Introduction
Skin cancer is one of the main causes of
cancer-related mortality worldwide and cutaneous melanoma is one of
the most aggressive forms of skin cancer in humans (1,2).
Although melanoma accounts for approximately 4% of all
dermatological cancers, >80% of deaths from skin cancer are
associated with melanoma. Furthermore, the ten-year survival rate
for patients with metastatic melanoma is <10% (3,4). The
cure rate for patients with melanoma has not achieved clinically
satisfactory response rates. Therefore, several studies have
focused on the discovery of novel therapeutic agents derived form
natural products for cancer prevention and therapy. The induction
of tumor cell apoptosis is one of the pursued strategies in
chemotherapy (5,6). In particular, molecules associated
with the regulation of apoptosis are clinically relevant targets
for chemical intervention (7,8).
Triptolide (TPL), a diterpenoid triepoxide, derived
from the herb Tripterygium wilfordii, has been used as a
herbal medicine in China for hundreds of years (9,10). TPL
has been shown to induce apoptosis in a number of cancer cells,
such as breast (11,12), lung (13), pancreatic (14,15)
and colon cancer (16,17). It has been reported that TPL-induced
apoptosis is associated with the death receptor and
mitochondrial-mediated pathways in leukemic (18–20)
and pancreatic cancer cells (14,15).
Recently, it was reported that TPL induced the apoptosis of
pancreatic cancer cells via the downregulation of decoy receptor 3
(DcR3) expression (15). TPL has
been shown to inhibit colon cancer cell proliferation by the
induction of G1 phase arrest through the upregulation of p21
(17). In our laboratory, we found
that TPL induced the apoptosis of NCI-H295 human adrenal cancer
cells through a mitochondrial-dependent pathway (21).
In the present study, A375.S2 human melanoma cells
were employed as a cell model to evaluate the in vitro
anti-melanoma potential of TPL. We found that the cytotoxic effect
of TPL on A375.S2 cell growth was associated with cell cycle arrest
at the S phase and the induction of apoptosis through the caspase-
and mitochondrial-dependent signaling pathways. The underlying
molecular mechanisms leading to these profound effects were also
investigated.
Materials and methods
Chemicals and reagents
TPL, dimethyl sulfoxide (DMSO), RNase A, Triton
X-100 and propidium iodide (PI) were purchased from Sigma Chemical
Co. (St. Louis, MO, USA). Minimum essential medium (MEM), fetal
bovine serum (FBS), L-glutamine penicillin-streptomycin and
trypsin-EDTA were purchased from Gibco/Invitrogen (Carlsbad, CA,
USA). The Annexin V-FITC apoptosis detection kit was from
Gibco/Invitrogen. 2,7-Dichlorodihydrofluorescein diacetate
(DCFH-DA) for determination of reactive oxygen species (ROS)
levels, DiOC6 for determination of mitochondrial
membrane potential (ΔΨm) and Fluo-3/AM for determination of
intracellular Ca2+ levels were purchased from
Oncoimmunin, Inc. (Gaithersburg, MD, USA).
Cell culture
The A375.S2 human malignant melanoma cell line was
purchased from the Food Industry Research and Development Institute
(Hsinchu, Taiwan). The A275.S2 cells were cultured in 75
cm2 tissue culture flasks with MEM supplemented with 10%
FBS (Gibco-BRL, Gaithersburg, MD, USA), penicillin-streptomycin
(100 U/ml penicillin and 100 μg/ml streptomycin) and 2 mM
L-glutamine and grown in a humidified atmosphere of air containing
5% CO2 at 37°C as previously described (22,23).
Cell morphological changes examinations,
viability and cell cycle distribution assays
The A375.S2 cells (2×105 cells/well) were
maintain in 12-well plates and incubated at 37°C for 24 h before
being treated with 0, 15, 20, 25, 30 and 35 nM of TPL for 24 and 48
h. DMSO (solvent; 0.5%) was used for the control regimen. Cells
were observed and photographed under a contrast phase microscope at
×400 magnification for the determination of morphological changes.
Cells were harvested by centrifugation at 1,000 × g for 5 min and
the cell pellets were then dissolved with 0.5 ml of PBS containing
5 μg/ml PI and viable cells were determined using a flow cytometer
(Becton-Dickinson, San Jose, CA, USA) as previously described
(13,14). Cells were then stained with PI/RNase
Staining Buffer (BD Biosciences Pharmingen, San Diego, CA, USA) and
analyzed for cell cycle distribution including sub-G1 phase using a
FACScan flow cytometer (Becton-Dickinson) as previously described
(22,23).
Annexin V-FITC/PI flow cytometric
analysis for apoptotic cell death
Following treatment with TPL for 48 h, the A375.S2
cells (2×105) were harvested and washed twice with
ice-cold PBS. The Annexin V-FITC apoptosis detection kit (Molecular
Probes, Eugene, OR, USA) was used for staining the
phosphatidylserine on the outside of the apoptotic cells. Briefly,
20 μl aliquots of Annexin V-FITC and 40 μl PI buffer were added to
400 μl of Annexin V-FITC binding buffer and after each treatment
the cells were incubated at room temperature for 15 min in the
dark. All samples were analyzed with fluorescence-activated cell
sorting (FACS; Becton-Dickinson) as described previously (24,25).
Determination of ROS production,
intracellular Ca2+ release and ΔΨm
A375.S2 cells (2×105 cells/well) were
placed onto 12-well plates and treated with 20 nM TPL for 0, 12, 24
and 48 h for ROS, Ca2+ and ΔΨm measurements.
Cells were harvested after each treatment then re-suspended in 500
μl of DCFH-DA (10 μM) for ROS (H2O2)
determination, re-suspended in 500 μl of Fluo-3/AM (2.5 μg/ml) for
intracellular Ca2+ concentrations and suspended in 500
μl of DiOC6 (4 μmol/l) for ΔΨm followed by
incubation at 37°C for 30 min. The cells were then analyzed by flow
cytometry as described previously (24,25).
Caspase-3, -8 and -9 activity assay
The A375.S2 cells (2×105 cells/well) were
maintained in 12-well plates for 24 h and then 0 and 20 nM of TPL
were individually added to the wells followed by incubation for 0,
12, 24 and 48 h. All cells were trypsinized, collected and
centrifuged and washed twice with PBS. All samples were
re-suspended in 50 μl of 10 μM substrate solution (CaspaLux
8-L1D2 for caspase-8,
CaspaLux9-M1D2 for caspase-9 and
PhiPhiLux-G1D1 for caspase-3) before being
incubated at 37°C for 60 min. All samples were washed with PBS and
analyzed by flow cytometry as described previously (26).
Western blot analysis for the
determination of apoptosis-associated proteins
The A375.S2 cells (1×106 cells/well) in
6-well plates were treated with 20 nM of TPL and then incubated for
0, 12, 24 and 48 h for the determination of proteins associated
with cell cycle arrest and apoptosis. Cells were harvested and
washed with cold PBS and then lysed with ice-cold lysis buffer
containing 50 mM HEPES (pH 7.7), 150 mM NaCl, 1 mM EDTA, 2.5 mM
EGTA, 1 mM DTT, 0.1% Tween-20, 10% (v/v) glycerol, 1 mM NaF,
protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim,
Germany) and phosphatase inhibitor cocktail (Sigma Chemical Co.).
The total proteins from each sample were quantified using the
Bio-Rad method. Each sample (50 μg protein) was resolved over 12%
sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE)
and transferred onto nitrocellulose membranes (Millipore,
Billerica, MA, USA). The blot was then soaked in blocking buffer
(5% non-fat dry milk/0.05% Tween-20 in 20 mM TBS at pH 7.6) at room
temperature for 1 h and then incubated with individual primary
monoclonal antibodies in blocking buffer at 4°C overnight, followed
by secondary antibody horseradish peroxidase conjugate and
detection by chemiluminescence and autoradiography using X-ray film
as described previously (26). To
ensure equal protein loading, each membrane was stripped and
re-probed with anti-β-actin antibody (24,26).
Dilutions of primary antibodies were 1:1,000 [antibodies specific
for caspase-9, -8, -3, cytochrome c, apoptosis-inducing
factor (AIF), endonuclease G (Endo G), Bcl-x, Bax, Fas, FasL and
glucose-regulated protein (GRP)78].
Statistical analysis
The quantitative data are presented as the means ±
SD. Statistical differences between the TPL-treated and control
samples were calculated using the Student’s t-test. A value of
P<0.05 was considered to indicate a statistically significant
difference. The results are representative of at least three
independent experiments (24,26).
Results
TPL induces cell morphological changes
and decreases the percentage of viable A375.S2 cells
In order to confirm the biological effects of TPL,
the A375.S2 cells were treated with various concentrations of TPL
for 24 and 48 h, and cell morphological changes and the percentage
of viable cells were determined. The results are shown in Fig. 1, indicating that TPL induced
morphological changes (Fig. 1A) and
decreased the percentage of viable cells in a
concentration-dependent manner (Fig.
1B).
TPL induces S phase arrest in A375.S2
cells
To investigate the inhibitory effect of TPL on cell
growth, we investigated the cell cycle distribution in A375.S2
cells by flow cytometry and the results are shown in Fig. 2. The number of cells in the G1, S,
G2/M and sub-G1 phase of the cell cycle showed that the A375.S2
cells accumulated in the S and Sub-G1 phases following exposure to
TPL, whereas the G1 cell population was decreased in a
concentration-dependent manner. Collectively, these observations
suggest that TPL causes S phase arrest and induces apoptosis in
A375.S2 cells.
TPL induces apoptosis in A375.S2
cells
To further confirm that TPL induces apoptosis in
A375.S2 cells, the cells were exposed to 20 nM of TPL for different
periods of time and then apoptosis was analyzed. The results are
shown in Fig. 3. The results
indicated that TPL induced apoptosis in time-dependent manner.
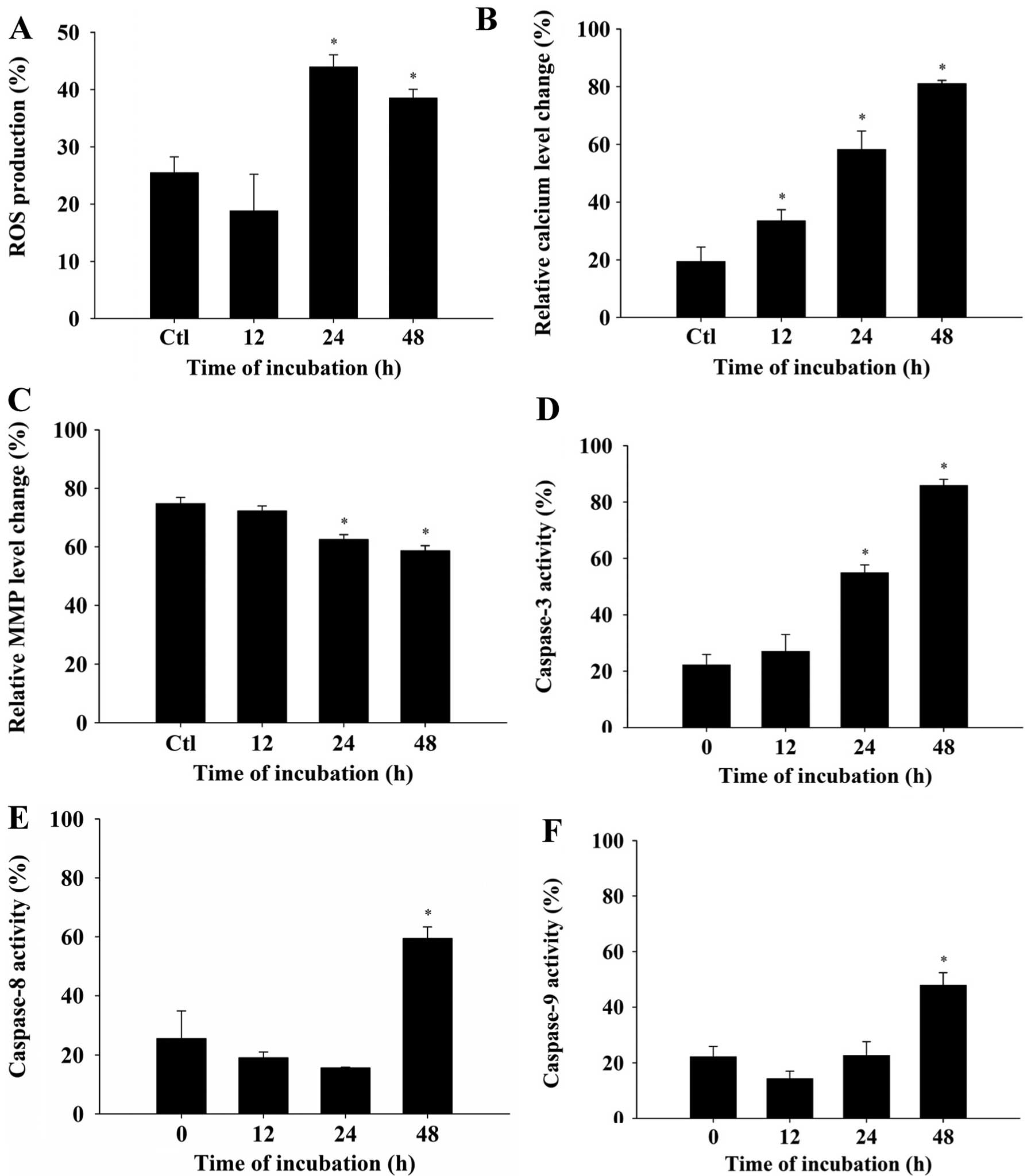
TPL promotes ROS production,
intracellular Ca2+ release and decreases ΔΨm in A375.S2
cells
To further investigate the role of ROS,
Ca2+ and the loss of ΔΨm in TPL-induced apoptosis
in A375.S2 cells, the cells were treated with 20 nM of TPL for
different periods of time. The harvested cells were analyzed for
ROS and Ca2+ production and the loss of ΔΨm. The
results are shown in Fig. 4A–C. The
results indicated that TPL promoted the production of ROS (Fig. 4A) and Ca2+ (Fig. 4B) but ΔΨm (Fig. 4C) in the A375.S2 cells. These
effects occurred in a time-dependent manner. These results show
that TPL-induced apoptosis in A375.S2 cells involves the production
of ROS and Ca2+ and the decrease in ΔΨm.
TPL promotes the activation of caspase-3,
-8 and -9 in A375.S2 cells
In order to investigate whether caspase-3, -8 and -9
are involved in TPL-induced apoptosis, the enzymatic activity of
caspases was detected using three foluorogenic peptide substrates
(CaspaLux 8-L1D2 for caspase-8,
CaspaLux9-M1D2 for caspase-9 and
PhiPhiLux-G1D1 for caspase-3). The results
are shown in Fig. 4C-E, indicating
that TPL induced a rapid increase in caspase-3, -8 and -9 activity.
The co-treatment of A375.S2 cells with pan-caspase inhibitor led to
a significant decrease in the activity of caspase-3, -8 and -9.
These findings show that TPL-induced apoptosis in A375.S2 cells
involves caspase activation.
TPL affects the cell cycle and
apoptosis-associated proteins in A375.S2 cells
In order to investigate whether the TPL-induced S
phase arrest and apoptosis involves apoptosis-associated proteins
in A375.S2 cells, the cells were treated with 20 nM TPL for 0, 12,
24 and 48 h and then examined by western blot analysis. The results
are shown in Fig. 5A–D, indicating
that TPL increased the levels of p21 and p27 but inhibited those of
CDK2, cyclin E and CDC25A (Fig.
5A), leading to S phase arrest. TPL promoted the expression of
GADD153, GRP78, caspase-4, IRE and calpain 1 (Fig. 5E), indicating that TPL induced
apoptosis through ER stress. Furthermore, TPL promoted the
expression of caspase-8, Fas and FasL (Fig. 5B and D), Bax and Bid, but inhibited
the levels of Bcl-2 (Fig. 5C).
However, it increased the levels of. cytochrome c, caspase-9
and -3, AIF and Endo G (Fig. 5B).
These findings show that TPL induces apoptosis via the caspase- and
mitochondrial-dependent pathway in A375.S2 cells.
 | Figure 5Triptolide affected the cell cycle
and apoptosis-associated proteins in A375.S2 cells. A total of
1×106 A375.S2 cells/ml cells in 6-well plate were
treated with 20 nM triptolide for 0, 12, 24 and 48 h. Cells were
harvested and apoptosis-associated proteins were measured by
western blot analysis. The protein levels of (A) p21, p27, CDK2,
cyclin E, cyclin A and CDC25A, (B) cytochrome c, caspase-9
and -3, AIF and Endo G, (C) Bax, Bad, Bcl-x, (D) caspase-8, Fas and
FasL, (E) GADD153, GRP78, caspase-4, IREα and β, calpain 1 and (F)
ERK1/2, JNK1/2 and p38 were examined using SDS-PAGE gel
electrophoresis and western blot analysis as described in Materials
and methods. |
Discussion
Previous studies have shown that melanomas are
resistant to conventional chemotherapy and metastasize to the
brain, lung, liver and skin (27,28).
Attention has been drawn to alternative options for the treatment
and prevention of cancer, including treatments derived from herbs.
Natural products for the treatment of cancer include antioxidants
and cancer preventative agents, or cancer therapeutic drugs
(29,30). TPL is one of the major active
compounds in Tripterygium wilfordii Hook F. extract, with
potent anti-inflammatory and anticancer activity (12,31,32).
In this study, we investigated Chinese herbal TPL for its effects
against human melanoma skin cancer cells in vitro. Our
results from flow cytometric analysis showed that TPL significantly
inhibited cell growth, inducing S phase arrest and apoptosis in
A375.S2 human melanoma cells (Figs.
1 and 2).
To our knowledge, our findings are the first to show
that TPL induces cytotoxic effects in human melanoma skin cancer
cells. These effects include cell morphological changes and the
decrease in the percentage of viable cells, as well as the
induction of S phase arrest and apoptosis (Figs. 1–3).
TPL also promoted caspase-8, -9 and -3 activity (Fig. 4D–F). We also used pan-caspase
inhibitor, which led to an increase in the percentage of viable
cells compared to the cells treated with TPL alone. These results
are in agreement with those from previous studies, demonstrating
that caspase-8 and -9 can be activated by TPL in pancreatic cancer
cells (15,33,34).
It is well known that the activation of caspase-8 and -9 involves
two different apoptotic pathways (22,35).
Caspase-8 involves the extrinsic pathway which is triggered by Fas
and FasL and caspase-9 involves the intrinsic pathway which is
triggered by the increase in the ratio of Bax/Bcl-2, leading to
mitochondrial dysfunction through the caspase-dependent pathway or
directly leading to the release of AIF and Endo G from the
mitochondria (mitochondrial-dependent pathway; also termed
caspase-dependent pathway) (22,35).
It has been reported that the TPL-induced apoptosis is related to
its ability to reduce DcR3 expression and increase FasL expression
(36,37). In this study, we found TPL induced
caspase-3, -8 and -9 activation (Fig.
4D–F) and increased the expression of Fas and FasL (Fig. 5D). These results further support the
notion that TPL is a potent activator of caspases in A375.S2
cells.
Our results also showed that TPL inhibited the
phosphorylation of ERK1/2 and JNK1/2 (Fig. 5F); however, it did not alter the
level of phosphorylation of MEK1 and MEK1/2. These results are in
agreement with those from previous studies, showing that TPL
inhibits the phosphorylation of ERK1/2 and affects ERK1/2
activation directly (17,38). Our findings also showed that TPL
promoted the expression of p21 and p27 in A375.S2 cells. It has
been reported that p21 is a well-characterized CDK inhibitor; high
levels of p21 can inhibit cyclin D1 expression, resulting in the
decline of pRb phosphorylation (39,40).
In this study, we did not observe any significant changes in the
A375.S2 cells following exposure to TPL. However, we did observe
the inhibition of cyclin A and CDC25A in the A375.S2 cells
following exposure to TPL, which may be the mechanism of action
behind the TPL-induced S phase arrest (Fig. 5A).
In conclusion, the results from the present study
demonstrated that the exposure of A375.S2 cells to TPL led to S
phase arrest and the induction of apoptosis (Fig. 6). TPL induced S phase arrest via the
promotion of p21 and p27 and the inhibition of cyclin A and CDC25A
expression. TPL induced the apoptosis of A375.S2 cells via Fas and
FasL, leading to the activation of caspase-dependent and
-independent signaling pathways. Apoptosis was also induced, in
part, through the ER stress pathways.
Acknowledgements
This study was supported by CMU-101-Asia-04 from the
China Medical University, Taichung, Taiwan, R.O.C.
References
|
1
|
Freedman DM, Dosemeci M and McGlynn K:
Sunlight and mortality from breast, ovarian, colon, prostate, and
non-melanoma skin cancer: a composite death certificate based
case-control study. Occup Environ Med. 59:257–262. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodriguez-Villanueva J and McDonnell TJ:
Induction of apoptotic cell death in non-melanoma skin cancer by
interferon-alpha. Int J Cancer. 61:110–114. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cromwell KD, Ross MI, Xing Y, et al:
Variability in melanoma post-treatment surveillance practices by
country and physician specialty: a systematic review. Melanoma Res.
22:376–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ascierto PA, Gogas HJ, Grob JJ, et al:
Adjuvant interferon alfa in malignant melanoma: An
interdisciplinary and multinational expert review. Crit Rev Oncol
Hematol. Aug 5–2012.(Epub ahead of print).
|
|
5
|
Chen GG and Lai PB: Role of apoptosis in
chemotherapy. Curr Drug Targets. 11:650–651. 2010.PubMed/NCBI
|
|
6
|
Guerriero JL, Ditsworth D, Fan Y, Zhao F,
Crawford HC and Zong WX: Chemotherapy induces tumor clearance
independent of apoptosis. Cancer Res. 68:9595–9600. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oberoi-Khanuja TK, Karreman C, Larisch S,
Rapp UR and Rajalingam K: Role of melanoma inhibitor of apoptosis
(ML-IAP) protein, a member of the baculoviral IAP repeat (BIR)
domain family, in the regulation of C-RAF kinase and cell
migration. J Biol Chem. 287:28445–28455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Günther C, Neumann H, Neurath MF and
Becker C: Apoptosis, necrosis and necroptosis: cell death
regulation in the intestinal epithelium. Gut. Jun 27–2012.(Epub
ahead of print).
|
|
9
|
Liu Y, Chen Y, Lamb JR and Tam PK:
Triptolide, a component of Chinese herbal medicine, modulates the
functional phenotype of dendritic cells. Transplantation.
84:1517–1526. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leuenroth SJ, Okuhara D, Shotwell JD, et
al: Triptolide is a traditional Chinese medicine-derived inhibitor
of polycystic kidney disease. Proc Natl Acad Sci USA.
104:4389–4394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan BJ, Tan BH and Chiu GN: Effect of
triptolide on focal adhesion kinase and survival in MCF-7 breast
cancer cells. Oncol Rep. 26:1315–1321. 2011.PubMed/NCBI
|
|
12
|
Messina ME Jr and Halaby R: Does
triptolide induce lysosomal-mediated apoptosis in human breast
cancer cells? Med Hypotheses. 77:91–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee KY, Park JS, Jee YK and Rosen GD:
Triptolide sensitizes lung cancer cells to TNF-related
apoptosis-inducing ligand (TRAIL)-induced apoptosis by inhibition
of NF-kappaB activation. Exp Mol Med. 34:462–468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu W, Li J, Wu S, et al: Triptolide
cooperates with Cisplatin to induce apoptosis in
gemcitabine-resistant pancreatic cancer. Pancreas. 41:1029–1038.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang W, Li X, Sun W, et al: Triptolide
triggers the apoptosis of pancreatic cancer cells via the
downregulation of Decoy receptor 3 expression. J Cancer Res Clin
Oncol. 138:1597–1605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Song F, Wu WK, et al: Triptolide
inhibits colon cancer cell proliferation and induces cleavage and
translocation of 14-3-3 epsilon. Cell Biochem Funct. 30:271–278.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Shen M, Yue Z, et al: Triptolide
inhibits colon-rectal cancer cells proliferation by induction of G1
phase arrest through upregulation of p21. Phytomedicine.
19:756–762. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi X, Jin Y, Cheng C, et al: Triptolide
inhibits Bcr-Abl transcription and induces apoptosis in
STI571-resistant chronic myelogenous leukemia cells harboring T315I
mutation. Clin Cancer Res. 15:1686–1697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pigneux A, Mahon FX, Uhalde M, et al:
Triptolide cooperates with chemotherapy to induce apoptosis in
acute myeloid leukemia cells. Exp Hematol. 36:1648–1659. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao GH, Luan JF, Ye D, et al: Effects of
triptolide on proliferation and apoptosis of Jurkat cell line in
acute T lymphocytic leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
16:506–509. 2008.(In Chinese).
|
|
21
|
Wu PP, Liu KC, Huang WW, et al: Triptolide
induces apoptosis in human adrenal cancer NCI-H295 cells through a
mitochondrial-dependent pathway. Oncol Rep. 25:551–557.
2011.PubMed/NCBI
|
|
22
|
Hsiao YP, Yu CS, Yu CC, et al: Triggering
apoptotic death of human malignant melanoma a375.s2 cells by
bufalin: involvement of caspase cascade-dependent and independent
mitochondrial signaling pathways. Evid Based Complement Alternat
Med. 2012:5912412012. View Article : Google Scholar
|
|
23
|
Huang SH, Wu LW, Huang AC, et al: Benzyl
isothiocyanate (BITC) induces G2/M phase arrest and apoptosis in
human melanoma A375.S2 cells through reactive oxygen species (ROS)
and both mitochondria-dependent and death receptor-mediated
multiple signaling pathways. J Agric Food Chem. 60:665–675. 2012.
View Article : Google Scholar
|
|
24
|
Lu CC, Yang JS, Chiang JH, et al: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang WW, Yang JS, Lin MW, et al:
Cucurbitacin E induces G(2)/M phase arrest through STAT3/p53/p21
signaling and provokes apoptosis via Fas/CD95 and
mitochondria-dependent pathways in human bladder cancer T24 cells.
Evid Based Complement Alternat Med. 2012:9527622012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar
|
|
27
|
Nowak M, Strzelczyk A, Reif PS, et al:
Minocycline as potent anticonvulsant in a patient with astrocytoma
and drug resistant epilepsy. Seizure. 21:227–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fidler IJ: The role of the organ
microenvironment in brain metastasis. Semin Cancer Biol.
21:107–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eichhorn T and Efferth T: P-glycoprotein
and its inhibition in tumors by phytochemicals derived from Chinese
herbs. J Ethnopharmacol. 141:557–570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schneekloth JS Jr and Crews CM: Natural
product inhibitors of the ubiquitin-proteasome pathway. Curr Drug
Targets. 12:1581–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao H, Yang Z, Wang X, et al: Triptolide
inhibits ovarian cancer cells invasion by repression of MMP7, MMP19
and upregulation of E-cadherin. Exp Mol Med. Aug 20–2012.(Epub
ahead of print).
|
|
32
|
Wang Y, Lu JJ, He L and Yu Q: Triptolide
(TPL) inhibits global transcription by inducing
proteasome-dependent degradation of RNA polymerase II (Pol II).
PLoS One. 6:e239932011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang SW, Wang W, Xie XY, Zhu WP and Li FQ:
In vitro synergistic cytotoxic effect of triptolide combined with
hydroxycamptothecin on pancreatic cancer cells. Am J Chin Med.
39:121–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mujumdar N, Mackenzie TN, Dudeja V, et al:
Triptolide induces cell death in pancreatic cancer cells by
apoptotic and autophagic pathways. Gastroenterology. 139:598–608.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chernigovskaia EV, Iamova LA, Atochin D,
Huang P and Glazova MV: Interaction of neuronal NOS and
catecholamines in regulation of expression of proteins of apoptosis
by vasopressinergic hypothalamic neurons. Zh Evol Biokhim Fiziol.
47:232–238. 2011.(In Russian).
|
|
36
|
Chen YW, Lin GJ, Chia WT, Lin CK, Chuang
YP and Sytwu HK: Triptolide exerts anti-tumor effect on oral cancer
and KB cells in vitro and in vivo. Oral Oncol. 45:562–568. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang M, Huang J, Pan HZ and Jin J:
Triptolide overcomes dexamethasone resistance and enhanced
PS-341-induced apoptosis via PI3k/Akt/NF-κB pathways in human
multiple myeloma cells. Int J Mol Med. 22:489–496. 2008.PubMed/NCBI
|
|
38
|
Li W, Liu Y, Li XX, et al: MAPKs are not
involved in triptolide-induced cell growth inhibition and apoptosis
in prostate cancer cell lines with different p53 status. Planta
Med. 77:27–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ferrandiz N, Caraballo JM,
Garcia-Gutierrez L, et al: p21 as a transcriptional co-repressor of
S-phase and mitotic control genes. PLoS One. 7:e377592012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mauro M, Rego MA, Boisvert RA, et al: p21
promotes error-free replication-coupled DNA double-strand break
repair. Nucleic Acids Res. 40:8348–8360. 2012. View Article : Google Scholar : PubMed/NCBI
|