Introduction
Neuropathic pain is induced by various kinds of
etiologies and share similar clinical characteristics, such as
persistent spontaneous pain, hyperalgesia and allodynia. Because of
the limited understanding of the mechanisms of neuropathic pain,
current therapeutic methods are not ideal. Instead of the taking
therapeutic measures after the establishment of the pain, treating
it during the development of neuropathic pain might allow patients
to have a better prognosis. Therefore, determining the potential
mechanisms responsible for the induction of neuropathic pain is
required to develop efficacious therapies.
As a constitutive transcription factor, CREB has
been proved to be involved in the maintenance of neuropathic pains
(1,2), though its specific function is not
clear. It has been shown that increased pCREB played an important
role in many kinds of pain models, such as inflammation pain
(3,4), neuropathic pain (1,2,5–7)
and chronic muscle pain (8). What
is more, the nociceptive behavior induced by inflammation and
neuropathic pain of these models have been shown accompanied with
the increase of pCREB (7,9). Ma et al(1) and Wang et al(2) reported that interrupting the induction
of pCREB by intrathecal injection of CREB antisense inhibited the
peripheral nerve injury-evoked nociceptive behavior in the
maintenance of neuropathic pain.
An increasing body of evidence indicates that pCREB
is crucial for the transmission of activities occurring at
membranes to regulate the expression of downstream genes, such as
c-fos (10), c-jun (11), BDNF (brain-derived neurotrophic
factor), tyrosine hydroxylase, and many neuropeptides (such as
somatostatin, enkephalin and corticotropin-releasing hormone)
(12), NR1, NR2B (13–21).
Among them, NR1 and NR2B encode two NMDA receptor subunits, which
may be regulated by Ca2+-sensitive signaling pathway.
Long-term potentiation, learning and memory storage in neurons may
rely on Ca2+-influx through NMDA receptors, which act
mainly via the induction of calcium-calmodulin kinases and the
activation of CREB. Klein et al(13) found that reporter gene driven by the
NR2B promoter could be expressed in both neuronal and non-neuronal
cell lines. NR2B expression is possibly regulated via Sp1 binding
sites and a CREB binding site, linked to Ca2+ -signaling
pathways. The findings of Lau et al(14) also suggested that the transcription
of NR1 is regulated by the c-AMP signaling pathway, most likely
through CREB and its activation by phosphorylation in cortical
neurons. CREB might also be involved in mediating ethanol-induced
upregulation of NR2B gene in fetal mouse cortical cells (15). Inhibiting CREB will affect the
expression of NR2B (16) and
constitutively active CREB can increase both NR2B mRNAs and
NR2B proteins. The functional CREB regulate learning and long-term
plasticity (22–24).
Our previous study showed that NR1 and NR2B are
essential in the development and maintenance of neuropathic pain
(25–27). Injecting fibrosarcoma cells into the
femur could increase the expression of NR2B in the spinal cord
accompanied by mechanical allodynia and thermal hyperalgesia. Both
are inhibited by the NR2B selective antagonist ifenprodil (28). Evidence from other investigations
indicated that the phosphorylation of CREB in neurons may
contribute to the plastic changes in the spinal cord and to the
development and maintenance of neuropathic pain eventually
(1,2,5,6).
However, the effect of CREB on the expression of NMDAR in spinal
cord in the development of neuropathic pain has not been previously
determined. Thus, we proposed that CREB might regulate the
expression of NR1 and NR2B within spinal cord and lead to chronic
pain. In order to test this hypothesis, we used intrathecal
antisense oligonucleotide treatment to reduce the production of
CREB proteins as well as the phosphorylated of CREB.
Materials and methods
Experimental animals
Adult (7–8 weeks old) male C57BL/6 mice weighing
20–25 g were obtained from the Model Animal Research Center of the
Nanjing University, housed on a 12-h light/dark schedule with
standard rodent chow and water ad libitum at room
temperature (21–24°C). The experimental protocol was approved by
the Animal Care and Use Committee at the Medical College of Nanjing
University and complied with the guidelines for the use of
laboratory animals (10). All
efforts were made to minimize animal suffering and to reduce the
number of animals used.
Intrathecal catheter implantation
An intrathecal catheter was implanted in each mouse
under the same surgical condition. Under deep sodium pentobarbital
(40 mg/kg, i.p.) anesthesia, the surgical procedures of intrathecal
catheterization in this study was based on minor modifications of
previous methods (11). After
intrathecal catheterization, the mice were allowed to recover for 3
days before being used experimentally. At the experimental day, the
mice appeared to be neurologically normal and complete paralysis of
the bilateral hind legs after intrathecal administration of 2%
lidocaine (2 μl) were used for surgery. The mice were kept in
individual cages after catheterization.
Chronic constriction injury (CCI)
Male mice were anesthetized with sodium
pentobarbital (40 mg/kg, i.p.) for surgical procedures. Chronic
constriction of the sciatic nerve was performed according to the
method described by Bennett and Xie (12). Briefly, the right sciatic nerve was
exposed at the level of the mid-thigh. Three ligatures (5-0 chromic
gut; Ethicon, Rome, Italy) were tied loosely around the sciatic
nerve with a 1.0–1.5 mm interval between each ligature. The wound
was then closed with 4-0 Ethicon silk suture in layers. Then, the
injured right hind paw was named as ipsilateral paw and the
uninjured left hind paw was named as contralateral paw.
Intrathecal CREB antisense ODN
administration
The sequences of these sense, missense and antisense
CREB ODNs were designed as previously reported (17). Sequences for the ODNs were as
follows: antisense, 5′-TGGTCATCTAGTCACCGG TG-3′; sense,
5′-CACCGGTGACTAGATGACCA-3′; and CREB missense,
5′-GACCTCAGGTAGTCGTCGTT-3′. The ODNs were phosphorothioate-modified
and synthesized by Sangon Biotechnology Co. (Shanghai, China). The
ODNs were reconstituted in saline before administration. The mice
were injected intrathecally with saline 5 μl, sense 5 μl/5 μg,
missense 5 μl/5 μg and antisense ODN 5 μl/5 μg, respectively, every
24 h for 6 days. The four groups were called NS, A, S and M groups.
Finally, the injection was followed by a flush of 5 μl of
saline.
Mechanical allodynia
Mice were habituated to the behavioral testing
conditions daily for 3 days before initiating baseline testing.
Each mouse was placed in an individual transparent plastic
compartment (8.5×11.5×14 cm) on a metal mesh floor and allowed to
acclimatize for 30 min each time. Von Frey filaments (Stoelting
Co., Wood Dale, IL, USA) with incremental stiffness (0.16–1.4 g)
were applied serially to the paw in ascending or descending order
of stiffness depending on the foot withdrawal response of the
mouse. The maximum and minimum cut-offs were at 1.4 and 0.16 g,
respectively. The filaments were poked vertically against the
plantar surface with sufficient force to cause slight bending
against the paw and held for 4–5 sec with an stimuli interval of
~15 sec. A withdrawal of hind paw upon the stimulus (at least three
times out of five applications) was considered as a positive
response, the paw withdrawal mechanical threshold (PWMT) was
determined by sequentially increasing and decreasing the stimulus
strength (the up-and-down) method (29). Each mouse was tested five times per
stimulus strength. The lowest Von Frey filaments which had three or
more positive responses were regarded as PWMT.
Thermal hyperalgesia
Mice were habituated to the behavioral testing
conditions for 3 days before initiating baseline testing. Each
mouse was placed in an individual transparent plastic compartment
(8.5×11.5×14 cm) on a thin glass platform and allowed to
acclimatize for 30 min each time. A radiant thermal stimulator (Ugo
Basile 7370; Plantar Test Apparatus, Comerio, Italy) was placed
onto the plantar surface of the hind paw through the glass
platform. There were five trials per mouse and 5-min intervals
between trials. The infrared intensity was set at 50 (corresponding
to 196 mW/cm2), which produced baseline paw withdrawal
latencies of 5–10 sec. A cut-off time of 20 sec was used to avoid
tissue damage. The mean latency of withdrawal response of each
hindpaw was determined by 5 tests.
Western blot analysis
Mice were sacrificed rapidly by decapitation and the
lumbar spinal cord segments were dissected out and frozen on dry
ice in collecting tubes. Samples were then stored at −80°C until
further processing. Tissue samples were homogenized in lysis
buffer. The homogenate was centrifuged at 12,000 rpm for 10 min at
4°C and supernatant was removed. The protein concentration was
determined by the BCA Protein Assay kit, following the
manufacturer’s instructions. Samples (50 μg) were separated on
SDS-PAGE (6–12% gradient gel; KeyGen, Nanjing, China) and
subsequently transferred to polyvinylidene difluoride membranes
(Millipore Corp., Billerica, MA, USA). The filter membranes were
blocked with 5% non-fat milk in Tris-buffered saline (TBS; pH 7.4;
Sigma) for 1 h at room temperature and incubated respectively with
rabbit anti-CREB (1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA), anti-pCREB (1:1,000; Cell Signaling Technology),
anti-NR1 (1:1000; Cell Signaling Technology), anti-NR2B (1:1000;
Cell Signaling Technology), and anti-β-actin (1:2,000, Cell
Signaling Technology) primary antibody at 4°C overnight. Following
three consecutive 5-min washes with TBS, the membrane was incubated
with anti-goat or mouse IgG (1:5,000; KeyGen). The blots were
visualized in ECL solution (DuPont NEN, Boston, MA, USA) for 1 min
and exposed to hyperfilm (Amersham Biosciences, Piscataway, NJ,
USA) for 1–10 min. Immunopositive bands were quantified using
Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA).
The density of specific bands was measured with a computer-assisted
imaging analysis system and normalized against corresponding
loading β-actin bands.
Reverse transcriptase-PCR
Mice were rapidly (<1 min) sacrificed through
decapitation after being anesthetized with pentobarbital and the
L3–L5 lumbar spinal cord segments were immediately frozen in liquid
nitrogen and stored at −80°C. Previous studies using a retrograde
neuronal tracer showed that neurons innervating the distal femur
originated from the L3–L5 dorsal root ganglions (DRGs) (18). Total RNA was isolated with TRIzol
(15596–026; Invitrogen, Carlsbad, CA, USA), and a 5 μg portion of
it was used for cDNA synthesis with M-MLV reverse transcriptase
(PC0002; Fermentas, Vilnius, Lithuania). The cDNA was used as
template for PCR amplification with Taq DNA polymerase (EP0702;
Fermentas). NR1 primers (upstream primer, 5′-ACAACAAGCTGCACGC
CTTTA-3′ and downstream primer, 5′-TGTTGTTCGACGTG CGGAAAT-3′), NR2B
primers (upstream primer, 5′-GTGG G TACGGGAGGGATAGG-3′, and
downstream primer, 5′-CA CCCATGCCCTCCCTATCC-3′) and GAPDH primers
(upstream primer, 5′-GAGACCTTCAACACCCCAGC-3′, and downstream
primer, 5′-CACAGAGTACTTGCGCTCAG-3′) designed by Nanjing KeyGen
Biological Technology Development Co. The amplified cDNA was
electrophoresed on 2% agarose gel and stained with ethidium
bromide. The intensity of each PCR band was analyzed using gel
imaging analytical system (Gel Doc XR; Bio-Rad Laboratories).
Samples without the addition of reverse transcriptase (negative
controls) yielded no detectable product.
Statistical analysis
Western blotting, behavioral and RT-PCR data were
analyzed using ANOVA. The post hoc (Student Newman-Keuls) tests
were performed to determine sources of differences, when
significant main effects were observed. In all cases, P<0.05 is
considered to indicate statistically significant result.
Results
CCI induces pain behavior
Before CCI operation (D0), no differences of the paw
withdrawal threshold were observed between sham group (group Sham)
and CCI group (group CCI) (Fig. 1,
P>0.05). Pain behavior threshold from both sides of hind paw in
sham group and the contralateral hind paw in CCI group have no
significant change. The paw withdrawal threshold (PWMT) with the
strength of the Von Frey filament stimulation of the ipsilateral
hind limb significantly decreased after CCI in mice from D1 to D28,
and the paw withdrawal threshold latency to thermal stimulation
(PWTL) prominently reduced from D1 to D21 (Fig. 1, P<0.05). We considered the
behavior test from D0 to D28, because the PWTL recovered to
preoperative level on D28.
The expression of pCREB increases after
CCI
In CCI, but not in sham group, the expression of
pCREB within the spinal cord increased after peripheral nerve
injury (Fig. 2) (n=3–4). The
protein level for pCREB began to increase on postoperative day 1 in
CCI mice and remained elevated when examined on postoperative day
21 (P<0.05). There was no significant difference in the CREB
expression in the spinal cord between CCI and sham mice (P>0.05)
detected by western blot analysis. Thus, CCI induced a
time-dependent expression of pCREB at the protein level, but not
CREB.
Intrathecal administration of CREB
antisense ODN attenuates pain behavior induced by CCI
From 1 to 7 days after intrathecal injection of
antisense CREB ODN, the pain behavior in the ipsilateral hindpaw
was attenuated to the pre-lesion value (Fig. 3, P>0.05). From 10 to 21 days
after first injection of antisense CREB ODN, the PWMT and PWTL were
significantly reduced when compared to pre-lesion value. However,
PWMT and PWTL in the ipsilateral hindpaw of mice with injection of
saline, sense and missense CREB ODN remained at regular level at
all the time points tested after CCI. The PWMT and PWTL in the
ipsilateral hindpaw remained significantly attenuated 10–21 days
after first intrathecal injection of antisense CREB ODN, when
compared to the other three groups (Fig. 3, P<0.05), but was never
eliminated to the pre-lesion value.
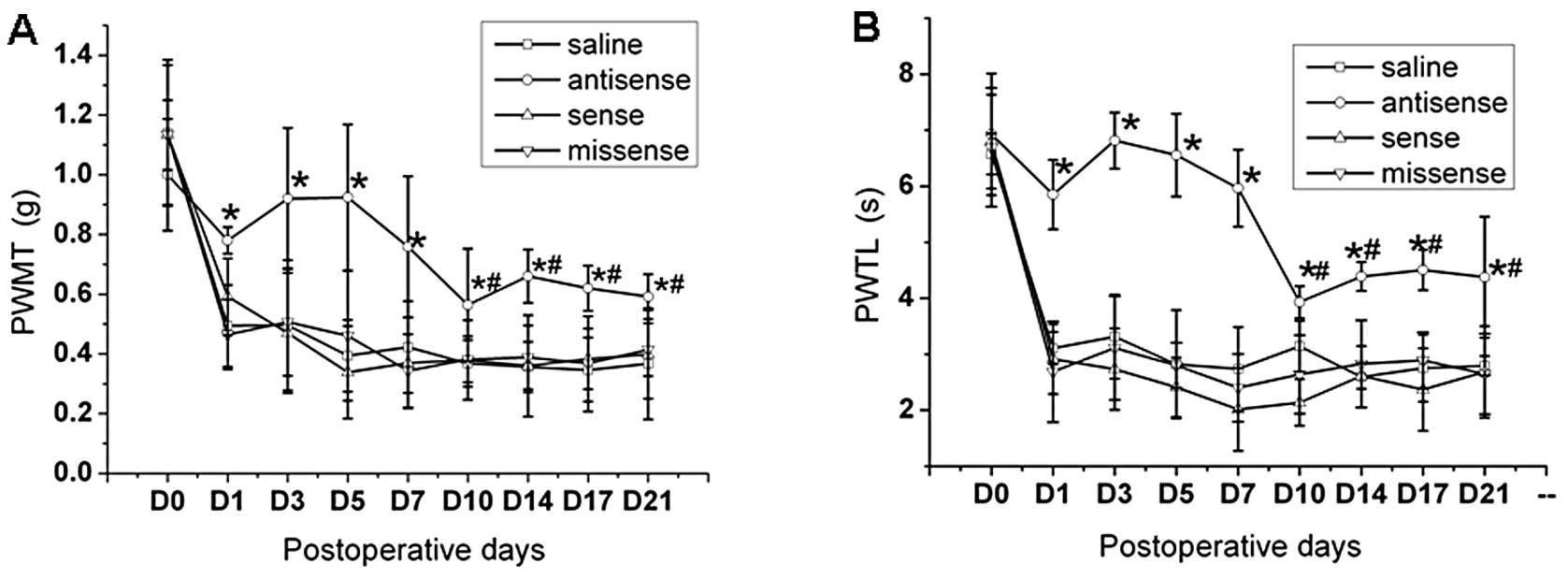 | Figure 3Effects of intrathecal administration
of antisense CREB ODN on pain behavior induced by CCI. Tests of the
(A) PWMT and (B) PWTL after intrathecal injection of CREB
antisense, sense, missense ODN and saline once a day from 1–6 days
after CCI. Data were obtained from four groups, with each using 6
mice. D0, D1, D3, D5, D7, D10, D14, D17 and D21 indicate days of
CCI. *P<0.05 vs. saline group. #P<0.05
vs. pre-lesion value. |
Intrathecal administration of CREB
antisense ODN causes the reduction of expression of pCREB and
CREB
We were interested in examining whether the effect
of CREB antisense ODN on allodynia during the development of
neuropathic pain actually caused the reduction of CREB or pCREB
protein within the spinal cord. Western blot analysis showed that
both the expression levels of CREB and pCREB within the spinal cord
were significantly reduced after the application of CREB antisense
ODN at day 7 and 14 after CCI compared with control groups
(Fig. 4, P<0.05). In addition,
the expression of CREB and pCREB at day 14 after CCI was higher
than the expression at day 7 (Fig.
4, P<0.05). No significant difference was found between the
saline group, CREB sense ODN group and CREB missense ODN group,
consistent with previous studies (1,2).
NMDAR mRNA levels in spinal cord are
reduced after intrathecal administration of CREB antisense ODN
To investigate whether the interruption of CREB
expression would influence NMDAR mRNA transcription in CCI mice, we
conducted reverse transcriptase PCR. In this experiment, each CCI
mouse received 5 μg/5 μl a CREB antisense OND, sense OND, missense
OND, or saline (n=6–8), all of which were given once daily via an
intrathecal catheter on postoperative days 1 to 6. The spinal cord
samples were harvested on postoperative day 7 and 14 (n=3–4 per
group per day). The mRNA levels of NR1 and NR2B within the spinal
cord were significantly reduced on postoperative day 7 and 14 in
CCI mice after the injection of the CREB antisense OND, compared
with the saline group (Fig. 5)
(P<0.05; n=3–4). NR1 and NR2B mRNAs at day 14 within the spinal
cord were evidently increased compared to day 7 (Fig. 5) (P<0.05; n=3–4). No significant
difference was observed between the saline group, CREB sense ODN
group and CREB missense ODN group at each time point.
NMDAR expression increases in spinal cord
after intrathecal administration of CREB antisense ODN
To understand whether the interruption of CREB
expression would influence NMDAR within spinal cord in CCI mice, we
tested the expression level of NMDAR using western blot analysis.
In this experiment, each CCI mice received 5 μg/5 μl of CREB
antisense OND, sense OND, missense OND, or saline (n=6–8), all of
which were given once daily via an intrathecal catheter on
postoperative days 1 to 6. The spinal cord samples were harvested
on postoperative day 7 and 14 (n=3–4 per group/day). The expression
of NR1 and NR2B subunits within the spinal cord from CCI mice
receiving the CREB antisense OND was significantly reduced on
postoperative day 7 and 14 compared with the saline group (Fig. 6) (P<0.05; n=3–4). The expression
levels of NR1 and NR2B at day 14 within the spinal cord were
clearly increased compared to day 7 (Fig. 6) (P<0.05; n=3–4). The expression
of NR2B on day 14 in saline group did not change compared with day
7. No significant difference was found between the saline group,
CREB sense ODN group and CREB missense ODN group at each time
point.
Discussion
Many previous studies have suggested that the
activation of CREB in the spinal dorsal horn plays an important
role in the process of pain induced by inflammation (3,4,9) and
nerve injury (5). However, the role
of CREB in sensitization of the spinal neurons still remains
unclear. It is known that CCI causes neuropathic pain associated
with behavioral changes. After CCI, the PWMT of the ipsilateral
hind limb was significantly reduced, although the PWTL prominently
decreased from D1 to D21 and not as much afterwards. Consistent
with the PWTL trend, the expression of the pCREB was activated
during the same time period. It is known that pCREB is the
functional form which is associated with thermal hyperalgesia and
mechanical allodynia after CCI. Our study found that the expression
of pCREB increased after CCI while the expression of CREB had no
significant changes at that stage. These findings are in line with
previous research stating that pCREB in the spinal dorsal horn of
rats increases following formalin injection (4,9),
carrageenan injection (3), partial
sciatic nerve ligation (1,5), chronic constriction injury of the
sciatic nerve (6) and spared nerve
injury (2). On the basis of
previous studies, our study used mice after CCI to study CREB and
thus revealed that the pCREB was highly expressed during the whole
process of neuropathic pain, suggesting its role in the induction
and maintenance of neuropathic pain induced by CCI.
The present study further demonstrated that chronic
daily intrathecal administration of CREB antisense ODN during the
development of neuropathic pain alleviated the CCI-induced
mechanical allodynia and thermal hyperalgesia possibly due to its
function in repressing the expression of CREB in spinal cord of CCI
mice. Intrathecal treatment with CREB antisense ODN in the
development of neuropathic pain induced by CCI completely relieved
pain behavior during the course of injection, while after all the
treatment tactile allodynia and thermal hyperalgesia elicited by
CCI was attenuated, but not eliminated. Ma et al(1) indicated that 3 weeks following partial
sciatic nerve ligation, the tactile allodynia in the ipsilateral
hindpaw was significantly attenuated 3–7 days after intrathecal
injection of antisense CREB ODN for 5 days, when compared with
pre-injection value, but never eliminated to the pre-lesion value
and expression of total CREB and pCREB was reduced concomitantly.
In another study, Wang et al(2) suggested that chronic injection of CREB
antisense ODN for 5 days after 2 weeks following spared nerve
injury significantly attenuated SNI-induced mechanical
(bilaterally) and cold allodynia (ipsilaterally) at the same time,
but never eliminated to the pre-lesion value and also reduced total
CREB and pCREB to an almost equal extent in both the ipsilateral
and the contralateral dorsal horn neurons. Previous studies
suggested that the CREB antisense ODN could not reverse the PWMT
and PWTL to pre-lesion value and lasted four days after injection.
In our study, we took intrathecal injection of antisense CREB ODN
from D1 to D6, once a day. Collectively, these findings suggested
that interruption of the expression of CREB by CREB antisense ODN
during the induction of neuropathic pain plays a definitively role
in the whole process of neuropathic pain.
Once CREB is phosphorylated, it can bind to specific
DNA consensus sequences to activate the expression of its
downstream genes, such as NR1 and NR2B (NMDA subunits) shown in
this study. NMDA receptors contain heteromeric combinations of the
NR1 subunit and one or more of NR2A-D subunits (19). Among all subunits, NR1 and NR2B are
predominant and their dynamic patterns determine many of the
biophysical and pharmacological properties of NMDA receptors
(20). Previous studies showed that
CREB affects NR1 and NR2B (13–16,21,30–33).
It is known that NR1 and NR2B play important roles in neuropathic
pain (25–27). Our study revealed that intrathecal
injection of CREB antisense after CCI attenuated neuropathic pain
by repressing NR1 and NR2B, downstream of CREB.
Zhuo in his study (34) showed that activation of NMDA
receptor triggers calcium influx. In adult anterior cingulate
cortex (ACC) pyramidal cells, most of NMDA receptors comprise of
NR1-NR2A, NR1-NR2B and bits of NR1-NR2A-NR2B. Ca2+
influx postsynaptically activates Ca2+-calmodulin (CaM)
dependent pathways. Then, Ca2+ and CaM start to
stimulate AC1 to generate the key second messenger cAMP, which
subsequently activates PKA. The catalytic subunit of PKA may
relocate to the nucleus and phosphorylate CREB at ser-133 site
(35). Phosphorylated CREB
activates NR2B gene expression. Subsequently, NR2B is increased,
and together with endogenous motor protein KIF17, these new NR2B
subunits are added to postsynaptic NMDA receptors. NMDA NR2B
receptor-AC1-cAMP-CREB-NR2B might form a positive feedback to
reinforce the NMDA receptor functions in the ACC neurons, thus, may
further enhance neuronal excitability within the ACC and contribute
to chronic pain.
Our study suggested that the NMDA-AC1-cAMP-CREB-NMDA
loop may function in spinal cord as well. Some results showed that
not only NR2 but also NR1 of the NMDAR was activated after CCI in
the ipsilateral spinal cord dorsal horn (36). Their mRNA level began to increase at
postoperative day 3 in CCI mice and remained elevated when examined
on postoperative day 7 and 14. CCI induced a time-dependent and
region-specific expression of NMDARs at both the mRNA level and the
protein level (36). In our study,
we also found that intrathecal injection of CREB antisense
inhibited mechanical allodynia and thermal hyperalgesia as well as
the increase of NR1 mRNA and protein levels in the spinal cord of
mice after CCI. Our results provide novel evidence that increased
expression of spinal NMDAR induced by neuropathic pain depends on
the activation of the CREB.
Previous studies have suggested that the activation
of spinal NMDAR can also potentiate CREB phosphorylation, producing
a positive feedback to the hypersensitization of spinal nociceptive
neurons (34). In an inflammation
model, the NMDAR antagonist of MK-801 inhibits the increased
phosphorylation of CREB in the dorsal horn neurons (9). It has been found that the NMDAR
antagonist of MK-801 could increase CREM/ICER activity, which
opposed CREB (29,37,38).
The activation of CREB increases both NMDAR-mediated synaptic
currents and surface level of NMDAR, and vice versa, the inhibition
of NMDAR abolishes the effect of CREB (32). Our study provides important evidence
that the positive feedback of NMDAR NR2B-CREB-NR2B may contribute
to the hypersensitization of spinal nociceptive neurons and
inhibiting CREB can interrupt this positive feedback.
Collectively, the above data demonstrated that
mechanical allodynia and thermal hyperalgesia induced by CCI as
well as the upregulation of pCREB expression levels in the spinal
cord could be inhibited by intrathecal injection of the CREB
antisense ODN. Interrupting the expression of CREB and pCREB during
the induction of neuropathic pain could impact in the development
of neuropathic pain. Additionally, the lack of pCREB prevented the
activation of NR1 and NR2B expression induced by CCI. CREB may be
involved in the development of neuropathic pain and regulates
expression of NR1 and NR2B subunit of NMDAR in the process. The
present study might shed some light on future therapeutic methods
on chronic pain.
Acknowledgements
The present research was supported by the National
Natural Foundation of China (30872439/c160202) and
(81171047/H0903).
References
|
1
|
Ma W, Hatzis C and Eisenach JC:
Intrathecal injection of cAMP response element binding protein
(CREB) antisense oligonucleotide attenuates tactile allodynia
caused by partial sciatic nerve ligation. Brain Res. 988:97–104.
2003. View Article : Google Scholar
|
|
2
|
Wang YY, Wu SX, Zhou L, Huang J, Wang W,
Liu XY and Li YQ: Dose-related antiallodynic effects of cyclic AMP
response element-binding protein-antisense oligonucleotide in the
spared nerve injury model of neuropathic pain. Neuroscience.
139:1083–1093. 2006. View Article : Google Scholar
|
|
3
|
Messersmith DJ, Kim DJ and Iadarola MJ:
Transcription factor regulation of prodynorphin gene expression
following rat hindpaw inflammation. Brain Res Mol Brain Res.
53:260–269. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anderson LE and Seybold VS: Phosphorylated
cAMP response element binding protein increases in neurokinin-1
receptor-immunoreactive neurons in rat spinal cord in response to
formalin-induced nociception. Neurosci Lett. 283:29–32. 2000.
View Article : Google Scholar
|
|
5
|
Ma W and Quirion R: Increased
phosphorylation of cyclic AMP response element-binding protein
(CREB) in the superficial dorsal horn neurons following partial
sciatic nerve ligation. Pain. 93:295–301. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miletic G, Pankratz MT and Miletic V:
Increases in the phosphorylation of cyclic AMP response element
binding protein (CREB) and decreases in the content of calcineurin
accompany thermal hyperalgesia following chronic constriction
injury in rats. Pain. 99:493–500. 2002. View Article : Google Scholar
|
|
7
|
Wang Y, Cheng X, Xu J, Liu Z, Wan Y and Ma
D: Anti-hyperalgesic effect of CaMKII inhibitor is associated with
downregulation of phosphorylated CREB in rat spinal cord. J Anesth.
25:87–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoeger-Bement MK and Sluka KA:
Phosphorylation of CREB and mechanical hyperalgesia is reversed by
blockade of the cAMP pathway in a time-dependent manner after
repeated intramuscular acid injections. J Neurosci. 23:5437–5445.
2003.PubMed/NCBI
|
|
9
|
Ji RR and Rupp F: Phosphorylation of
transcription factor CREB in rat spinal cord after formalin-induced
hyperalgesia: relationship to c-fos induction. J Neurosci.
17:1776–1785. 1997.PubMed/NCBI
|
|
10
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu WP, Xu XJ and Hao JX: Chronic lumbar
catheterization of the spinal subarachnoid space in mice. J
Neurosci Methods. 133:65–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bennett GJ and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar
|
|
13
|
Klein M, Pieri I, Uhlmann F, Pfizenmaier K
and Eisel U: Cloning and characterization of promoter and 5′-UTR of
the NMDA receptor subunit epsilon 2: evidence for alternative
splicing of 5′-non-coding exon. Gene. 208:259–269. 1998.
|
|
14
|
Lau GC, Saha S, Faris R and Russek SJ:
Up-regulation of NMDAR1 subunit gene expression in cortical neurons
via a PKA-dependent pathway. J Neurochem. 88:564–575. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rani CS, Qiang M and Ticku MK: Potential
role of cAMP response element-binding protein in ethanol-induced
N-methyl-D-aspartate receptor 2B subunit gene transcription in
fetal mouse cortical cells. Mol Pharmacol. 67:2126–2136. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin X, Takei Y, Kido MA and Hirokawa N:
Molecular motor KIF17 is fundamental for memory and learning via
differential support of synaptic NR2A/2B levels. Neuron.
70:310–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guzowski JF and McGaugh JL: Antisense
oligodeoxynucleotide-mediated disruption of hippocampal cAMP
response element binding protein levels impairs consolidation of
memory for water maze training. Proc Natl Acad Sci USA.
94:2693–2698. 1997. View Article : Google Scholar
|
|
18
|
Edoff K, Grenegard M and Hildebrand C:
Retrograde tracing and neuropeptide immunohistochemistry of sensory
neurones projecting to the cartilaginous distal femoral epiphysis
of young rats. Cell Tissue Res. 299:193–200. 2000. View Article : Google Scholar
|
|
19
|
Nakanishi S: Molecular diversity of
glutamate receptors and implications for brain function. Science.
258:597–603. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lau CG and Zukin RS: NMDA receptor
trafficking in synaptic plasticity and neuropsychiatric disorders.
Nat Rev Neurosci. 8:413–426. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai G and Kusiak JW: Cloning and analysis
of the 5′ flanking sequence of the rat N-methyl-D-aspartate
receptor 1 (NMDAR1) gene. Biochim Biophys Acta. 1152:197–200.
1993.
|
|
22
|
Yin JC and Tully T: CREB and the formation
of long-term memory. Curr Opin Neurobiol. 6:264–268. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mayr B and Montminy M: Transcriptional
regulation by the phosphorylation-dependent factor CREB. Nat Rev
Mol Cell Biol. 2:599–609. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carlezon WA, Duman RS and Nestler EJ: The
many faces of CREB. Trends Neurosci. 28:436–445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma ZL, Zhang W, Gu XP, Yang WS and Zeng
YM: Effects of intrathecal injection of prednisolone acetate on
expression of NR2B subunit and nNOS in spinal cord of rats after
chronic compression of dorsal root ganglia. Ann Clin Lab Sci.
37:349–355. 2007.PubMed/NCBI
|
|
26
|
Gu X, Yang L, Wang S, Sung B, Lim G, Mao
J, Zeng Q and Yang C: A rat model of radicular pain induced by
chronic compression of lumbar dorsal root ganglion with SURGIFLO.
Anesthesiology. 108:113–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang W, Shi CX, Gu XP, Ma ZL and Zhu W:
Ifenprodil induced antinociception and decreased the expression of
NR2B subunits in the dorsal horn after chronic dorsal root ganglia
compression in rats. Anesth Analg. 108:1015–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu X, Zhang J, Ma Z, Wang J, Zhou X, Jin
Y, Xia X, Gao Q and Mei F: The role of N-methyl-D-aspartate
receptor subunit NR2B in spinal cord in cancer pain. Eur J Pain.
14:496–502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Storvik M, Linden AM, Kontkanen O, Lakso
M, Castren E and Wong G: Induction of cAMP response element
modulator (CREM) and inducible cAMP early repressor (ICER)
expression in rat brain by uncompetitive N-methyl-D-aspartate
receptor antagonists. J Pharmacol Exp Ther. 294:52–60.
2000.PubMed/NCBI
|
|
30
|
Lonze BE and Ginty DD: Function and
regulation of CREB family transcription factors in the nervous
system. Neuron. 35:605–623. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
West AE, Griffith EC and Greenberg ME:
Regulation of transcription factors by neuronal activity. Nat Rev
Neurosci. 3:921–931. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang YH, Lin Y, Brown TE, et al: CREB
modulates the functional output of nucleus accumbens neurons: a
critical role of N-methyl-D-aspartate glutamate receptor (NMDAR)
receptors. J Biol Chem. 283:2751–2760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jung WR, Kim HG, Shin MK, Park DI and Kim
KL: The effect of ganglioside GQ1b on the NMDA receptor signaling
pathway in H19-7 cells and rat hippocampus. Neuroscience.
165:159–167. 2010. View Article : Google Scholar
|
|
34
|
Zhuo M: Plasticity of NMDA receptor NR2B
subunit in memory and chronic pain. Mol Brain. 2:42009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gonzalez GA and Montminy MR: Cyclic AMP
stimulates somatostatin gene transcription by phosphorylation of
CREB at serine 133. Cell. 59:675–680. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang S, Lim G, Zeng Q, Sung B, Yang L and
Mao J: Central glucocorticoid receptors modulate the expression and
function of spinal NMDA receptors after peripheral nerve injury. J
Neurosci. 25:488–495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Storvik M, Tiikkainen P, van Iersel M and
Wong G: Distinct gene expression profiles in adult rat brains after
acute MK-801 and cocaine treatments. Eur Neuropsychopharmacol.
16:211–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Konopka D, Szklarczyk AW, Filipkowski RK,
Trauzold A, Nowicka D, Hetman M and Kaczmarek L: Plasticity- and
neurodegeneration-linked cyclic-AMP responsive element
modulator/inducible cyclic-AMP early repressor messenger RNA
expression in the rat brain. Neuroscience. 86:499–510. 1998.
View Article : Google Scholar : PubMed/NCBI
|




















