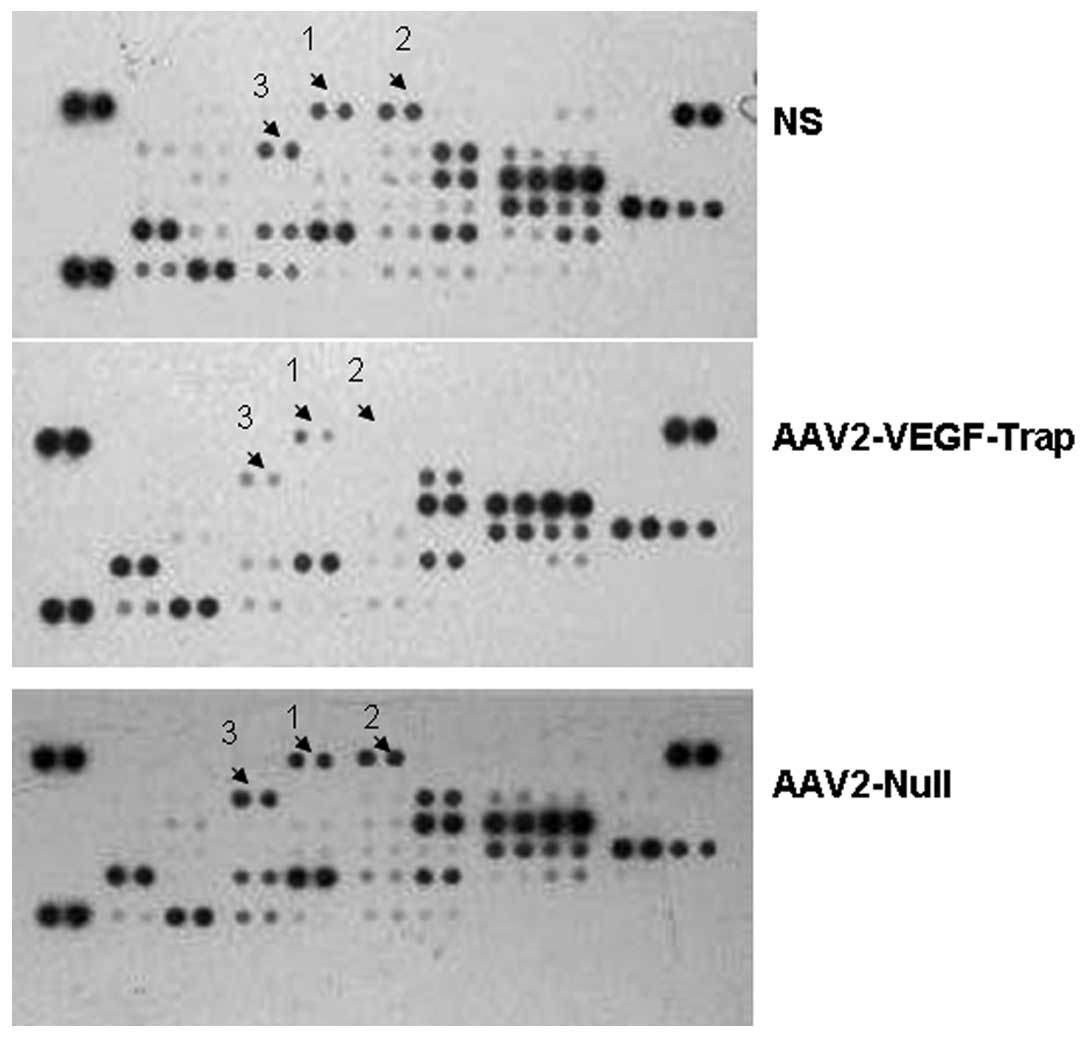
AAV-VEGF-Trap reduces the expression of
angiogenesis-related factors
To analyze the relative levels of
angiogenesis-related proteins, serum samples were detected via the
Proteome Profiler Mouse Angiogenesis Array kit. Our results
revealed that the expression of many angiogenic growth factors had
been obviously reduced in mice treated with AAV2-VEGF-Trap than the
control groups (Fig. 4, Table I), which suggested that the
decreased tumor volumes and metastasis of AAV2-VEGF-Trap group may
be caused by decreased expression of the angiogenesis-related
proteins.
 | Table IMean pixel density of angiogenesis
factors of each group. |
Table I
Mean pixel density of angiogenesis
factors of each group.
| Mean pixel
density |
|---|
|
|
|---|
| NS | AAV2-VEGF-Trap | AAV2-Null |
|---|
| 1 Angiopoietin-3 | 23285.42743 | 0 | 54752.14071 |
| 2 CXCL16 | 47495.42495 | 0 | 127174.0433 |
| 3 Cyr61 | 51711.83539 | 0 | 50608.70028 |
| 4 DLL4 | 36233.71378 | 0 | 42994.55948 |
| 5 DPPIV | 105744.736 | 67509.88204 | 105357.301 |
| 6 Endothelin-1 | 67219.70701 | 0 | 78493.54819 |
| 7 FGF acidic | 43989.51959 | 0 | 67765.62207 |
| 8 FGF basic | 49741.49019 | 0 | 37905.64895 |
| 9 Fractalkine | 39346.7841 | 0 | 58738.60613 |
| 10 HB-EGF | 34086.33856 | 0 | 54464.60068 |
| 11 HGF | 43835.77957 | 0 | 53129.75554 |
| 12 Leptin | 32774.45842 | 0 | 53819.95561 |
| 13 PlGF-2 | 45577.01475 | 0 | 41604.89934 |
| 14 Prolactin | 109857.0365 | 67141.787 | 91828.23958 |
| 15 PDGF-AA | 87302.40411 | 57683.83602 | 78166.29315 |
| 16 Serpin F1 | 72810.73259 | 52744.2355 | 77245.32806 |
| 17 Endoglin | 36370.85379 | 0 | 59343.50619 |
| 18 Angiogenin | 103220.4658 | 64170.16169 | 160876.2768 |
| 19 IGFBP-3 | 349666.8265 | 43272.03951 | 397592.7965 |
| 20
Angiopoietin-1 | 118544.2774 | 0 | 181185.0739 |
Toxicity of AAV2-VEGF-Trap
In general, the viruses have security issues. We
examined the potential toxicity of AAV2-VEGF-Trap by measuring
changes in body weight every 3 days, and morphological analyses of
major organs. There was no obvious difference in body weight
between the experimental group and the control groups (Fig. 5A). Sections of heart, liver, spleen
and kidney were stained with hematoxylin and eosin. Overall, no
obvious or significant differences were detected in response to
AAV2-VEGF-Trap and AAV2-Null compared with NS group (Fig. 5B).
Discussion
Angiogenesis plays a key role in tumor growth and
metastasis. Tumor angiogenesis is regulated by a variety of active
substances. Many angiogenic factors have been implicated in the
regulation of the growth and metastasis of solid tumors. VEGF is a
main angiogenic factor (27,28),
both in physiological or pathological processes it has important
implications (29). In
angiogenesis, tumor growth and metastasis, VEGF plays a critical
role and is overexpressed in the majority of solid tumors,
consistent with its role as a key mediator of tumor
neoangiogenesis. Most tumors induce new vessel formation,
suggesting that this neovascularization is required for their
growth (30–32). Thus, blocking the formation of
neovascularization is critical for inhibiting tumor growth. VEGF is
an important and effective target for use against cancer, by
binding and blocking VEGF to inhibit the growth and metastasis of
tumors.
We have constructed a recombinant adeno-associated
virus (AAV) expressing VEGF-Trap for the first time as shown both
in vitro and in vivo models of angiogenesis.
VEGF-Trap, a high-affinity soluble decoy receptor, comprises
portions of the extracellular domains of both the second Ig domain
of VEGFR1 with the third Ig domain of VEGFR2 fused to an Fc segment
of human IgG. In clinical trials, VEGF-Trap has been shown to
require repeated intravenous administration to maintain adequate
therapy levels with the accompanying high cost, and the patient’s
resistance compared to a single administration. AAV vectors have
not shown any danger and are very simple, so it can persist stably
in transduced cells and achieve long-term transgene expression.
Thus, in the present study, we chose recombinant AAV2 as gene
delivery system to study the efficacy of VEGF-Trap in the mouse
tumor model.
Transfected with AAV2-VEGF-Trap the cells led to
expression of VEGF-Trap compared with the control, in vitro.
Treatment of tumor-bearing mice with a single intravenous
administration of 6×109 vg of AAV2-VEGF-Trap caused a
tumor volume reduction. In addition, lung metastases markedly
decreased in both size and number in the 4T1 model. Analyzing tumor
microvessels with CD31 immunohistochemistry showed an obvious
reduction of microvessel density in AAV2-VEGF-Trap, compared with
the NS and AAV2-Null groups. Furthermore, AAV2-VEGF-Trap increased
the extent of apoptosis over that produced by the control groups.
We demonstrated that injection of AAV2-VEGF-Trap resulted in
persistent high levels of VEGF-Trap in serum (0, 1, 3, 7, 14, 21
and 28 days after administration of AAV2-VEGF-Trap). The normal
female Balb/c mice with a single intravenous administration of
6×109 vg of AAV2-VEGF-Trap can be achieved obvious
long-term expression for at least three months. No VEGF-Trap was
detected in the injection of AAV2-Null. Through detecting the
relative levels of angiogenesis-related proteins in the sera, the
result showed obvious decrease not only of the concentration of
VEGF in sera, but also the concentration of other angiogenic
factor, such as aFGF, bFGF and angiopoietin-1.
Most preclinical and clinical studies of anti-VEGF
agents have focused on targeting VEGF or its receptors. Several
agents have shown promise in controlling tumor growth and lung
metastasis, for example, VEGF-Trap. In previous studies, VEGF-Trap
had minimum interactions with the extracellular matrix, and this
property obviously accounts for its satisfactory pharmacokinetic
profile, superior to soluble forms of VEGFR-1 to prevent VEGF from
binding to its normal receptors by administering decoy VEGF
receptor to block the VEGF signaling pathway (16,33–36).
In previous studies, purified VEGF-Trap proteins were administered,
with the major problem in pharmacotherapy of side effects of the
constant VEGF-Trap administration and the limited half-life of
VEGF-Trap (37), requiring multiple
injections. Gene therapy has advantages of maintaining sustained
levels of VEGF-Trap in vivo, which enhanced antitumor
efficacy. AAV vectors are widely used in preclinical and clinical
gene therapy because of the low pathogenicity and toxicity, and
sustained long-term expression of the target gene.
Taken together, AAV2-VEGF-Trap ultimately limits the
blood supply and markedly increases tumor cell apoptosis.
AAV2-mediated sustained expression of VEGF-Trap was safe and
efficacious of gene therapy. This treatment dramatically reduces
tumor burden and inhibits spontaneous metastasis via systematic
effect of VEGF-Trap. In addition, long-term expression of a single
administration of AAV-VEGF-Trap simultaneously reduces the
expression of angiogenesis-related factors.
Acknowledgements
This study was funded by the Program for New Century
Excellent Talents in University (NCET) (NCET-09-0575) and the
National Key Basic Research Program (973 Program) of China
(2010CB529900).
References
|
1
|
Sharma RA, Harris AL, Dalgleish AG,
Steward WP and O’Byrne KJ: Angiogenesis as a biomarker and target
in cancer chemoprevention. Lancet Oncol. 2:726–732. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Teo NB, Shoker BS, Martin L, Sloane JP and
Holcombe C: Angiogenesis in pre-invasive cancers. Anticancer Res.
22:2061–2072. 2002.PubMed/NCBI
|
|
3
|
Kieran MW and Billett A: Antiangiogenesis
therapy. Current and future agents. Hematol Oncol Clin North Am.
15:835–851. viii2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pour L, Hajek R, Buchler T, Maisnar V and
Smolej L: Angiogenesis and antiangiogenic cancer therapy. Vnitr
Lek. 50:930–938. 2004.(In Czech).
|
|
6
|
O’Reilly MS, Holmgren L, Shing Y, et al:
Angiostatin: a novel angiogenesis inhibitor that mediates the
suppression of metastases by a Lewis lung carcinoma. Cell.
79:315–328. 1994.PubMed/NCBI
|
|
7
|
O’Reilly MS, Boehm T, Shing Y, et al:
Endostatin: an endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997.
|
|
8
|
Moses MA and Langer R: A metalloproteinase
inhibitor as an inhibitor of neovascularization. J Cell Biochem.
47:230–235. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rastinejad F, Polverini PJ and Bouck NP:
Regulation of the activity of a new inhibitor of angiogenesis by a
cancer suppressor gene. Cell. 56:345–355. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jain RK: Molecular regulation of vessel
maturation. Nat Med. 9:685–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leung DW, Cachianes G, Kuang WJ, Goeddel
DV and Ferrara N: Vascular endothelial growth factor is a secreted
angiogenic mitogen. Science. 246:1306–1309. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim KJ, Li B, Winer J, et al: Inhibition
of vascular endothelial growth factor-induced angiogenesis
suppresses tumour growth in vivo. Nature. 362:841–844. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar
|
|
15
|
Millauer B, Shawver LK, Plate KH, Risau W
and Ullrich A: Glioblastoma growth inhibited in vivo by a
dominant-negative Flk-1 mutant. Nature. 367:576–579. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holash J, Davis S, Papadopoulos N, et al:
VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl
Acad Sci USA. 99:11393–11398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim ES, Serur A, Huang J, et al: Potent
VEGF blockade causes regression of coopted vessels in a model of
neuroblastoma. Proc Natl Acad Sci USA. 99:11399–11404. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gomez-Manzano C, Holash J, Fueyo J, et al:
VEGF Trap induces antiglioma effect at different stages of disease.
Neuro Oncol. 10:940–945. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zaiss AK and Muruve DA: Immunity to
adeno-associated virus vectors in animals and humans: a continued
challenge. Gene Ther. 15:808–816. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Herzog RW, Yang EY, Couto LB, et al:
Long-term correction of canine hemophilia B by gene transfer of
blood coagulation factor IX mediated by adeno-associated viral
vector. Nat Med. 5:56–63. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Takabe K, Bidlingmaier SM, Ill CR
and Verma IM: Sustained correction of bleeding disorder in
hemophilia B mice by gene therapy. Proc Natl Acad Sci USA.
96:3906–3910. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ponnazhagan S, Mahendra G, Kumar S, et al:
Adeno-associated virus 2-mediated antiangiogenic cancer gene
therapy: long-term efficacy of a vector encoding angiostatin and
endostatin over vectors encoding a single factor. Cancer Res.
64:1781–1787. 2004. View Article : Google Scholar
|
|
23
|
Collins SA, Buhles A, Scallan MF, et al:
AAV2-mediated in vivo immune gene therapy of solid tumours. Genet
Vaccines Ther. 8:82010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Casanovas O, Hicklin DJ, Bergers G and
Hanahan D: Drug resistance by evasion of antiangiogenic targeting
of VEGF signaling in late-stage pancreatic islet tumors. Cancer
Cell. 8:299–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paley PJ, Staskus KA, Gebhard K, et al:
Vascular endothelial growth factor expression in early stage
ovarian carcinoma. Cancer. 80:98–106. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heist RS, Zhai R, Liu G, et al: VEGF
polymorphisms and survival in early-stage non-small cell lung
cancer. J Clin Oncol. 26:856–862. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johnson DE, Lee PL, Lu J and Williams LT:
Diverse forms of a receptor for acidic and basic fibroblast growth
factors. Mol Cell Biol. 10:4728–4736. 1990.PubMed/NCBI
|
|
28
|
Basilico C and Moscatelli D: The FGF
family of growth factors and oncogenes. Adv Cancer Res. 59:115–165.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ferrara N: VEGF and the quest for tumour
angiogenesis factors. Nat Rev Cancer. 2:795–803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferrara N and Alitalo K: Clinical
applications of angiogenic growth factors and their inhibitors. Nat
Med. 5:1359–1364. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van Hinsbergh VW, Collen A and Koolwijk P:
Angiogenesis and anti-angiogenesis: perspectives for the treatment
of solid tumors. Ann Oncol. 10(Suppl 4): S60–S63. 1999.PubMed/NCBI
|
|
33
|
Kuo CJ, Farnebo F, Yu EY, et al:
Comparative evaluation of the antitumor activity of antiangiogenic
proteins delivered by gene transfer. Proc Natl Acad Sci USA.
98:4605–4610. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ferrara N, Chen H, Davis-Smyth T, et al:
Vascular endothelial growth factor is essential for corpus luteum
angiogenesis. Nat Med. 4:336–340. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gerber HP, Vu TH, Ryan AM, Kowalski J,
Werb Z and Ferrara N: VEGF couples hypertrophic cartilage
remodeling, ossification and angiogenesis during endochondral bone
formation. Nat Med. 5:623–628. 1999. View
Article : Google Scholar
|
|
36
|
Gerber HP, Kowalski J, Sherman D, Eberhard
DA and Ferrara N: Complete inhibition of rhabdomyosarcoma xenograft
growth and neovascularization requires blockade of both tumor and
host vascular endothelial growth factor. Cancer Res. 60:6253–6258.
2000.
|
|
37
|
Folkman J: Antiangiogenic gene therapy.
Proc Natl Acad Sci USA. 95:9064–9066. 1998. View Article : Google Scholar
|