Introduction
We have classified the wall invasion pattern of
gallbladder carcinoma (GBC) into two groups, i.e., the infiltrative
growth type (IG type) and destructive growth type (DG type)
(1). The DG type was significantly
associated with poor differentiation, aggressive infiltration, and
decreased postoperative survival in histological differentiation,
lymphatic invasion, venous invasion, lymph node status, neural
invasion, and mode of subserosal infiltration. Therefore, the
classification of the IG/DG growth pattern is thought to be a
useful indicator of the local aggressiveness of GBC. There has been
no definition or classification of the wall invasion pattern, and
it was defined mainly based on the invasive phenomenon through the
muscle layer. We also demonstrated that the wall invasion pattern
was correlated with the overall aggressiveness of cancer, i.e.,
cell proliferation and local aggressiveness of cancer, such as the
stromal infiltration of GBC using an immunohistochemical procedure
(2). High-grade cell proliferation
employing the Ki-67 labeling index (Ki-67 LI) and invasiveness with
stromal laminin-5 γ2 chain staining were significantly correlated
with an aggressive wall invasion pattern indicating the DG type of
GBC. In this study, we analyzed the prognostic value of the wall
invasion pattern at a clinically significant depth of tumor
invasion, i.e., the subserosal layer.
From the viewpoints of surgical pathology and
anatomy, it is sometimes difficult to resect advanced GBC radically
(3). There is controversy regarding
the surgical indication, such as partial resection of the liver,
bile duct resection, and pancreatoduodenectomy for dissecting
regional lymph nodes (4–8). Since preoperative evaluation of the
tumor spread of subserosa-invasive GBC was difficult, the risk of
excessive or inadequate surgery is relatively high. Therefore, it
is important to reconsider the surgical procedure to ensure an
appropriate operation avoiding oversurgery. Previous studies
reported that the presence of lymph node metastases represented the
main marker of a poor prognosis (9–12).
Intraoperative frozen sections provide important information on
lymph node metastasis in surgery. In addition, we propose the
clinical use of the wall invasion pattern as a new prognostic
predictor which is also available using ordinary hematoxylin-eosin
sections. Classification according to the wall invasion pattern and
degree of lymph node metastasis would be helpful to re-examine the
necessity of an additional surgical procedure in or after the
surgery.
Materials and methods
Gallbladder tissue specimens
All tissue specimens were obtained on the surgical
resection of gallbladder adenocarcinomas at Tokai University
Hospital. The subjects were 42 patients (24 men and 18 women; age
range 40–93 years; mean age 65.4±10.7 years) with gallbladder
tumors invading the subserosal layer, i.e., the perimuscular
connective tissue (pT2), at surgery. The stages of GBC were based
on the TNM classification. The median postoperative follow-up
duration was 852.5 (332.0–2,027.8) days.
Histological examination
The gallbladder tissue specimens for histological
analysis were rapidly fixed in 10% buffered formalin for 24–48 h
and routinely embedded in paraffin. Tumor invasion was examined
using 4 μm sections stained with hematoxylin and eosin. The degree
of venous invasion was classified as: v0, no venous invasion; v1+,
minimal venous invasion, i.e., 1 or 2 foci of venous invasion in
one histological section; v2+, moderate venous invasion, i.e., 3 or
4 foci; and v3+, marked venous invasion with ≥5 foci. The degree of
lymphatic invasion was classified as: ly0, no lymphatic invasion;
ly1+, mild lymphatic invasion, ly2+, moderate lymphatic invasion;
and ly3+, marked lymphatic invasion. The degree of perineural
invasion was classified as: ne0, no perineural invasion; ne1+, mild
perineural invasion; ne2+, moderate perineural invasion; and ne3+,
marked perineural invasion. The mode of subserosal infiltration was
classified into three groups, according to the General Rules for
Gastric Cancer Study of the Japanese Gastric Cancer Association
(13), i.e., INF alpha α), a cancer
nest showing expansive growth and presenting a clear borderline
between itself and adipose tissue; INF beta (β), growth and
invasive patterns intermediate of those of α and γ; and INF gamma
(γ): scirrhous growth, a cancer nest showing invasive growth, while
the borderline between the tumor and adipose tissue is unclear. The
degree of biliary invasion was classified as: binf0, no invasion to
the hepatoduodenal ligament; binf1, uncertain invasion to the
hepatoduodenal ligament; binf2, mild invasion to the hepatoduodenal
ligament; or binf3, moderate to marked invasion to the
hepatoduodenal ligament.
Definition and histological
identification of invasion pattern
The following terminology was used to define and
classify the two patterns of invasion through the muscle layer.
Infiltrative growth (IG) type: cancer cells show infiltrative
growth in the muscle layer (through the intermuscular space)
without muscle layer destruction (Fig.
1A and C) (1). Destructive
growth (DG) type: cancer cells show massive growth with destruction
of the muscle layer (Fig. 1B and D)
(1). The cases showing both DG and
IG components were classified as the DG type because aggressive
growth patterns were present.
Azan staining was helpful for distinguishing the DG
from the IG type of GBC. The DG type usually showed aggressive
growth, and included a stromal desmoplastic reaction with activated
fibroblasts and dense collagen fibers, which were aniline
blue-positive with Azan staining (Fig.
1F). The IG type revealed a lower-level reaction of
desmoplasia, which was weakly positive for aniline blue (Fig. 1E).
Statistical analysis
Descriptive statistical analyses were employed to
examine the demographic characteristics of the study population.
Data are expressed as means ± SD and medians (25th and 75th
percentiles). The baseline characteristics, disease, and
pathological variables were compared between patients with the IG
and DG types by means of the χ2 test for continuous and
categorical variables. Univariate analyses (χ2 test)
were primarily used for selecting variables on the basis of a
p<0.05. The significant variables in univariate analyses and
clinically significant factors were subjected to Cox’s proportional
hazard regression modeling to assess the effect that independent
covariates had on the dependent variable of survival. Odds ratios
(ORs) and their 95% confidence intervals (CIs) were used to assess
the independent contributions of significant factors. A p<0.05
was considered to indicate significance.
Survival times were measured from the date of
surgery, and death from all causes (without differentiating between
deaths resulting from GBC or other causes) was taken as the
outcome. Survival curves were traced with the Kaplan-Meier method,
and the comparison of survival curves was carried out using the
log-rank test. All analyses were performed using the statistical
software package SPSS II (version 11.0; SPSS, Tokyo, Japan).
Results
Of the 42 subserosa-invasive GBCs (pT2), 24 (57.1%)
cases showed the IG type and 18 (42.9%) the DG type. Well to
moderately differentiated adenocarcinoma was the most frequent
histological type (85.7%). Poorly differentiated adenocarcinoma and
other histological types such as signet ring cell carcinoma and
adenosquamous cell carcinoma were also observed. We analyzed the
relationship between the wall invasion patterns through the muscle
layer and clinicopathological features (Table I). Lymphatic invasion (p=0.021),
venous invasion (p=0.020), mode of subserosal infiltration
(p<0.001), histological differentiation, (p=0.030) and biliary
infiltration (p=0.007) were noted, respectively, at a significantly
higher incidence in more aggressive infiltration or poor
differentiation in the DG type. In addition, cases with the DG type
tended to show a higher incidence of neural invasion (p=0.094) and
lymph node metastasis (p=0.103). The overall survival rate in the
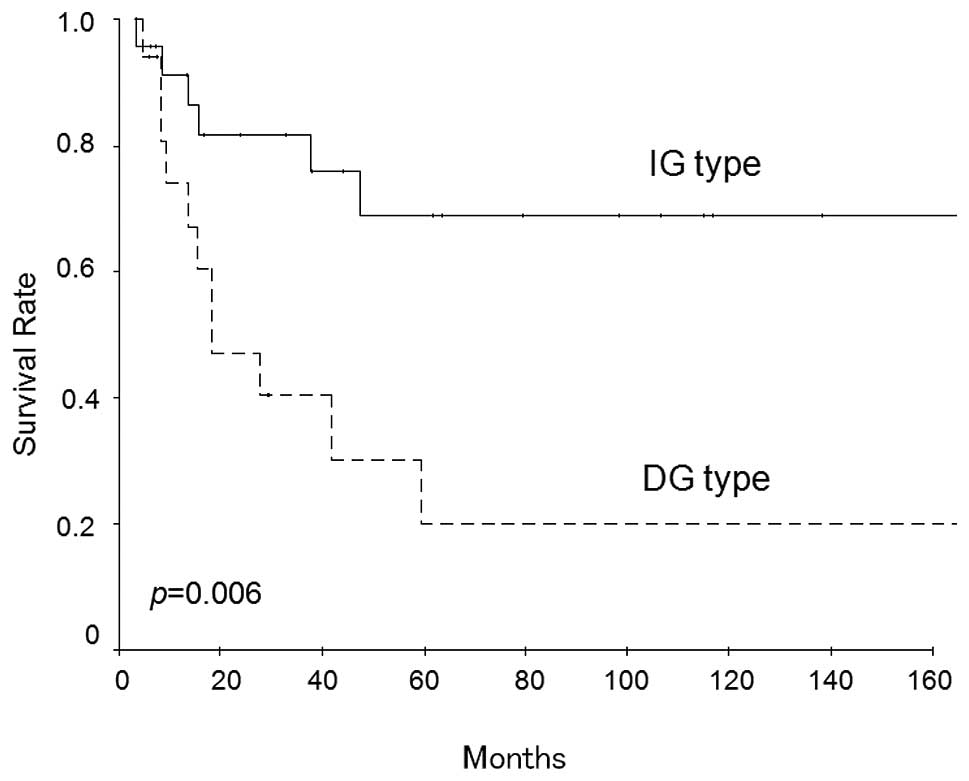
series was 48.7%. Fig. 2 shows the
survival curves of patients with each wall invasion pattern. The
cumulative 5-year survival rate of curative resection cases was
lower in patients with the DG type than in those with the IG type
(20.2 versus 68.9%, respectively, p=0.006, log-rank test).
 | Table IThe invasion pattern and
clinicopathological features of human subserosa-invasive
gallbladder cancer. |
Table I
The invasion pattern and
clinicopathological features of human subserosa-invasive
gallbladder cancer.
| | Invasion pattern | | |
|---|
| |
| | |
|---|
| Clinicopathological
features | No. of patients | IG | DG | Rate of DG pattern
(%) | p-value χ2
test |
|---|
| Histological
differentiation |
| Well, mod. | 36 | 23 | 13 | 36.1 | 0.030 |
| Poor, other | 6 | 1 | 5 | 83.3 | |
| Lymphatic
invasion |
| ly0, 1+ | 29 | 20 | 9 | 31.0 | 0.021 |
| ly2+, 3+ | 13 | 4 | 9 | 69.2 | |
| Venous invasion |
| v0, 1+ | 27 | 19 | 8 | 29.6 | 0.020 |
| v2+, 3+ | 15 | 5 | 10 | 66.7 | |
| Nodal status |
| pN0, 1 | 33 | 21 | 12 | 36.4 | 0.103 |
| pN2 | 9 | 3 | 6 | 66.7 | |
| Neural invasion |
| ne0, 1+ | 27 | 18 | 9 | 33.3 | 0.094 |
| ne2+, 3+ | 15 | 6 | 9 | 60.0 | |
| Subserosal
infiltration |
| INFα, β | 29 | 22 | 7 | 24.1 | <0.001 |
| INFγ | 13 | 2 | 11 | 84.6 | |
| Biliary invasion |
| binf0, 1 | 32 | 22 | 10 | 31.3 | 0.007 |
| binf2, 3 | 10 | 2 | 8 | 80.0 | |
| Overall | 42 | 24 | 18 | 42.9 | |
To define the significance of prognostic factors, a
Cox’s proportional hazard regression model was designed to assess
factors which were significant on univariate analysis. In this
model, the low degree of venous/perineural invasion (v0,1+/pn0,1+)
and the IG type of wall invasion pattern were associated with a
significant improvement in overall survival (Table II).
 | Table IICox’s proportional hazards model of
human subserosa-invasive gallbladder cancer. |
Table II
Cox’s proportional hazards model of
human subserosa-invasive gallbladder cancer.
| Factor | Risk ratio | p-value | 95% confidence
interval |
|---|
| Sex | 0.566 | 0.280 | 0.201–1.589 |
| Venous invasion | 0.154 | 0.042 | 0.025–0.931 |
| Perineural
invasion | 20.079 | 0.002 | 2.959–136.241 |
| Invasion pattern | 3.691 | 0.020 | 1.232–11.058 |
All the cases of subserosa-invasive GBC were
categorized into two groups in terms of the wall invasion pattern
and lymph node status, and their survival rates were compared using
the Kaplan-Meier method and log-rank test. The overall survival
rate in patients with the DG type and/or N2 metastasis (n=21) was
lower than those with the IG type and N0, 1 metastasis (n=21)
(p=0.0023, log-rank test) (Fig.
3).
Discussion
The radical resection of advanced GBC is sometimes
difficult because of frequent lymph node metastasis. In this study,
we reviewed 42 surgically resected cases of GBC to clarify the
relationship between the wall invasion pattern and
clinicopathological features, especially in subserosa-invasive GBC.
Lymphatic invasion, venous invasion, distant lymph node metastases,
poor differentiation, subserosal scirrhous infiltration (INFγ), and
biliary infiltration were more frequently detected in the DG type
cases, compared with the IG type cases. This is the first report to
describe the relationship between the wall invasion pattern and
clinicopathological features of subserosa-invasive GBC.
The layers of the gallbladder wall include the
surface epithelium, lamina propria, smooth muscle, perimuscular
subserosal connective tissue, and serosa, but they lack the
muscularis mucosae and submucosa. The smooth muscle layer consists
of loosely arranged bundles of muscle fibers, and is thin compared
with other parts of the digestive tract (14,15).
Therefore, GBCs can easily invade the subserosal layer through the
smooth muscle layer, and show frequent vascular permeation and
perineural invasion. Our previous study demonstrated that the wall
invasion pattern through the muscle layer is correlated with
histological aggressiveness and the survival rate of patients with
GBC. The cases in our previous study included not only pT2 GBCs
(subserosa-invasive GBCs) but also pT3-4 GBCs together. The bias
affected by the depth of tumor invasion in the afore-mentioned
clinicopathological relationship could not be excluded. In this
study, we clarified that our concept was adequate, according to the
greater significance at the same tumor invasion depth, i.e., the
subserosal layer is the critical depth both clinically and
histologically.
Most GBCs are adenocarcinomas that exhibit the
well-differentiated type in the mucosal layer whilst growing
laterally and superficially, but display the moderately to poorly
differentiated type in the gallbladder wall; therefore, advanced
GBCs usually show invasive growth with a desmoplastic reaction,
especially from the muscle to subserosal layer (16–22).
We propose that the DG type is associated with a more intensive
desmoplastic reaction than the IG type; i.e., DG and IG types
showed different wall invasion patterns throughout the muscle
layers, as well as different subserosal stromal desmoplastic
reactions of GBC.
Finally, we discuss the clinical applications of the
concept we demonstrated in this study. Surgeons try to perform a
potentially curative resection for advanced GBC. However, the true
benefits of these radical resections have not been completely
established because long-term survivors of advanced GBC are
limited. Radical surgery should improve not only survival in early
GBC, but should also promote long-term benefits in advanced GBC,
which shows high mortality and morbidity. Previous studies have
reported the importance of radical lymph node dissection for GBC,
and many surgeons have encountered cases in which lymph node
dissection improved survival. However, we have encountered cases
showing a poor subserosa-invasive GBC prognosis even after radical
surgery with lymph node dissection regardless of resection of the
other organs, such as the bile duct and liver (6–12). The
wall invasion pattern of GBC is easily diagnosed using ordinary
hematoxylin-eosin sections, and is applicable to intraoperatively
frozen sections. Our data clarified that the wall invasion pattern
was an independent predictor of survival in subserosa-invasive GBC,
i.e., cases of the DG type and/or N2 metastasis showed a
significantly poorer prognosis than those of the IG type and N0, 1
metastasis. Therefore, the wall invasion pattern could contribute
to decision-making concerning curative resection for advanced GBC
(4,5,23–25).
In conclusion, our study provided evidence to
support the concept of a wall invasion pattern in
subserosa-invasive GBC. The DG invasion pattern is an indicator of
a high malignant potential and indirectly worsens the prognosis of
patients with gallbladder adenocarcinoma. To reduce the mortality
rate after surgery, we can indicate cases with the IG type and N0,
1 metastasis for radical resection in subserosa-invasive GBC.
References
|
1
|
Okada K, Kijima H, Imaizumi T, Hirabayashi
K, Matsuyama M, Yazawa N, Oida Y, Dowaki S, Tobita K, Ohtani Y,
Tanaka M, Inokuchi S and Makuuchi H: Wall-invasion pattern
correlates with survival of patients with gallbladder
adenocarcinoma. Anticancer Res. 29:685–691. 2009.PubMed/NCBI
|
|
2
|
Okada K, Kijima H, Imaizumi T, Hirabayashi
K, Matsuyama M, Yazawa N, Oida Y, Dowaki S, Tobita K, Ohtani Y,
Tanaka M, Inokuchi S and Makuuchi H: Stromal laminin-5gamma2 chain
expression is associated with the wall-invasion pattern of
gallbladder adenocarcinoma. Biomed Res. 30:53–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kimura W, Nagai H, Kuroda A and Morioka Y:
Clinicopathologic study of asymptomatic gallbladder carcinoma found
at autopsy. Cancer. 64:98–103. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kokudo N, Makuuchi M, Natori T, Sakamoto
Y, Yamamoto J, Seki M, Noie T, Sugawara Y, Imamura H, Asahara S and
Ikari T: Strategies for surgical treatment of gallbladder carcinoma
based on information available before resection. Arch Surg.
138:741–750. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chijiiwa K, Nakano K, Ueda J, Noshiro H,
Nagai E, Yamaguchi K and Tanaka M: Surgical treatment of patients
with T2 gallbladder carcinoma invading the subserosal layer. J Am
Coll Surg. 192:600–607. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Aretxabala X, Roa I, Burgos L, Losada
H, Roa JC, Mora J, Hepp J, Leon J and Maluenda F: Gallbladder
cancer: an analysis of a series of 139 patients with invasion
restricted to the subserosal layer. J Gastrointest Surg.
10:186–192. 2006.PubMed/NCBI
|
|
7
|
Kosuge T, Sano K, Shimada K, Yamamoto J,
Yamasaki S and Makuuchi M: Should the bile duct be preserved or
removed in radical surgery for gallbladder cancer?
Hepatogastroenterology. 46:2133–2137. 1999.PubMed/NCBI
|
|
8
|
Shimizu Y, Ohtsuka M, Ito H, Kimura F,
Shimizu H, Togawa A, Yoshidome H, Kato A and Miyazaki M: Should the
extrahepatic bile duct be resected for locally advanced gallbladder
cancer? Surgery. 136:1012–1017. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsukada K, Kurosaki I, Uchida K, Shirai Y,
Oohashi Y, Yokoyama N, Watanabe H and Hatakeyama K: Lymph node
spread from carcinoma of the gallbladder. Cancer. 80:661–667. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagakura S, Shirai Y, Yokoyama N and
Hatakeyama K: Clinical significance of lymph node micrometastasis
in gallbladder carcinoma. Surgery. 129:704–713. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sasaki E, Nagino M, Ebata T, Oda K, Arai
T, Nishio H and Nimura Y: Immunohistochemically demonstrated lymph
node micrometastasis and prognosis in patients with gallbladder
carcinoma. Ann Surg. 244:99–105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shirai Y, Wakai T and Hatakeyama K:
Radical lymph node dissection for gallbladder cancer: indications
and limitations. Surg Oncol Clin North Am. 16:221–232. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Classification of gastric carcinoma - 2nd
English edition. Gastric Cancer. 1:10–24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Albores-Saavedra J, Henson DE and Sobin
LH: The WHO Histological Classification of Tumors of the
Gallbladder and Extrahepatic Bile Ducts. A commentary on the second
edition. Cancer. 70:410–414. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Albores-Saavedra J, Henson DE and Klimstra
DS: Tumors of the Gallbladder, Extrahepatic Bile Ducts and Ampulla
of Vater. Atlas of Tumor Pathology. 3rd Series, Fasc. 27. Armed
Forces Institute of Pathology; Washington, DC: pp. 37–111. 2000
|
|
16
|
Nishime C, Ohnishi Y, Suemizu H, Tamaoki
N, Suematsu M, Oida Y, Yamazaki H, Nakamura M, Ueyama Y and Kijima
H: Gallbladder small cell carcinoma Xenograft established by serial
transplantation in nude mice. Anticancer Res. 26:79–83.
2006.PubMed/NCBI
|
|
17
|
Kashiwagi H, Kijima H, Dowaki S, Ohtani Y,
Tobita K, Yamazaki H, Nakamura M, Ueyama Y, Tanaka M, Inokuchi S,
et al: Clinicopathological significance of sialyl Lex expression in
human gallbladder carcinoma. Oncol Rep. 11:1139–1143.
2004.PubMed/NCBI
|
|
18
|
Kashiwagi H, Kijima H, Dowaki S, Ohtani Y,
Tobita K, Yamazaki H, Nakamura M, Ueyama Y, Tanaka M, Inokuchi S
and Makuuchi H: MUC1 and MUC2 expression in human gallbladder
carcinoma: a clinicopathological study and relationship with
prognosis. Oncol Rep. 8:485–489. 2001.PubMed/NCBI
|
|
19
|
Kijima H, Kashiwagi H, Dowaki S, Ohtani Y,
Tobita K, Matsubayasi H, Ajioka Y, Watanabe H, Tsuchida T, Yamazaki
H, Nakamura M, Ueyama Y, Tanaka M and Makuuchi H: Stromal sialyl
Le(a) expression is correlated with vascular invasion of human
gallbladder adenocarcinoma. Int J Oncol. 17:55–60. 2000.PubMed/NCBI
|
|
20
|
Kashiwagi H, Kijima H, Dowaki S, Ohtani Y,
Tobita K, Tsukui M, Tanaka Y, Matsubayasi H, Tsuchida T, Yamazaki
H, Nakamura M, Ueyama Y, Tanaka M, Tajima T and Makuuchi H: DF3
expression in human gallbladder carcinoma: significance for
lymphatic invasion. Int J Oncol. 16:455–459. 2000.PubMed/NCBI
|
|
21
|
Dowaki S, Kijima H, Kashiwagi H, Ohtani Y,
Tobita K, Tsukui M, Tanaka Y, Tazawa K, Matsubayashi H, Tsuchida T,
et al: CEA immunohistochemical localization is correlated with
growth and metastasis of human gallbladder carcinoma. Int J Oncol.
16:49–53. 2000.PubMed/NCBI
|
|
22
|
Ohtani Y, Kijima H, Dowaki S, Kashiwagi H,
Tobita K, Tsukui M, Tanaka Y, Tsuchida T, Tokunaga T, Yamazaki H,
et al: Stromal expression of thrombospondin-1 is correlated with
growth and metastasis of human gallbladder carcinoma. Int J Oncol.
15:453–457. 1999.PubMed/NCBI
|
|
23
|
Nakata T, Kobayashi A, Miwa S, Soeda J and
Miyagawa S: Impact of tumor spread to the cystic duct on the
prognosis of patients with gallbladder carcinoma. World J Surg.
31:155–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaneoka Y, Yamaguchi A, Isogai M, Harada T
and Suzuki M: Hepatoduodenal ligament invasion by gallbladder
carcinoma: histologic patterns and surgical recommendation. World J
Surg. 27:260–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kayahara M and Nagakawa T: Recent trends
of gallbladder cancer in Japan: an analysis of 4770 patients.
Cancer. 110:572–580. 2007. View Article : Google Scholar : PubMed/NCBI
|