Introduction
Desmoids are benign fibromatoses, composed
histologically of mature fibroblasts. The tumors usually grow
slowly. For extra-abdominal desmoids, local control may be mainly
achieved by surgical intervention and may be improved with the
addition of radiation therapy, considering functional and cosmetic
outcomes (1,2). However, intra-abdominal desmoid tumors
are life-threatening. Although desmoids do not metastasize to other
organs, they grow and invade locally and infiltrate into
surrounding organs such as the intestine, ureters and major
vessels, causing bowel and ureteric obstruction and intra-tumoral
hemorrhage.
In patients with familial adenomatous polyposis
(FAP), desmoid tumors develop approximately 1,000 times more
frequently than in the general population, among whom they are rare
(3). Desmoid tumors develop in
3.6–13% of patients with FAP (4),
and account for 10–14% of deaths of FAP patients, making it the
second leading cause of death after colorectal carcinoma (3,5).
Surgery is usually indicated for extra-abdominal desmoids such as
those in the abdominal wall, but it is not recommended for
mesenteric desmoid tumors, which account for the majority of
intra-abdominal desmoids, since they are difficult to curatively
resect. Surgery carries a high risk of serious complications such
as bleeding, injury of the involved organs and short bowel
syndrome, and it is associated with a high incidence of recurrence
(6,7).
Dacarbazine (DTIC)-doxorubicin (DOX) therapy (D-D
therapy) is a hopeful therapeutic option against desmoid tumors,
providing a high clinical response (8–12). DOX
interferes with the function of topoisomerase II and DTIC is an
N-methyl-type compound that produces alkylating species. It was
demonstrated that adding DTIC to DOX to treat sarcoma patients
resulted in a better clinical outcome than DOX alone (13). The adverse effects of this protocol
are well documented (14).
Myelosuppression occurs as one of the major problems of this
therapy. In addition, the risk of heart failure due to DOX limits
the number of administration cycles although it usually takes a
long time for patients with desmoid tumors to reach remission.
Based on the original protocol proposed by Patel et
al(10), DTIC (750–1,000
mg/m2/cycle) and DOX (60–90 mg/m2/cycle) are
administered every 3–4 weeks for a total of 4–6 times (Fig. 1A), since cardiac muscle damage may
occur if the DOX dose exceeds 550 mg/m2(14).
To reduce the myelosuppression and assure an
extended therapeutic period, we modified the protocol adopting
low-dose DTIC (600–700 mg/m2/cycle) and DOX (50
mg/m2/cycle). We aimed to accomplish 10–11 therapeutic
cycles. In this study, we report the successful treatment of 3
patients with intra-abdominal desmoids by the modified regimen that
was safe and effective against desmoid tumors.
Case reports
General treatment and results
Low-dose DTIC-DOX (D-D) therapy was approved by the
Institutional Review Board for Patients, and all expenses for DTIC
and DOX therapy were paid by the Medical School Experience of the
Osaka University Hospital. Low-dose D-D therapy was administered to
3 patients with intra-abdominal desmoid tumors: one sporadic and
two FAP-associated tumors. For each cycle, DTIC and DOX were
administered by continuous infusion by the central vein route for 3
days (72 h), repeated every 4–5 weeks (Fig. 1B). A total of 10–11 cycles of
low-dose D-D therapy was administered. Two patients whose follow-up
period after therapy was more than 4 years achieved a complete
response (CR). The third patient whose last administer rate was
completed in September 2011 had a partial response (PR). To monitor
for heart dysfunction caused by DOX, electric cardiography and
echocardiogram were repeated periodically, but no adverse effects
were detected. Other adverse events were only slight ones: nausea,
vomiting, alopecia and leukocytopenia without fever. All patients
were able to spent ~20 days at home in a month during low-dose D-D
therapy.
Individual treatments
Case 1
In 1997, a 23-year-old female was found to have a
5.5-cm size sporadic desmoid tumor in the pelvic space and she
underwent surgery. Three years later the recurrent tumor appeared
in her pelvis, and she underwent a second surgery. In September
2005, she again had recurrent disease, and the tumor was no longer
resectable due to invasion to the vagina and rectum. Her principal
doctor consulted our team. In October 2005, the computed tomography
(CT) scan image indicated that the desmoid tumor (maximum diameter,
8 cm) occupied the right half of the lower pelvis and it compressed
the rectum far to the left side (Fig.
2B). From December 2005 to November 2006, low-dose D-D therapy
was performed every 4–5 weeks and 500–600 mg/m2 DTIC and
50 mg/m2 DOX was administered 10 times (Fig. 2A). According to the Common
Terminology Criteria for Adverse Events (CTCAE) v4.0 by the
National Cancer Institute (15),
the patient experienced grade 1 vomiting and grade 1 nausea
(Fig. 2A). The patient had grade
1–2 leukocytopenia (Fig. 2A, CTCAE
v3.0) (15) but had no febrile
neutropenia (CTCAE v4.0). An anti-estrogen receptor (tamoxifen,
40–20 mg/day) and the NSAID sulindac (Clinoril, 300 mg/day) were
subsequently administered orally. After 10 cycles, the tumor
diameter was reduced to 5.4 cm, and the tumor volume decreased by
36.6% when analyzed by CT volumetry (Fig. 2B). In February 2011, the desmoid
tumor almost disappeared, and the rectum was located back to the
middle site (Fig. 2B). The patient
has not had a recurrent tumor up until August 2012.
Case 2
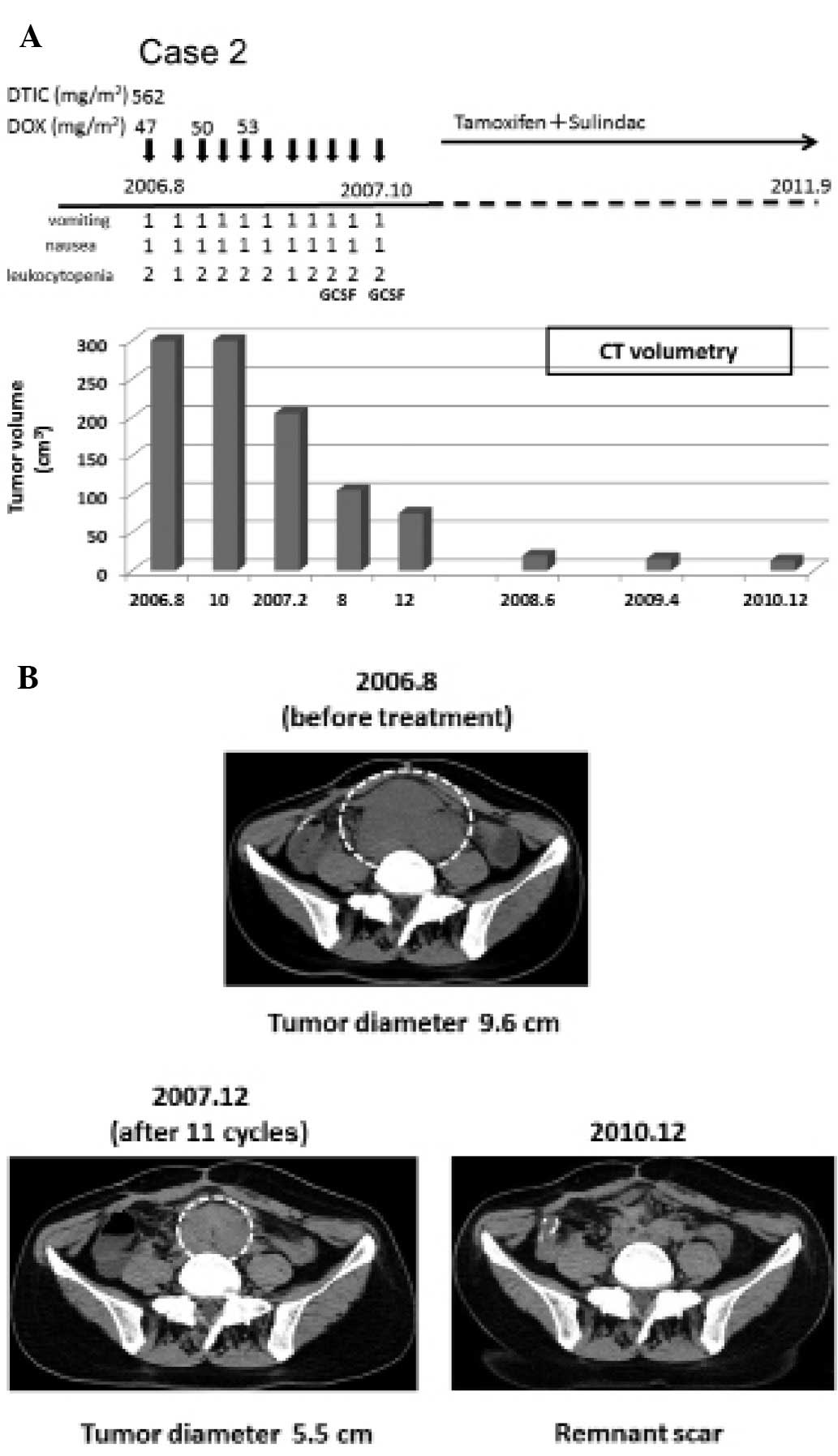
In November 2004, a 33-year-old female underwent
proctocolectomy due to FAP. In August 2006, an intra-abdominal
desmoid tumor (9.6 cm in diameter) derived from the mesenterium was
found (Fig. 3A). From August 2006
to October 2007, low-dose D-D therapy (562.5 mg/m2 DTIC
and 47.0–53.0 mg/m2 DOX per cycle) was executed for a
total of 11 cycles, followed by oral administration of an
anti-estrogen receptor (tamoxifen, 40–20 mg/day), and the NSAID
sulindac (Clinoril, 300 mg/day) (Fig.
3A). During the therapeutic period, the patient experienced
grade 1 nausea and grade 1 vomiting (CTCAE v4.0, Fig. 3A). She had no febrile neutropenia.
Instead, the patient had grade 1 or 2 leukocytosis (CTCAE v3.0)
which was alleviated promptly by treatment with granulocyte
stimulating factor (GCSF) twice (Fig.
3A). In December 2007, the tumor volume was decreased by 83.75%
(Fig. 3B). The desmoid tumor
continued to shrink and in December 2010, CT scan imaging showed
only a remnant scar (Fig. 3B). The
patient has not had a recurrent tumor up until June 2012.
Case 3
In February 2006, a 33-year-old male underwent
proctocolectomy due to FAP. In September 2010, an intra-abdominal
desmoid tumor (9.0 cm in diameter) generated from the mesenterium
was found by CT scan (Fig. 4B).
From November 2010 to September 2011, low-dose D-D therapy (700
mg/m2 DTIC and 50 mg/m2 DOX) was administered
10 times, followed by oral administration of the NSAID sulindac
(Clinoril, 300 mg/day) (Fig. 4A).
During the therapeutic period, blood toxicity was not experienced.
The patient experienced grade 2 vomiting and grade 1 alopecia
(CTCAE v4.0) (Fig. 4A). In August
2011, the tumor volume decreased by 81.4% (Fig. 4B).
Discussion
Dacarbazine-doxorubicin (DTIC-DOX) therapy achieved
better results against desmoid tumors (8–12) than
other potential treatments such as prednisolone, interferon γ,
vinorelbine, vinblastine and methotrexate (16–20).
We previously reported the case of a patient who repeatedly had
aggressive intra-abdominal mesenteric desmoids after
proctocolectomy due to FAP. He was administered 667–800
mg/m2 DTIC and 67–80 mg/m2 DOX in each cycle.
Although this DTIC-DOX therapy in a total of 7 courses achieved
tumor inhibitory effects, the patient experienced grade 3 to 4
leukocytopenia (CTCAE v3.0) 5 times in 7 therapies, and at the time
GCSF was necessary. Moreover, during the tumor regression, the
patient had a fever of higher than 38°C, which persisted for 2
months.
Apart from severe leukocytopenia, one adverse effect
of DTIC-DOX therapy is heart dysfunction due to DOX. Following the
original regimen by Patel et al(10) (DTIC, 750–1000 mg/m2; DOX,
60–90 mg/m2), drug administration is limited to 4–6
cycles for fear of heart failure. Paradoxically, it usually takes
an extended time for the desmoid tumor to reach complete remission.
Therefore, we planned to administer a reduced dose of DTIC and DOX
at 600–700 and 50 mg/m2, respectively, per cycle.
According to this protocol, we were able to continue therapy every
4–5 weeks for 10–13 months (10–11 cycles).
The first two patients after a long follow-up period
achieved a complete response (CR). Case 2 patient had no febrile
neutropenia and had an absolute neutrophil count <1000 without
fever, which was treated with GCSF twice. Case 2 patient also did
not have febrile neutropenia but did not require GCSF throughout
the therapeutic period. Both patients spent 1 week in the hospital
and 3–4 weeks at home during each cycle period.
According to RECIST guidelines, a 30% reduction in
tumor diameter (2r) for partial response is equivalent to
65% reduction in tumor volume (4/3πr3) (21). However, the desmoid tumor of case 1
patient had only a 12.2% reduction in volume 4 months after
commencement of the therapy, and the desmoid tumor of case 2
patient had a 14.3% reduction in volume after 5 months (Figs. 2A and 3A). Many physicians may discontinue
therapy at this point as these patients did not achieve a partial
response even after 4–5 months from the start of the treatment.
However, considering our previous experience of patients treated
with a standard dose of D-D therapy, the desmoid tumors of the
patients did not respond to therapy until 4 months and subsequently
continued to shrink for a long time. Therefore, we continued
low-dose D-D therapy by monitoring the tumor volume carefully and
their desmoid tumors continued to shrink, eventually achieving
almost a CR. Consequently, case 1 patient received 10 cycles for 12
months, and case 2 patient received 11 cycles of low-dose D-D
therapy for 14 months. CR was maintained for 1.5 and 2.5 years,
respectively.
After D-D therapy, we administered NSAID sulindac
and an anti-estrogen receptor tamoxifen to facilitate tumor
shrinkage. These agents are reportedly shown to be effective in
15–30% of desmoid tumor cases (22,23).
Since the desmoid tumor of case 1 patient markedly shrank after
low-dose D-D therapy with the aid of sulindac and tamoxifen, we
cannot deny the possibility that additional administration of these
supplementary agents might also be of importance for treating
desmoid tumors.
We are currently treating the third patient who had
intra-abdominal desmoid after proctocolectomy due to FAP with
low-dose D-D therapy (Fig. 4A). The
tumor volume of this patient decreased by 32.6% at 2 months and by
81.4% at 9 months following commencement of low-dose D-D therapy,
without any serious adverse events (Fig. 4).
Taken together, we showed that low-dose DTIC-DOX
therapy is safe and effective and ensures an acceptable quality of
life to the patients who need to endure a long therapy period.
Further large scale confirmation study is essential.
Abbreviations:
|
CT
|
computed tomography
|
|
DOX
|
doxorubicin
|
|
DTIC
|
dacarbazine
|
|
D-D therapy
|
DTIC-DOX therapy
|
|
FAP
|
familial adenomatous polyposis
|
|
NSAID
|
non-steroidal anti-inflammatory
drug
|
References
|
1
|
de Bree E, Keus R, Melissas J, Tsiftsis D
and van Coevorden F: Desmoid tumors: need for an individualized
approach. Expert Rev Anticancer Ther. 9:525–535. 2009.PubMed/NCBI
|
|
2
|
Berri RN, Baumann DP, Madewell JE, Lazar A
and Pollock RE: Desmoid tumor: current multidisciplinary
approaches. Ann Plast Surg. 67:551–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gurbuz AK, Giardiello FM, Petersen GM,
Krush AJ, Offerhaus GJ, Booker SV, et al: Desmoid tumors in
familial adenomatous polyposis. Gut. 35:377–381. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clark SK and Phillips RK: Desmoids in
familial adenomatous polyposis. Br J Surg. 83:1494–1504. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arvanitis ML, Jagelman DG, Fazio VW,
Lavery IC and McGannan E: Mortality in patients with familial
adenomatous polyposis. Dis Colon Rectum. 33:639–642. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clark SK, Neale KF, Landgrebe JC and
Phillips RK: Desmoid tumours complicating familial adenomatous
polyposis. Br J Surg. 86:1185–1189. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heiskanen I and Jarvinen HJ: Occurrence of
desmoid tumours in familial adenomatous polyposis and results of
treatment. Int J Colorectal Dis. 11:157–162. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gega M, Yanagi H, Yoshikawa R, Noda M,
Ikeuchi H, Tsukamoto K, et al: Successful chemotherapeutic modality
of doxorubicin plus dacarbazine for the treatment of desmoid tumors
in association with familial adenomatous polyposis. J Clin Oncol.
24:102–105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lynch HT, Fitzgibbons R Jr, Chong S,
Cavalieri J, Lynch J, Wallace F, et al: Use of doxorubicin and
dacarbazine for the management of unresectable intra-abdominal
desmoid tumors in Gardner’s syndrome. Dis Colon Rectum. 37:260–267.
1994.PubMed/NCBI
|
|
10
|
Patel SR, Evans HL and Benjamin RS:
Combination chemotherapy in adult desmoid tumors. Cancer.
72:3244–3247. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poritz LS, Blackstein M, Berk T, Gallinger
S, McLeod RS and Cohen Z: Extended follow-up of patients treated
with cytotoxic chemotherapy for intra-abdominal desmoid tumors. Dis
Colon Rectum. 44:1268–1273. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ezumi K, Yamamoto H, Takemasa I, Nomura M,
Ikeda M, Sekimoto M and Monden M: Dacarbazine-doxorubicin therapy
ameliorated an extremely aggressive mesenteric desmoid tumor
associated with familial adenomatous polyposis: report of a case.
Jpn J Clin Oncol. 38:222–226. 2008. View Article : Google Scholar
|
|
13
|
Gottlieb JA, Benjamin RS, Baker LH,
O’Bryan RM, Sinkovics JG, Hoogstraten B, et al: Role of DTIC
(NSC-45388) in the chemotherapy of sarcoma. Cancer Treat Rep.
60:199–203. 1976.PubMed/NCBI
|
|
14
|
Gottieb JA, Baker LH, Quagliana JM, Luce
JK, Whitecar JP Jr, Sinkovics JG, et al: Chemotherapy of sarcoma
with a combination of adriamycin and dimethyl triazeno imidazole
carboxamide. Cancer. 30:1632–1638. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
CTCAE v3.0 and v4.0 provided by the
National Cancer Institute. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.
|
|
16
|
Nakada I, Ubukata H, Goto Y, Watanabe Y,
Sato S, Tabuchi T, et al: Prednisolone therapy for intra-abdominal
desmoid tumors in a patient with familial adenomatous polyposis. J
Gastroenterol. 32:255–259. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bauernhofer T, Stoger H, Schmid M, Smola
M, Gurtl-Lackner B, Hofler G, et al: Sequential treatment of
recurrent mesenteric desmoid tumor. Cancer. 77:1061–1065. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kono T, Tomita I, Chisato N, Matsuda M,
Kakisaka A and Kasai S: Successful low-dose chemotherapy using
vinblastine and methotrexate for the treatment of an ileoanal pouch
mesenteric desmoid tumor: report of case. Dis Colon Rectum.
47:246–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weiss AJ, Horowiz S and Lackman RD:
Therapy of desmoid tumors and fibromatosis using vinorelbine. Am J
Clin Oncol. 22:193–195. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okuno SH and Edmonson JH: Combination
chemotherapy for desmoid tumors. Cancer. 97:134–135. 2003.
View Article : Google Scholar
|
|
21
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Glabbeke MV, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hansmann A, Adolph C, Vogel T, Unger A and
Moeslein G: High-dose tamoxifen and sulindac as first-line
treatment for desmoid tumors. Cancer. 100:612–620. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patel SR and Benjamin RS: Desmoid tumors
respond to chemotherapy: defying the dogma in oncology. J Clin
Oncol. 24:11–12. 2006. View Article : Google Scholar : PubMed/NCBI
|