Introduction
Advancements in the diagnosis and treatment of
primary cancers have paradoxically led to an increased incidence of
metastatic brain tumors (MBTs) (1)
that have an unfavorable clinical prognosis (2). Since patients with metastasis
typically have multiple brain lesions, local treatment modalities
such as surgery and radiation therapy are impractical. Furthermore,
chemotherapeutic agents typically cannot penetrate through the
blood-brain barrier (BBB), making systemic chemotherapy ineffective
(3). Palliative whole-brain
irradiation or stereotactic radiosurgery is currently the sole
viable option for MBTs.
The tumor-tropic properties of neural stem cells
(NSCs) provide a novel approach that can overcome the
aforementioned challenges in developing chemotherapy regimens for
MBTs (4). NSCs can be genetically
engineered to carry therapeutic genes to tumor lesions. For
example, cytosine deaminase (CD), which converts 5-fluorocytosine
(5-FC) to 5-fluorouracil (5-FU), can be engineered into NSCs to
specifically target the multiple lesion sites of MBTs. This is
significant since 5-FU, although effective, cannot penetrate the
BBB and have toxic effects (3,5–8), while
5-FC is BBB-permeable and nontoxic allowing systemic
administration. We previously used an immortalized human NSC line
expressing CD and showed its significant therapeutic effects
against brain tumors (5,7).
In order to ensure therapeutic effects, there must
be a high local concentration of 5-FU. However, 5-FU itself does
not act as a strong cytotoxic compound until it is converted to
5-FU ribonucleoside (5-FUR) by endogenous enzymes (9,10). As
one strategy to enhance this conversion, a previous report showed
that the expression of uracil phosphoribosyl transferase in
small-cell lung cancers led to superior therapeutic effects
(11). For therapeutic purposes,
however, such genetic manipulation of cancer cells is not
plausible. As an alternative strategy, the level of
ribose-1-phosphate in cancer cells can be increased by
supplementing excess levels of ribonucleosides to drive the
endogenous biochemical reaction of 5-FU to 5-FUR (Fig. 2). Therefore in this study, we
explored the effects of systemic administration of ribonucleosides,
in addition to the administration of 5-FU pro-drug 5-FC, to
potentiate the therapeutic effects of genetically engineered NSCs
for the treatment of MBTs in in vitro and in vivo
models.
Materials and methods
Cell culture
Immortalized human fetal NSCs (F3), F3 NSCs
expressing Escherichia coli CD (F3.CD), and a human breast
cancer cell line MDA-MB-435 (American Type Culture Collection,
Manassas, VA) were cultured in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine,
100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml
amphotericin B (Invitrogen).
Genetic engineering of F3.CD
Preparation of the F3.CD from the parental F3 line
was previously described (5,12,13).
Briefly, an expression plasmid encoding CD was constructed using
the retroviral pBABE-puro backbone and 1.5-kDa CD cDNA. Vectors
were packaged by co-transfecting PA317 cells with the expression
plasmid and the MV12 envelope-coding plasmid. The resulting
supernatant was used for multiple infections of F3 cells.
Transduced F3.CD cells were selected with 3 μg/ml puromycin
(Invitrogen) over four weeks.
Cytotoxic activities of 5-FU and
5-FUR
To confirm the differential cytotoxic activity of
5-FU and 5-FUR, 1×104 MDA-MB-435 cells were plated in
96-well plates. Twenty-four hours after seeding, 5-FU or 5-FUR
(Sigma) (0, 0.05, 0.5, 5, 50 or 500 nM) was applied for 48 h. The
status of the cells was analyzed using a microscope, and their
viability was determined with a colorimetric assay (Cell Counting
Kit-8; Dojindo Molecular Technologies).
In vitro effects of ribonucleoside
supplementation
MDA-MB-435 (2.5×103) and F3.CD (or F3)
(2.5×103) cells were co-cultured in 96-well plates for
24 h. 5-FU (or 5-FC) (0.5 mM) and 0.2 mM ribonucleoside (adenosine,
guanosine or uridine) was added. After a 48-h treatment, viability
was measured as previously described. To confirm the enhanced
production of 5-FUR, 1.5×106 F3.CD cells were suspended
in 2 ml PBS with 2 mM 5-FC with or without 10 mM adenosine at 37°C
for 48 h. The resulting conditioned media were collected, mixed
with an equal volume of methanol, and filtered by sterilized PVDF
filters. The filtrates were analyzed by HPLC (Younglin; Solvent
Delivery Pump SP930D, UV/730D absorbance detector, rheodyne
injector) using Capcell Pak C18 column (type UG120, 5
μm, size 4.6 mm ID × 250 mm; Shiseido Co., 1 ml/min flow rate, 265
nm detection).
In vivo effects of systemic adenosine
administration on the F3.CD and 5-FC treatment against MBTs
The animal experiments were approved by the Review
Board of Samsung Biomedical Research Institute (Seoul, Korea).
MDA-MB-435 cells (1×105) in 10 μl HBSS were directly
implanted into the brains of anesthetized BALB/c-nu mice (6-weeks
old) using a rodent stereotactic frame [co-ordinates:
anterior/posterior (AP) +1.0 mm, medial/lateral (ML) +1.7 mm,
dorsal/ventral (DV) −3.2 mm, depth from the dura matter 2 mm].
Thirteen and twenty days after MDA-MB-435 cell implantation,
animals were subjected to contralateral injection (AP +1.0 mm, ML
−1.7 mm, DV −3.2 mm) of 5 μl HBSS (Group I/II, n=10 for each group)
or F3.CD cells (1×105) in 5 μl HBSS (Group III/IV, n=10
for each group). The Group II/IV and Group III/IV received
intraperitoneal (i.p.) injection of adenosine (200 mg/kg in 200 μl
PBS) and 5-FC (500 mg/kg in 200 μl normal saline), respectively,
every day for 5 days after each contralateral injection. Two days
after the last i.p. injection, brains were removed and cut into 4-
to 6-mm slices. For tumor volume measurement (largest
width2 × largest length × 0.5), the brain slices were
fixed in 10% formalin/PBS, embedded in paraffin, sectioned into
4-μm coronal sections, and stained with hematoxylin and eosin
(H&E). A rabbit anti-CD polyclonal antibody (1:1,000; Dr K.S.
Aboody, City of Hope Medical Center, Duarte, CA, USA) was used for
immunostaining.
Statistics
Statistical comparisons were performed using the
Student’s t-test. Survival analysis was performed using the
Kaplan-Meier and the log-rank tests. P-values <0.05 were
considered to indicate a statistically significant result.
Results
Superior cytotoxic activity of 5-FUR
against MDA-MB-435 cells
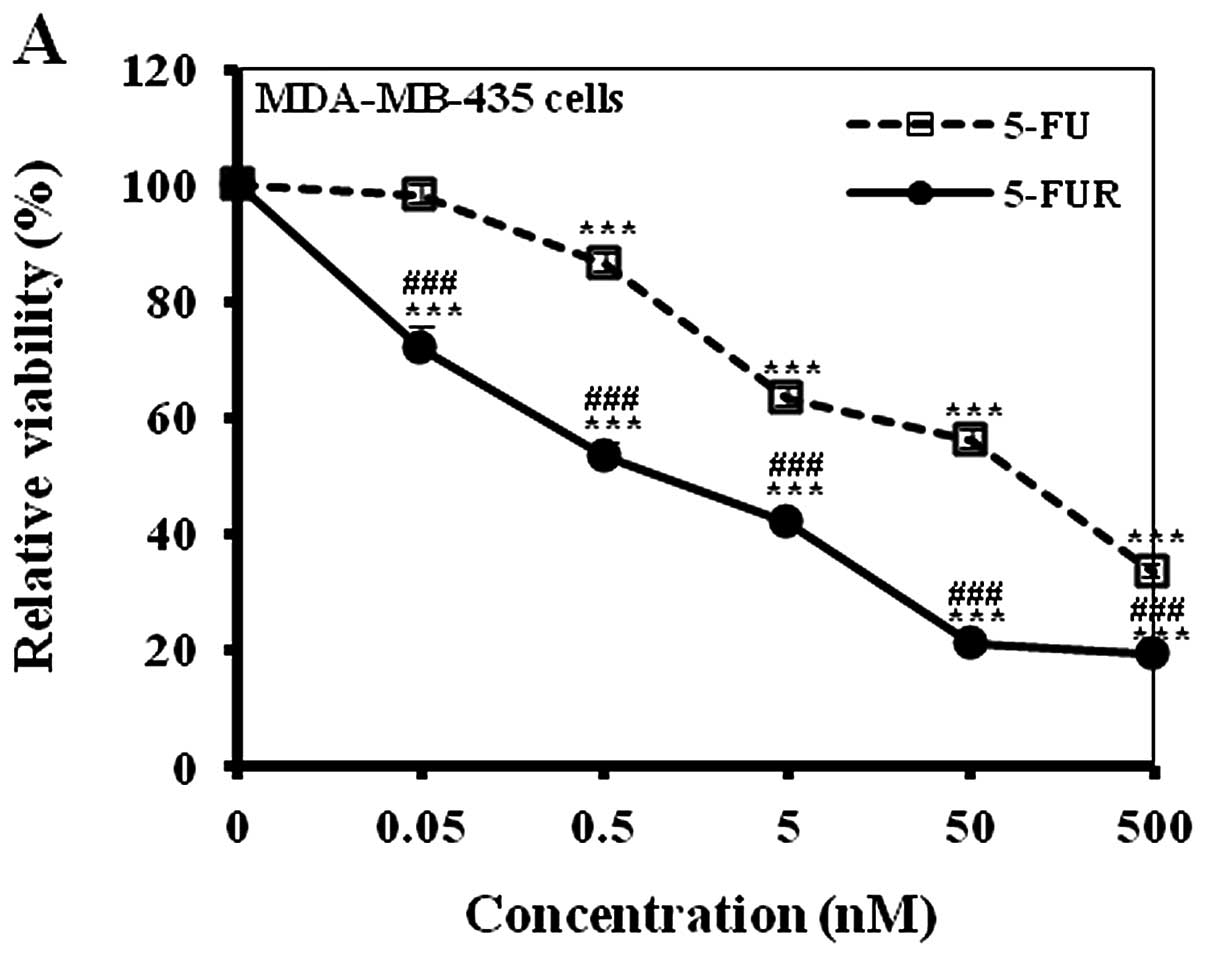
To confirm the differential cytotoxic activity of
5-FU and 5-FUR, MDA-MB-435 cells were treated with 5-FU or 5-FUR
(0, 0.05, 0.5, 5, 50 or 500 nM) for 48 h. When cell viability was
determined with a colorimetric assay, 5-FUR showed significantly
higher cytotoxic effects than 5-FU at all tested doses (Fig. 3A). These results correlate well with
a prior report, which showed that the cytotoxic effect of 5-FU is
mediated by its toxic metabolite 5-FUR (10).
In vitro effects of ribonucleoside
supplementation on the cytotoxicity of 5-FU
Sufficient ribose-1-phosphate supplementation
facilitates the conversion reaction of 5-FU to 5-FUR. When
MDA-MB-435 or F3.CD cells were treated with 0.02, 0.2 or 2 mM
ribonucleoside (adenosine, guanosine, or uridine) for 48 h,
adenosine and guanosine showed toxicity to the cells at 2 mM
(Fig. 1). In doses <2 mM, cells
tolerated the addition of all ribonucleosides (Fig. 1). Sensitivity of F3.CD, F3, and
MDA-MB-435 cells to 5-FC and 5-FU was tested. 5-FU decreased the
viability of all tested cells, but 5-FC only decreased the
viability of the F3.CD cells (data not shown). Viability of F3 and
MDA-MB-435 cells was not affected by 5-FC addition up to 0.5 mM
(data not shown). Adenosine was selected for further experiments
since it exerted the greatest reduction in viability (Fig. 1). The combination of 5-FU and
adenosine increased the cytotoxic effect in both MDA-MB-435 and
F3.CD cells (Fig. 3B and C),
suggesting that adenosine supplementation potentiated the
therapeutic effects of F3.CD and 5-FC. To confirm the facilitated
production of 5-FUR, the 48-h conditioned media from F3.CD cells
with 2 mM 5-FC and with or without 10 mM adenosine were analyzed by
HPLC. 5-FU was detected in both groups, whereas 5-FUR was found
only in the adenosine-treated group (Fig. 3D). These results indicate that F3.CD
cells have the ability to convert 5-FC to 5-FU and that the
production of toxic 5-FUR was facilitated by the supplementation of
adenosine.
In vitro effect of adenosine
supplementation on the bystander effects of F3.CD cells
MDA-MB-435 (2.5×103) and F3.CD (or F3)
cells (2.5×103) were co-cultured for 24 h. 5-FU (or
5-FC) (0.5 mM) and adenosine (0, 125, 250 or 500 nM) were added for
48 h. When MDA-MB-435 and F3.CD cells were co-cultured, the
cytotoxic effect of 5-FU was elevated dose-dependently in regards
to the adenosine addition (P<0.001, Fig. 4A), whereas adenosine treatment
without 5-FU showed only mild toxicity at 500 nM (Fig. 4A). In vitro cytotoxic effects
of 5-FC and/or adenosine were not observed in the MDA-MB-435 and F3
co-culture (Fig. 4B). However,
addition of adenosine increased the bystander cytotoxicity of F3.CD
and 5-FC on MDA-MB-435 cells dose-dependently (P<0.01, Fig. 4B). Therefore, the adenosine
treatment improved the therapeutic activities of the NSCs
expressing CD.
In vivo effects of adenosine
supplementation on the F3.CD and 5-FC treatment against brain
metastatic tumors
MDA-MB-435 cells (1×105) were directly
implanted into the brains of BALB/c-nu mice (Fig. 5A). Thirteen and twenty days after
the tumor cell implantation, animals were subjected to
contralateral injection of 5 μl HBSS (Group I/II, n=10 for each
group) or 1×105 F3.CD cells in 5 μl HBSS (Group III/IV,
n=10 for each group) (Fig. 5A).
Group II/IV and Group III/IV received i.p. injection of adenosine
(200 mg/kg) and 5-FC (500 mg/kg), respectively, every day for 5
days after each contralateral injection (Fig. 5A). Two days after the last i.p.
injection, tumor volumes and migration of implanted F3.CD cells
were measured (Fig. 5A). A large
number of CD-immunoreactive F3.CD cells were identified in the
tumor bed and at the tumor normal parenchyma interface. Some
migrating F3.CD cells were also found in the corpus callosum
(Fig. 5B), confirming the
tumor-tropic activity of F3.CD cells. Histological analysis showed
significantly (P<0.05) reduced tumor volumes in the brains of
the F3.CD and 5-FC-treated animals (Group III) compared with the
control groups [Group I, 18.3±5.4 mm3 (mean ± SE); Group
II, 17.3±4.9 mm3; Group III, 6.9±1.6 mm3]
(Fig. 5C and D). Systemic
administration of adenosine (Group II) had no significant effects
on the tumor volume, compared with the control group (Group I)
(Fig. 5C and D). Group IV, which
underwent F3.CD + 5-FC treatment and additional systemic adenosine
supplementation, showed a further decrease in the tumor volume
(3.5±0.9 mm3) at a statistically significant level
compared with that of Group III (Fig.
5C and D).
Discussion
Recent studies have found that immortalized and
genetically engineered NSCs display tumor-tropic activities that
may be exploited for tumor-specific gene therapy for various types
of tumors including MBTs (3,5,7,8,14).
Previously, F3.CD cells, immortalized human fetal NSCs expressing
the therapeutic CD gene, led to a marked reduction in tumor burden
and significantly prolonged the survival of brain tumor-bearing
animals (5). In various animal
models, the safety, feasibility and efficacy of NSCs to track
invasive tumor cells and distant microtumor foci, and their ability
to deliver therapeutic gene products to tumor cells have been
confirmed. Thus, an effective antitumor modality overcoming the
obstacles of current gene therapy strategies have been provided
(8), leading to the FDA approval of
the first human NSC clinical trial to treat glioma patients in
2010.
We previously reported this advantage on brain
metastatic tumors using the same animal model as this study
(5). The cytotoxic drug 5-FU is
effective in the treatment of various primary tumors, but it
suffers from the inability to penetrate across the BBB. However,
5-FC, the prodrug form of 5-FU, readily penetrates across the BBB
into the brain parenchyma. 5-FC is converted to 5-FU by CD, an
enzyme which is not encoded by the human genome. Therefore, if CD
could be delivered to or locally expressed in MBTs, the prodrug
would have great potential in the treatment of MBTs. Brain
metastases are different from primary brain tumors such as gliomas
and medulloblastomas since they originate from cells that do not
reside in the brain. We showed that NSCs injected into the
contralateral hemisphere migrate to breast tumor brain metastases.
This suggests that breast tumor brain metastases attract NSCs.
In the present study, we tested the hypothesis that
sufficient ribonucleoside could potentiate the tumor cell
inhibitory effect of F3.CD and 5-FC treatment. 5-FU locally
produced by F3.CD cells could be degraded by a cell detoxification
system before it is converted to its toxic metabolites, 5-FU
ribonucleoside (5-FUR) or 5-FU ribonucleotide (9,10). The
putative mechanism of ribonucleoside addition is that it would
deviate the balance of the chemical reaction by the endogenous
enzyme nucleoside phosphorylase to produce 5-FUR (Fig. 2). In case of no adenosine
(ribonucleoside) supplementation, nucleoside phosphorylase uses
endogenous ribose-1-phosphate. However, the concentration of 5-FUR
produced from endogenous ribose-1-phosphate was minimal, and could
not be detected in this study. In vitro and in vivo,
survival and/or proliferation of tumor cells were inhibited more
significantly by F3.CD and 5-FC when adenosine was supplied.
Simple adenosine or addition of another nucleoside
could improve the antitumor activity of NSCs carrying the
therapeutic gene CD. This supplementation method could be applied
to other suicide gene therapies against various tumor types. To the
best of our knowledge, this is the first report applying adenosine
supplementation therapy to NSC-based gene therapy. Our demonstrated
method may further increase therapeutic potential and thereby the
clinical applicability of NSC-based gene therapy further.
Acknowledgements
This study was supported by a grant from the
National R&D Program for Cancer Control, Ministry of Health and
Welfare, Republic of Korea (0820310) and by a grant of the Korea
Health Technology R&D Project, Ministry of Health and Welfare,
Republic of Korea (A120446).
References
|
1
|
Patchell RA: The management of brain
metastases. Cancer Treat Rev. 29:533–540. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seol HJ, Jin J, Seong DH, Joo KM, Kang W,
Yang H, Kim J, Shin CS, Kim Y, Kim KH, Kong DS, Lee JI, Aboody KS,
Lee HJ, Kim SU and Nam DH: Genetically engineered human neural stem
cells with rabbit carboxyl esterase can target brain metastasis
from breast cancer. Cancer Lett. 311:152–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aboody KS, Najbauer J, Schmidt NO, Yang W,
Wu JK, Zhuge Y, Przylecki W, Carroll R, Black PM and Perides G:
Targeting of melanoma brain metastases using engineered neural
stem/progenitor cells. Neuro Oncol. 8:119–126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmidt NO, Przylecki W, Yang W, Ziu M,
Teng Y, Kim SU, Black PM, Aboody KS and Carroll RS: Brain tumor
tropism of transplanted human neural stem cells is induced by
vascular endothelial growth factor. Neoplasia. 7:623–629. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Joo KM, Park IH, Shin JY, Jin J, Kang BG,
Kim MH, Lee SJ, Jo MY, Kim SU and Nam DH: Human neural stem cells
can target and deliver therapeutic genes to breast cancer brain
metastases. Mol Ther. 17:570–575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ito S, Natsume A, Shimato S, Ohno M, Kato
T, Chansakul P, Wakabayashi T and Kim SU: Human neural stem cells
transduced with IFN-beta and cytosine deaminase genes intensify
bystander effect in experimental glioma. Cancer Gene Ther.
17:299–306. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee SJ, Kim Y, Jo MY, Kim HS, Jin Y, Kim
SU, Jin J, Joo KM and Nam DH: Combined treatment of tumor-tropic
human neural stem cells containing the CD suicide gene effectively
targets brain tumors provoking a mild immune response. Oncol Rep.
25:63–68. 2011.PubMed/NCBI
|
|
8
|
Kim JH, Kim JY, Kim SU and Cho KG:
Therapeutic effect of genetically modified human neural stem cells
encoding cytosine deaminase on experimental glioma. Biochem Biophys
Res Commun. 417:534–540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Christensen CL, Zandi R, Gjetting T,
Cramer F and Poulsen HS: Specifically targeted gene therapy for
small-cell lung cancer. Expert Rev Anticancer Ther. 9:437–452.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rogulski KR, Kim JH, Kim SH and Freytag
SO: Glioma cells transduced with an Escherichia coli
CD/HSV-1 TK fusion gene exhibit enhanced metabolic suicide and
radiosensitivity. Hum Gene Ther. 8:73–85. 1997.PubMed/NCBI
|
|
11
|
Christensen CL, Gjetting T, Poulsen TT,
Cramer F, Roth JA and Poulsen HS: Targeted cytosine
deaminase-uracil phosphoribosyl transferase suicide gene therapy
induces small-cell lung cancer-specific cytotoxicity and tumor
growth delay. Clin Cancer Res. 16:2308–2319. 2010. View Article : Google Scholar
|
|
12
|
Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH,
Park IH, Ko Y, Jeong SW and Kim SU: Brain transplantation of
immortalized human neural stem cells promotes functional recovery
in mouse intracerebral hemorrhage stroke model. Stem Cells.
25:1204–1212. 2007. View Article : Google Scholar
|
|
13
|
Kim SU and de Vellis J: Stem cell-based
cell therapy in neurological diseases: a review. J Neurosci Res.
87:2183–2200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seol HJ, Yoo HY, Jin J, Joo KM, Kim HS,
Yoon SJ, Choi SH, Kim Y, Pyo HR, Lim DH, Kim W, Um HD, Kim JH, Lee
JI and Nam DH: The expression of DNA damage checkpoint proteins and
prognostic implication in metastatic brain tumors. Oncol Res.
19:381–390. 2011. View Article : Google Scholar : PubMed/NCBI
|