Introduction
Liver cancer in men is the fifth most frequently
diagnosed cancer worldwide but the second most frequent cause of
cancer death. In women, it is the seventh most commonly diagnosed
cancer and the sixth leading cause of cancer death. An estimated
748,300 new liver cancer cases and 695,900 cancer deaths occurred
worldwide in 2008 (1). Half of
these cases and deaths are estimated to occur in China (2). Liver cancer is also a genetic disease
developing from a multi-step process. Single or multiple mutations
in genes related to growth control, invasion and metastasis form
the molecular genetic basis of malignant transformation and tumor
progression (3). Therefore,
identification of key genes and targets in signaling pathways
related to tumorigenesis is indispensible for the diagnosis and
prevention of liver cancer.
High mobility group box 1 (HMGB1) is a DNA-binding
nuclear protein, released actively following cytokine stimulation
as well as passively during cell death. It is the prototypic
damage-associated molecular pattern (DAMP) molecule and has been
implicated in several inflammatory disorders (4). HMGB1 interacts with its receptors RAGE
and TLR that belong to family of pattern recognition receptors and
involves in activation of pathways leading to production of
pro-inflammatory cytokines, forming a positive feedback circuit of
inflammation. Serum HMGB-1 increases after major gastrointestinal
surgery, and its peak levels correlate with the duration of SIRS
and postoperative pulmonary dysfunction. It has been revealed in
mediation of sepsis and represents a potential target in therapy of
various disorders related to inflammation (5–7).
Moreover, HMGB1 is a nuclear protein that binds to a number of
molecules related to cancer and associates with each of the
hallmarks of cancer including unlimited replicative potential,
ability to develop angiogenesis, evasion of apoptosis,
self-sufficiency in growth signals, insensitivity to inhibitors of
growth, inflammation, tissue invasion and metastasis (8,9). It
has been reported that HMGB1 and its receptor RAGE are expressed in
many malignant tumors (10).
Increased expression of HMGB1 is associated with progression and
poor prognosis in human nasopharyngeal carcinoma (11). It is also a useful serological
biomarker for early diagnosis as well as evaluating the
tumorigenesis, stage, and prognosis of gastric cancer (12). HMGB1 protein may contribute to the
malignant progression of head and neck cell carcinoma, and present
as a novel prognostic marker and a potential therapeutic target for
cancer (13).
HMGB1 pathway plays an important role in the
metastasis of multiple cancers. They are closely associated with
metastasis of squamous cell carcinoma (14). HMGB1 secreted from primary tumors
spread to the regional lymph nodes decreases the number of
macrophages to attenuate the anti-metastatic defense of the lymph
nodes in patients with colorectal cancer (15,16).
It can activate TLR4 and RAGE signaling pathways to induce
caspase-1 with subsequent production of many inflammatory mediators
which in turn promotes cancer invasion and metastasis (17). Hypoxia-increased RAGE expression
also regulates tumor cell invasion and metastasis through
activation of Erk1/2, AKT and nuclear translocation of NF-κB
(18). Targeting HMGB1 inhibits
ovarian cancer growth and metastasis by RNA interference (RNAI),
Therefore, HMGB1 is a newly identified gene associated with cancer
growth and metastasis, representing a new therapeutic target for
the treatment of cancer (19).
Recently, it has been shown that reduced HMGB1
expression induced by RNAI inhibits the bioactivity of hepatic
carcinoma cells (20). However, the
expression and clinical significance of HMGB1 in liver cancer,
especially the molecular mechanisms of HMGB1 in tumorigenesis of
liver cancer have rarely been reported. In the present study, human
liver cancer and normal liver tissues were collected. The
expression of HMGB1 was assessed using RT-PCR and western blot
assays in biopsy samples. Using a loss of function approach, we
investigated in vitro and in vivo the role of HMGB1
signaling pathway in growth and metastasis of liver cancer, and
attempted to find a promising therapeutic target for liver
cancer.
Materials and methods
Materials
SMMC-7721 cell line used in the experiment was from
Institute of Biochemistry and Cell Biology (Shanghai, China);
six-week-old female immune-deficient nude mice (BALB/c-nu) were
purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai
Laboratory Animal Center of Chinese Academy Sciences).
Adenovirus-mediated HMGB1 small hairpin RNA vector, negative
control vector and virion-packaging elements were from Genechem
(Shanghai, China). The primers of HMGB1, p-AKT, Ki-67 and MMP-2
were synthesized by ABI Co., Ltd. (USA). All antibodies were from
Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Drugs and reagents
3-(4,5)-Dimethylthiahiazo(-z-yl)-3,5-di-phenytetrazolium
bromide (MTT) was from Dingguo biology (Shanghai, China);
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum
(FBS) were from Thermo Fisher Scientific Inc. (Waltham, MA, USA);
TRIzol Reagent and Lipofectamine 2000 were from Invitrogen
(Carlsbad, CA, USA); M-MLV Reverse Transcriptase was from Promega
(Madison, WI, USA); SYBR Green Master Mix was from Takara (Otsu,
Japan); Cell cycle analysis kit and apoptosis kit (Propidium Iodide
(PI), RNase A, Annexin V-FITC) were from KeyGen Biology (Nanjing,
China). ECL-PLUS/kit was from GE Healthcare (Piscataway, NJ,
USA).
Tissue samples
Forty freshly resected liver cancer and normal liver
tissue samples were collected at the Department of General Surgery
of Shanghai Rui Jin Hospital during 2010 and were classified
according to American Joint Committee on Cancer (AJCC) TNM staging
system. Tissues and clinical information were obtained as part of
an approved study at Shanghai Jiao Tong University School of
Medicine. There were 30 cases of liver cancer tissues and 10 cases
of normal liver tissues. A portion of each tissue sample was stored
in liquid nitrogen for RT-PCR and western blot examination. All
tumors and normal tissues were diagnosed by two independent
gastroenterologists.
RT-PCR
To quantitatively determine the mRNA expression
level of HMGB1, p-AKT, Ki-67 and MMP-2 in liver cancer, normal
liver tissues and SMMC-7721 cells, RT-PCR was used. Total RNA of
each clone was extracted with TRIzol according to the
manufacturer’s protocol. Reverse-transcription was carried out
using M-MLV and cDNA amplification was carried out using SYBR Green
Master Mix kit according to the manufacturer’s protocol. The genes
were amplified using specific oligonucleotide primer and human
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as
an endogenous control. The PCR primer sequences were: HMGB1, 5′-ATA
TGGCAAAAGCGGACAAG-3′ and 5′-AGGCCAGGATGTT CTCCTTT-3′; p-AKT,
5′-GGAGAUCAUGCAGCAUCGC dtdt-3′ and 5′-GCGAUGCUGCAUGAUCUCCdtdt-3′;
Ki-67, 5′-CTTTGGGTGCGACTTGACG-3′ and 5′-GTCGACCC CGCTCCTTTT-3′;
MMP-2, 5′-GGCCCTGTCACTCCTGA GAT-3′ and 5′-GGCATCCAGGTTATCGGGGA-3′;
GAPDH, 5′-CAACGAATTTGGCTACAGCA-3′ and 5′-AGGGGTCTA CATGGCAACTG-3′.
Data were analyzed using the comparative Ct method
(2−ΔΔCt). Three separate experiments were performed for
each clone.
Western blot assay
Liver cancer, normal liver tissues and SMMC-7721
cells were harvested and extracted using lysis buffer (Tris-HCl,
SDS, mercaptoethanol, glycerol). Cell extracts were boiled for 5
min in loading buffer and then equal amount of cell extracts were
separated on 15% SDS-PAGE gels. Separated protein bands were
transferred into polyvinylidene fluoride (PVDF) membranes and the
membranes were blocked in 5% skim milk powder. The primary
antibodies against HMGB1, p-AKT, Ki-67 and MMP-2 were diluted
according to the instructions of antibodies and incubated overnight
at 4°C. Then, horseradish peroxidase-linked secondary antibodies
were added at a dilution ratio of 1:1000, and incubated at room
temperature for 2 h. The membranes were washed with PBS for three
times and the immunoreactive bands were visualized using
ECL-PLUS/Kit according to the manufacturer’s instructions. The
relative protein level in different cell lines was normalized to
GAPDH concentration. Three separate experiments were performed for
each clone.
Cell culture and adenovirus
transfection
SMMC-7721 cells were cultured in DMEM medium
supplemented with 10% heat-inactivated FBS, 100 μg/ml of penicillin
and 100 μg/ml of streptomycin. They were all placed in a humidified
atmosphere containing 5% CO2 at 37°C. Recombinant
adenovirus vector rAd5-HMGB1 and negative control rAd5-GFP were
transfected into SMMC-7721 cells. Cells were subcultured at a 1:5
dilution in 300 μg/ml G418-containing medium. Positive stable
transfectants were selected and expanded for further study. The
clone in which the rAd5-HMGB1 virus vectors transfected was named
as rAd5-HMGB1 group, the negative control vectors transfected was
named as GFP group and SMMC-7721 cells were named as CON group.
Cell proliferation assay
Cell proliferation was analyzed with the MTT assay.
Briefly, cells infected with rAd5-HMGB1 were incubated in
96-well-plates at a density of 1×105 cells per well with
DEME medium supplemented with 10% FBS. Cells were treated with 20
μl MTT dye at 0, 24, 48, 72 h and then incubated with 150 μl of
DMSO for 5 min. The color reaction was measured at 570 nm with
enzyme immunoassay analyzer (Bio-Rad, Hercules, CA, USA). The
proliferation activity was calculated for each clone.
Transwell invasion assay
Transwell filters were coated with matrigel (3.9
μg/μl, 60–80 μl) on the upper surface of a polycarbonic membrane
(diameter 6.5 mm, pore size 8 μm). After incubating at 37°C for 30
min, the matrigel solidified and served as the extracellular matrix
for analysis of tumor cell invasion. Harvested cells
(1×105) in 100 μl of serum-free DMEM were added into the
upper compartment of the chamber. A total of 200 μl conditioned
medium derived from NIH3T3 cells was used as a source of
chemoattractant, and was placed in the bottom compartment of the
chamber. After 24 h of incubation at 37°C with 5% CO2,
the medium was removed from the upper chamber. The non-invaded
cells on the upper side of the chamber were scraped off with a
cotton swab. The cells that had migrated from the matrigel into the
pores of the inserted filter were fixed with 100% methanol, stained
with hematoxylin, and mounted and dried at 80°C for 30 min. The
number of cells invading through the matrigel was counted in three
randomly selected visual fields from the central and peripheral
portion of the filter using an inverted microscope (magnification,
×200). Each assay was repeated three times.
Cell apoptosis analysis
To detect cell apoptosis, cells infected with
rAd5-HMGB1 were trypsinized, washed with cold PBS and resuspended
in binding buffer according to the instruction of the apoptosis
kit. FITC-Annexin V and PI were added to the fixed cells for 20 min
in darkness at room temperature. Then, Annexin V binding buffer was
added to the mixture before the fluorescence was measured on
FACSort flow cytometer. The cell apoptosis was analyzed using the
CellQuest software (Becton Dickinson, USA). Three separate
experiments were performed for each clone.
Cell cycle analysis
To detect cell cycle variation, cells infected with
rAd5-HMGB1 were trypsinized, washed by PBS and fixed with 80% cold
ethanol overnight at −20°C. After PBS washing, the fixed cells were
stained with PI in the presence of RNase A for 30 min at room
temperature in darkness. Each sample was filtered through a 50 μm
nylon filter to obtain single-cell suspension. The samples were
then analyzed on FACsort flow cytometer (Becton Dickinson, Mountain
View, CA, USA). ModFit3.0 software (Verity Software House, Topsham,
ME, USA) was used for cell cycle analysis. Three separate
experiments were performed for each clone.
In vivo tumor xenograft studies
Two mice were injected subcutaneously with
1×108 SMMC-7721 cells in 50 μl of PBS pre-mixed with an
equal volume of matrigel matrix (Becton Dickinson). Mice were
monitored daily, and two mice developed subcutaneous tumors. When
the tumor size reached ~5 mm in length, they were surgically
removed, cut into 1–2 mm3 pieces, and re-seeded
individually into 12 other mice. When tumor size reached ~5 mm in
length, the mice were randomly assigned to control (CON), GFP and
rAd5-HMGB1 groups. In GFP and rAd5-HMGB1 groups, 15 μl of
adenovirus was injected into subcutaneous tumors using a multi-site
injection format. Mice in CON group received 15 μl of PBS only.
Injections were repeated on the third day after initial treatment.
The tumor volume every three days was measured with a caliper,
using the formula volume = (length × width)2/2.
Statistical analysis
The result of each experiment was shown as mean ± SD
when applicable. Statistically significant difference in each assay
was determined by SPSS version 11.5. Difference in each group was
tested for significance using t-test and ANOVA analysis of
variance. P<0.05 was considered significant.
Results
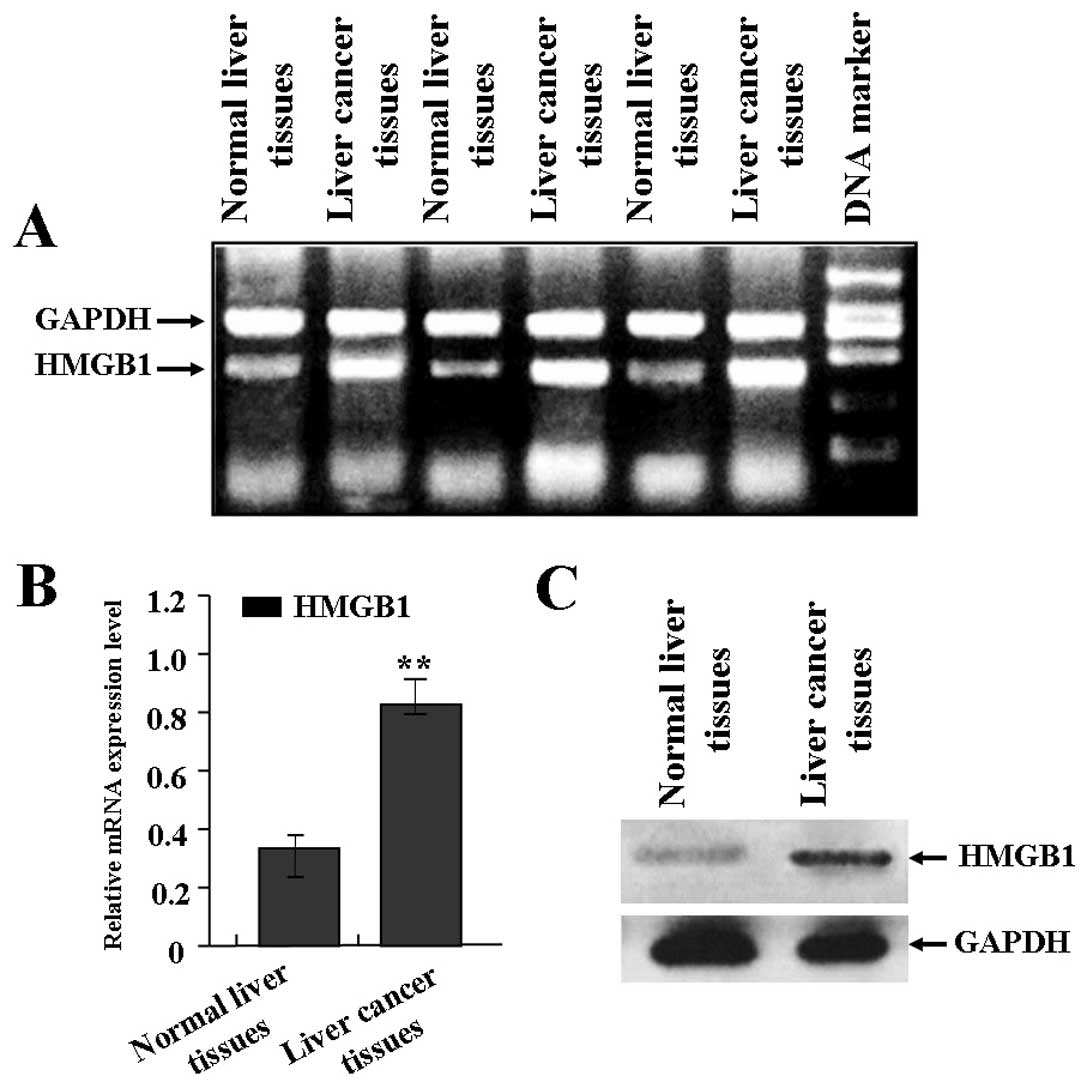
Expression of HMGB1 in human liver
cancer
The expression of HMGB1 in liver cancer was
evaluated using RT-PCR and western blot assays. As shown in
Fig. 1A and B, the mRNA expression
level of HMGB1 was significantly increased in liver cancer tissues
in comparison with normal liver tissues. Also, as shown in Fig. 1C, the protein expression level of
HMGB1 was also significantly increased in liver cancer tissues
compared with the normal liver tissues.
Correlation of HMGB1 mRNA expression with
the clinicopathologic characteristics
The relationship between HMGB1 mRNA expression and
various clinical and pathologic features was analyzed. As shown in
Table I, no significant correlation
was found between HMGB1 expression with age, pathological type and
classification, and serum AFP levels. However, the significant
correlation was found between HMGB1 expression with TNM,
pathological grade and distant metastasis of liver cancer.
 | Table IThe correlation of HMGB1 expression
with clinico-pathologic characteristics of human liver cancer. |
Table I
The correlation of HMGB1 expression
with clinico-pathologic characteristics of human liver cancer.
| Clinicopathological
factors | n | HMGB1 mRNA expression
(mean ± SD) | P |
|---|
| Age |
| >60 | 11 | 0.82±0.35 | |
| ≤60 | 19 | 0.84±0.19 | >0.05 |
| Pathological
grade |
| I+II | 9 | 0.49±0.16 | |
| III+IV | 21 | 0.92±0.55 | <0.01 |
| Pathological
type |
| Massive type | 22 | 0.81±0.24 | |
| Nodular type | 8 | 0.84±0.32 | >0.05 |
| AFP |
| ≤400 μg/l | 9 | 0.80±0.17 | |
| >400 μg/l | 21 | 0.83±0.26 | >0.05 |
| Cirrhosis |
| Yes | 20 | 0.78±0.13 | |
| No | 10 | 0.81±0.23 | >0.05 |
| Distant
metastasis |
| Yes | 11 | 0.94±0.33 | |
| No | 19 | 0.68±0.15 | <0.01 |
Suppression of HMGB1 and p-AKT expression
by rAd5-HMGB1 in SMMC-7721 cells
In order to efficiently knockdown the expression of
HMGB1 in liver cancer SMMC-7721 cells, an adenovirus-mediated RNAI
approach was used to construct the rAd5-HMGB1 vector. In pilot
studies, the transfection efficiency of rAd5-HMGB1 (MOI=100) in
SMMC-7721 cells was greater than 85.0%. After rAd5-HMGB1 was
transfected into SMMC-7721 cells, real-time PCR and western blot
assays were performed at 48 h recovery to measure HMGB1 and p-AKT
expression. As shown in Fig. 2A and
B, an obvious inhibition of HMGB1 and p-AKT expression was
observed in rAd5-HMGB1 group compared with the GFP group and CON
group.
Suppression of liver cancer cell
proliferation by rAd5-HMGB1
Deregulated cell proliferation is a hallmark of
cancer (21). In order to test the
effect of rAd5-HMGB1 on liver cancer cell proliferation, we
investigated the proliferative activities of SMMC-7721 cells by
MTT. As a result, it was found that knockdown of HMGB1 could
significantly reduce the proliferative activities of SMMC-7721
cells in a time-dependent manner compared with GFP group and CON
group (Fig. 3A). In addition, Ki-67
is at the very heart of many essential cellular processes and
determines the tumor progression and the outcome of anticancer
treatment. To determine whether knockdown of HMGB1 suppressed
endogenous Ki-67 through translational repression, the expression
of Ki-67 was examined by Real-time PCR and western blot assays. It
was shown that the amount of Ki-67 expression was decreased in
rAd5-HMGB1 group compared with GFP group and CON group (Fig. 3B and C), suggesting that knockdown
of HMGB1 inhibits liver cancer cell proliferation through
down-regulation of the Ki-67 expression.
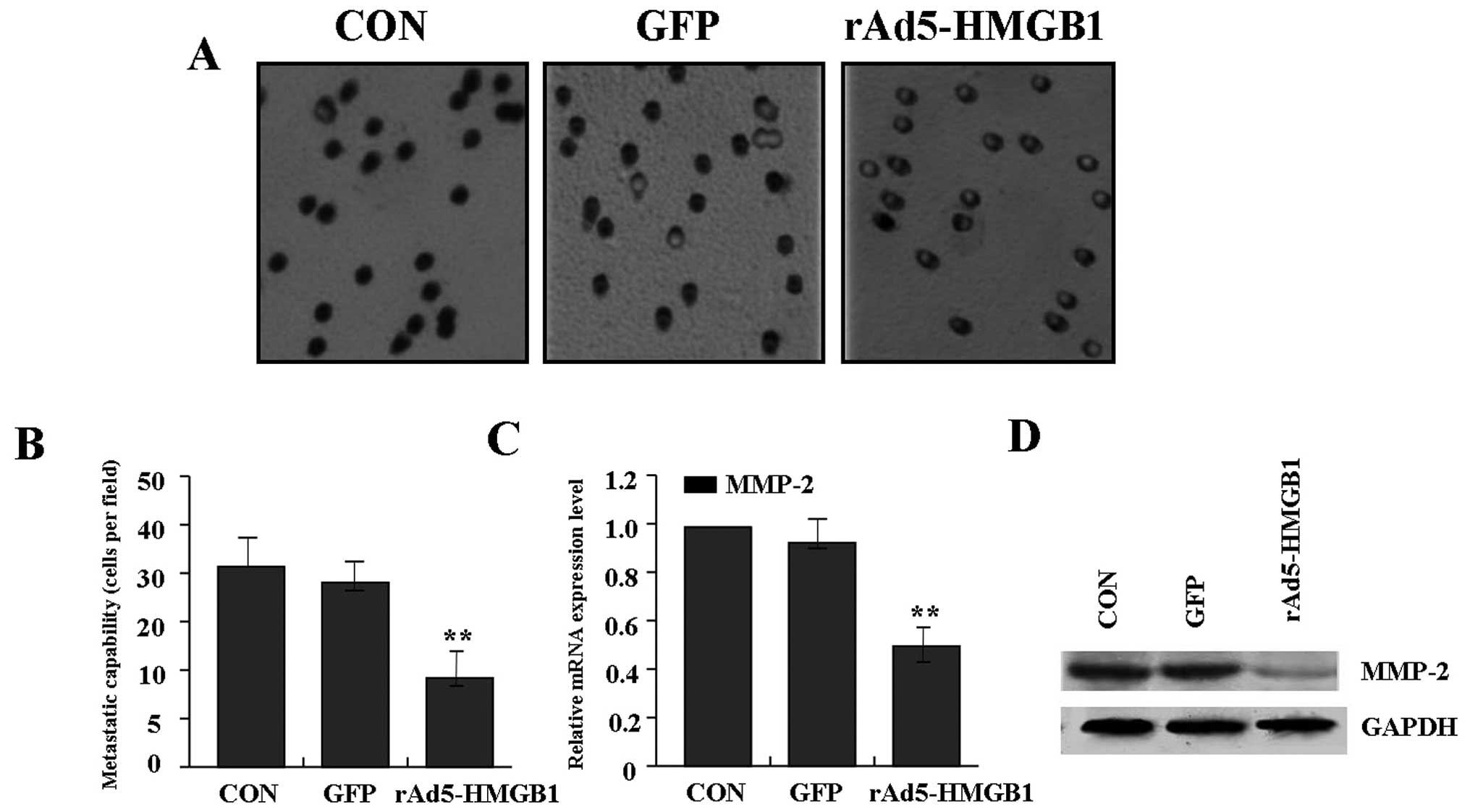
Suppression of liver cancer cell
metastasis by rAd5-HMGB1
To determine the effect of rAd5-HMGB1 on liver
cancer cell metastasis, transwell assay was carried out. The
results indicated that the invasive and metastatic potential was
determined on the basis of the ability of cells to invade a matrix
barrier containing laminin and type IV collagen, the major
components of the basement membrane. Representative micrographs of
Transwell filters can be seen in Fig.
4A. The invasive capability of SMMC-7721 cells was distinctly
decreased in rAd5-HMGB1 group compared with GFP group and CON group
(Fig. 4B). In terms of the
important role of MMP-2 in tumor metastasis, Real-time PCR and
western blot assays were performed to investigate the effect of
rAd5-HMGB1 on expression of MMP-2. As shown in Fig. 4C and D, the expression of MMP-2 was
significantly reduced in rAd5-HMGB1 group compared with GFP group
and CON group, indicating that knockdown of HMGB1 inhibits liver
cancer cell metastasis through down-regulation of the MMP-2
expression.
Induction of liver cancer cell apoptosis
and cycle arrest by rAd5-HMGB1
To determine whether knockdown of HMGB1 affected
SMMC-7721 cell apoptosis and cycle distribution, flow cytometry
with PI/FITC-Annexin V staining was performed. The results showed
that the apoptotic index of SMMC-7721 cells in rAd5-HMGB1 group was
markedly higher than the GFP and CON groups (Fig. 5A and C). The cycle distribution of
SMMC-7721 cells was also analyzed and cell cycle kinetics showed
that the G0/G1 phase fraction was increased,
S-phase fraction was decreased and cell cycle was arrested in
G0/G1 phase in rAd5-HMGB1 group compared with
GFP and CON groups (Fig. 5B and D).
Therefore, knockdown of HMGB1 can induce liver cancer cell
apoptosis and block cell cycle progression.
Suppression of xenograft tumor growth by
rAd5-HMGB1
Our in vitro experiments demonstrated that
knockdown of HMGB1 efficiently inhibited the growth and metastasis
of SMMC-7721 cells. Therefore, it is necessary to further
investigate the effect of knockdown of HMGB1 on xenograft tumor
growth in vivo. The mean volume of tumors in all
experimental mice before treatment was 67.01±14.38 mm3.
During the whole tumor growth period, the tumor growth activity was
measured. Tumors treated with rAd5-HMGB1 grew relatively slowly
compared with the CON and GFP groups (Fig. 6A and B). When the tumors were
harvested, the average weight of tumors in group rAd5-HMGB1 was
much less than that of the CON and GFP groups, respectively
(Fig. 6C). This result in
vivo indicated that knockdown of HMGB1 inhibited liver cancer
cell growth.
Discussion
HMGB1 pathway is closely associated with
tumorigenesis, expansion and invasion of multiple cancers, and
plays a critical role in the development and progression of many
malignant tumors. HMGB1 promotes the development and progression of
renal cell carcinoma via ERK1/2 activation, which is partially
mediated by RAGE (22). RAGE as a
pattern recognition receptor binds HMGB1 and is involved in cancer
development, progression, and metastasis (23). Also, HMGB1 may contribute to the
progression of cancer via modulation of the local immune response
and promote activity of regulatory T cells in cancer patients
(24,25). Thus, HMGB1 targeting is a potential
therapeutic strategy against cancer development, progression, and
especially metastasis. Technical breakthroughs in application of
HMGB1 targeting to human diseases are now urgently required
(23).
Of note, some studies have found a correlation
between serum HMGB1 and clinicopathological outcome of live cancer.
HMGB1 may be a useful marker for evaluating the tumor stage and
predicting prognosis in hepatocellular carcinoma. Targeting HMGB1
may be a potential approach for carcinoma treatment (26). In our study, it was found that HMGB1
expression was significantly increased in live cancer compared with
normal live tissues, and it was also closely correlated with
pathological grade and distant metastasis, representing an
important therapeutic target for liver cancer.
Reduced HMGB1 expression induced by RNAI inhibits
the bioactivity of hepatic carcinoma cells (20). Ethyl pyruvate is a potent inhibitor
of HMGB1 release and has a therapeutic role in the treatment of
cancer in conjunction with other therapeutic agents (27). To study the molecular mechanisms of
HMGB1 in liver growth and metastasis, in our study, a loss of
function experiment showed that knockdown of HMGB1 gene by
adenovirus-mediated RNAI inhibited the proliferative activities and
metastatic potential, induced cell apoptosis and cycle arrest, and
slowed xenograft tumor growth in SMMC-7721 cells. Combined with the
correlation of HMGB1 with pathological grade and distant metastasis
of liver cancer, HMGB1 might be considered as a potential
therapeutic target for liver cancer.
Ki-67 is a nuclear protein that is expressed in
proliferating cells and may be required for maintaining cell
proliferation, used as a marker for cell proliferation of gastric
cancer (28). MMP-2 is thought to
be a key enzyme involved in the degradation of type IV collagen and
high level of MMP-2 in tissues is associated with tumor growth and
invasion (29). It has been
reported that blockade of AKT pathway by RNAI inhibits the growth
and metastasis of malignant tumors via negative regulation of
Ki-67, MMP-9 and positive regulation of tissue inhibitor of
metalloproteinase-2 (TIMP-2) and p53 (30,31).
Importantly, in our study, it was found that knockdown of HMGB1
down-regulated the expression of p-AKT, Ki-67 and MMP-2, suggesting
that targeted blockade HMGB1 pathway might inhibit liver cancer
growth and metastasis through AKT-mediated regulation of Ki-67 and
MMP-2 expression.
In summary, the expression of HMGB1 is closely
correlated with pathological grade and distant metastases of liver
cancer, and targeted blockade of HMGB1 pathway inhibits liver
cancer growth and metastasis in vitro and in vivo,
suggesting HMGB1 may be involved in liver cancer development and
progression through AKT-mediated regulation of Ki-67 and MMP-2
expression, and represent a potential therapeutic target for this
aggressive malignancy.
Acknowledgements
This work was funded by Chinese National Programs
for High Technology Research and Development (No. 31170938).
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Ferlay J, Shin HR, Bray F, et al:
Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int
J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tajima Y, Yamazaki K, Makino R, et al:
Gastric and intestinal phenotypic marker expression in early
differentiated-type tumors of the stomach: clinicopathologic
significance and genetic background. Clin Cancer Res. 12:6469–6479.
2006. View Article : Google Scholar
|
|
4
|
Sims GP, Rowe DC, Rietdijk ST, et al:
HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol.
28:367–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okada H, Imai M, Ono F, et al: Novel
complementary peptides to target molecules. Anticancer Res.
31:2511–2516. 2011.PubMed/NCBI
|
|
6
|
Takahata R, Ono S, Tsujimoto H, et al:
Postoperative serum concentrations of high mobility group box
chromosomal protein-1 correlates to the duration of SIRS and
pulmonary dysfunction following gastrointestinal surgery. J Surg
Res. 170:e135–e140. 2011. View Article : Google Scholar
|
|
7
|
Naglova H and Bucova M: HMGB1 and its
physiological and pathological roles. Bratisl Lek Listy.
113:163–171. 2012.PubMed/NCBI
|
|
8
|
Lee H, Shin N, Song M, et al: Analysis of
nuclear high mobility group box 1 (HMGB1)-binding proteins in colon
cancer cells: clustering with proteins involved in secretion and
extranuclear function. J Proteome Res. 9:4661–4670. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang D, Kang R, Zeh HJ III and Lotze MT:
High-mobility group box 1 and cancer. Biochim Biophys Acta.
1799:131–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kostova N, Zlateva S, Ugrinova I and
Pasheva E: The expression of HMGB1 protein and its receptor RAGE in
human malignant tumors. Mol Cell Biochem. 337:251–258. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu D, Ding Y, Wang S, Zhang Q and Liu L:
Increased expression of high mobility group box 1 (HMGB1) is
associated with progression and poor prognosis in human
nasopharyngeal carcinoma. J Pathol. 216:167–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chung HW, Lee SG, Kim H, et al: Serum high
mobility group box-1 (HMGB1) is closely associated with the
clinical and pathologic features of gastric cancer. J Transl Med.
7:382009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Xie C, Zhang X, et al: Elevated
expression of HMGB1 in squamous-cell carcinoma of the head and neck
and its clinical significance. Eur J Cancer. 46:3007–3015. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi J, Lee MK, Oh KH, et al: Interaction
effect between the receptor for advanced glycation end products
(RAGE) and high-mobility group box-1 (HMGB-1) for the migration of
a squamous cell carcinoma cell line. Tumori. 97:196–202.
2011.PubMed/NCBI
|
|
15
|
Moriwaka Y, Luo Y, Ohmori H, et al: HMGB1
attenuates anti-metastatic defense of the lymph nodes in colorectal
cancer. Pathobiology. 77:17–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo Y, Ohmori H, Fujii K, et al: HMGB1
attenuates anti-metastatic defence of the liver in colorectal
cancer. Eur J Cancer. 46:791–799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan W, Chang Y, Liang X, et al: High
mobility group box 1 activates caspase-1 and promotes
hepatocellular carcinoma invasiveness and metastases. Hepatology.
55:1863–1875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tafani M, Schito L, Pellegrini L, et al:
Hypoxia-increased RAGE and P2X7R expression regulates tumor cell
invasion through phosphorylation of Erk1/2 and Akt and nuclear
translocation of NF-{kappa}B. Carcinogenesis. 32:1167–1175.
2011.PubMed/NCBI
|
|
19
|
Chen J, Liu X, Zhang J and Zhao Y:
Targeting HMGB1 inhibits ovarian cancer growth and metastasis by
lentivirus-mediated RNA interference. J Cell Physiol.
227:3629–3638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang W, Wang Z, Li X, et al: Reduced
high-mobility group box 1 expression induced by RNA interference
inhibits the bioactivity of hepatocellular carcinoma cell line
HCCLM3. Dig Dis Sci. 57:92–98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanahan D and Weinberg RA: The hallmarks
of cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin L, Zhong K, Sun Z, et al: Receptor for
advanced glycation end products (RAGE) partially mediates
HMGB1-ERKs activation in clear cell renal cell carcinoma. J Cancer
Res Clin Oncol. 138:11–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohmori H, Luo Y and Kuniyasu H:
Non-histone nuclear factor HMGB1 as a therapeutic target in
colorectal cancer. Expert Opin Ther Targets. 15:183–193. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng RQ, Wu XJ, Ding Y, et al:
Co-expression of nuclear and cytoplasmic HMGB1 is inversely
associated with infiltration of CD45RO+ T cells and
prognosis in patients with stage IIIB colon cancer. BMC Cancer.
10:4962010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wild CA, Brandau S, Lotfi R, et al: HMGB1
is overexpressed in tumor cells and promotes activity of regulatory
T cells in patients with head and neck cancer. Oral Oncol.
48:409–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng BQ, Jia CQ, Liu CT, et al: Serum
high mobility group box chromosomal protein 1 is associated with
clinicopathologic features in patients with hepatocellular
carcinoma. Dig Liver Dis. 40:446–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang X, Chavez AR, Schapiro NE, et al:
Ethyl pyruvate administration inhibits hepatic tumor growth. J
Leukoc Biol. 86:599–607. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Czyzewska J, Guzińska-Ustymowicz K, Lebelt
A, et al: Evaluation of proliferating markers Ki-67, PCNA in
gastric cancers. Rocz Akad Med Bialymst. 49(Suppl 1): 64–66.
2004.PubMed/NCBI
|
|
29
|
Cawston TE and Wilson AJ: Understanding
the role of tissue degrading enzymes and their inhibitors in
development and disease. Best Pract Res Clin Rheumatol.
20:983–1002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Semba S, Moriya T, Kimura W and Yamakawa
M: Phosphorylated Akt/PKB controls cell growth and apoptosis in
intraductal papillary-mucinous tumor and invasive ductal
adenocarcinoma of the pancreas. Pancreas. 26:250–257. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Zhang QY, Fu YC, et al:
Expression of p-Akt and COX-2 in gastric adenocarcinomas and
adenovirus mediated Akt1 and COX-2 ShRNA suppresses SGC-7901
gastric adenocarcinoma and U251 glioma cell growth in vitro and in
vivo. Technol Cancer Res Treat. 8:467–478. 2009. View Article : Google Scholar : PubMed/NCBI
|