Introduction
Breast cancer is the most prevalent cancer in women
and affects approximately one million women worldwide (1). One out of eight women will develop
breast cancer sometime during her life. The higher prevalence of
using complementary and alternative medicine (CAM) to treat cancer
patients (40–83%) receiving conventional treatment was recognized
due to the adverse toxic side-effects of chemotherapy during breast
cancer treatment (2). Among the
different CAM regimens, homeopathy, a nearly 200-year-old system of
medicine has been shown to alleviate the side-effects of
chemotherapy in cancer patients, and homeopathic drugs possess
antitumorigenic property (3,4).
Unfortunately, scientific studies corroborating these clinical
observations are few. There are only a few reports on the mechanism
of action of homeopathic drugs in experimental cancers and cell
cultures (5–7).
In the present study, we investigated the underlying
molecular mechanisms of the antitumorigenic effects of thuja, a
promising homeopathic remedy, on breast cancer cells. Thuja, a
bioactive derivative of the white cedar tree, Thuja
occidentalis, is used in homeopathy for treating polypus type
of tumors of the breast, skin and uterus and for other diseases of
the skin, blood, gastrointestinal tract, kidney, brain and warty
excrescences (8–10). The protective effect of T.
occidentalis has also been reported against radiation-induced
toxicity in mice (11). Studies
have found that thuja has promising cytotoxic effects on Dalton’s
lymphoma ascites (DLA), Ehrlich ascites carcinoma (EAC) and lung
carcinoma L929 cells (12). Dubey
and Batra also reported hepatoprotective activities (13) and antioxidant activity (14) of thuja in CCL4-treated
liver damage in rats. In another study, in vitro treatment
with the thujone-rich fraction showed cytotoxic, antiproliferative
and apoptosis in A375 melanoma cells (9). Moreover, an in vitro study by
Frenkel et al (7) revealed
the antitumorigenic effect of thuja on breast cancer cells.
However, the underlying mode of action involved in its professed
antitumorigenic effects on breast cancer is still largely
unidentified and warrants further study. To the best of our
knowledge, therefore, this is the first report elucidating the
detailed mechanism of action of the antitumorigenic effects of
thuja on breast cancer cells.
It has been well established that the development
and growth of tumor cells are controlled by complex signaling
pathways involved in the regulation of cell death, survival and
proliferation. In mammalian cells, the response to cellular stress
factors such as DNA damage involves activation of tumor-suppressor
p53, which translates stress signals into cell cycle arrest or
apoptosis, depending on the balance between pro-apoptotic and
antiproliferative genes. Tumor-suppressor p53 being the ‘the
guardian of the genome’ is one of the most frequent targets in the
therapy of human tumors (15).
Thus, drugs reviving the tumor-suppressor functions of p53 are
effective for targeted cancer therapy.
Tumor-suppressor p53 is a redox active transcription
factor that organizes and directs cellular responses in the face of
a variety of stresses that lead to genomic instability. Evidence
suggests that reactive oxygen species (ROS) generated by cells as
products or by-products, can function either as signaling molecules
in diverse physiological processes or as cellular toxicants
(16). Indeed, low levels of ROS
have been linked to cellular proliferation and cell cycle
progression. This provides an explanation for the pro-oxidant state
invariably associated with the transformed phenotype (17). In contrast, higher levels of ROS
stimulate multiple death pathways such as typical and atypical
apoptosis and necrosis, thereby enhancing therapeutic efficiency.
In our research the anti-apoptotic (18) and anti-migratory effects of ROS
(19) were validated. Recent
studies revealed that cellular concentration and distribution of
p53 has a distinct cellular function, and that ROS act as both an
upstream signal that triggers p53 activation and as a downstream
factor that mediates apoptosis (16). Therefore, a novel approach for
enhancing therapeutic drug-mediated tumor cell death is effective
when drug-induced oxidative stress triggers p53, a redox active
transcription factor, to allow efficient execution of
drug-dependent apoptosis.
The present study investigated the antitumorigenic
potential of thuja, a bioactive extract of Thuja
occidentalis, commonly known as Arbor vitae or white cedar. Our
findings revealed that thuja preferentially induced apoptosis in
functional p53-expressing mammary epithelial carcinoma cells
sparing normal cells, highlighting the imperative role of ROS in
initiating and amplifying the p53-dependent programmed suicide of
breast cancer cells. While revealing the detail molecular
mechanism, it was found that thuja manipulates the intracellular
redox state of functional p53-expressing breast cancer cells in an
ROS-p53 feedback loop manner to amplify cell death via the
mitochondrial death pathway. In summary, the present study for the
first time elucidates the molecular mechanisms underlying the
antitumorigenic activity of thuja against breast cancer that may be
exploited to achieve efficient and safe tumor regression.
Materials and methods
Cell culture
Human mammary epithelial carcinoma cell lines, MCF-7
and MDA-MB-231, were obtained from NCCS, India. Peripheral blood
collected from healthy human volunteers with informed consent
(Institutional Review Board 1382) was centrifuged over
Ficoll-Hypaque density gradient (Amersham Pharmacia, Uppsala,
Sweden) to obtain total peripheral blood mononuclear cells. Cells
were routinely maintained in DMEM supplemented with 10% heat
inactivated fetal bovine serum (Lonza, Portsmouth, NH, USA),
L-glutamine (2 mM), sodium pyruvate (100 mg/ml), non-essential
amino acids (100 mM), streptomycin (100 mg/ml), penicillin (50
U/ml; Invitogen, Carlsbad, CA, USA) at 37°C in a humidified 5%
CO2 incubator. Cells were maintained in an exponential
growth phase for all experiments. Viable cell numbers were
determined by trypan blue exclusion test.
Treatment of cells
The different strengths (6C, 30C and 200C) of
placebo and thuja were procured from Hahnemann Publishing Co. Pvt.,
Ltd. (Kolkata, India) (authorized manufacturing house sanctioned by
both GMP and certified by ISO). The drugs procured were colorless,
odorless and endotoxin-free. Drugs were stored at room temperature,
away from sunlight and vigorously shaken immediately before each
treatment. Cells were treated with thuja or placebo of potencies 6C
or 30C or 200C at different concentrations (10, 15, 20 and 30
μl/ml) for different time-points (0, 6, 8, 12, 24, 36 and 48 h) to
select the optimum time required for cell killing. To understand
the sequence of events leading to apoptosis, cancer cells were
treated with mitochondrial pore inhibitor cyclosporine A (CsA) (25
μM; Merck, Darmstadt, Germany) for 1 h prior to incubation with
thuja. For transcriptional blockage of p53, cells were treated with
pifithrin-α (30 μM; Sigma). NAC (40 mM; Sigma) treatment was
carried out for 1 h prior to incubation with thuja for
pharmacological inhibition of ROS while H2O2
(0.5 mM; Sigma) treatment was applied for ROS enhancement.
Flow cytometry
For the determination of cell death, cells were
stained with 7AAD and Annexin V-PE and analyzed by flow cytometry
(FACSCalibur; BD Biosciences, San Jose, CA, USA). Electronic
compensation of the instrument was conducted to exclude overlapping
of the emission spectra. A total of 10,000 events were acquired for
analysis using CellQuest software (BD Biosciences) (20). For the assessment of mitochondrial
transmembrane potential, cells were loaded with potential-sensitive
dye 3,3-dihexyloxacarbocyanine iodide (DiOC6; Merck)
during the last 30 min of treatment at 37°C in the dark.
Fluorescence of retained DiOC6 was determined by flow
cytometry using logarithmic amplification by CellQuest software
(21).
Assessment of ROS
For detection of intracellular ROS, the untreated
and thuja-treated cells were incubated during the last 20 min at
37°C in the dark with 10 μM of dichlorofluorescein diacetate
(DCF-DA; Sigma). DCF fluorescence was measured by flow cytometry
and subjected to analysis using CellQuest 3.2 software. The probe
was excited at 488 nm and emission was measured through a 530 nm
band-pass filter. For confocal microscopy a Leica fluorescence
microscope DM 900 was used to visualize the images of thuja-treated
cells incubated with DCF-DA (10 μM). DCF-DA is colorless and
non-fluorescent until both of the acetate groups are hydrolyzed and
the products are subsequently oxidized to fluorescein derivatives
(H2DCFDA). Digital images were captured with a cool
(−25°C) charged coupled device (CCD) camera (Princeton Instruments)
controlled with the Andor iQ BioImaging software (BT, London, UK)
(19).
Fluorescence imaging
Chromatin condensation and nuclear fragmentation
were analyzed using a standard protocol. Briefly, cells were grown
on coverslips, fixed with 3% p-formaldehyde for 10 min and then
permeabilized with 0.1% Triton X-100 for 5 min. Cells were then
incubated with 4′6-diamidino-2-phenylindole (DAPI; BD Pharmingen,
San Jose, CA, USA). The morphology of the cell nuclei was
visualized using a fluorescence microscope (Leitz microscope fitted
with epifluorescence illuminator through a ×60 aperture oil
immersion lens; Carl Zeiss, Oberkochen, Germany). For fluorescent
imaging, cells growing on a coverslip were fixed with 3%
p-formaldehyde and were stained with the anti-p53 antibody (Santa
Cruz Biotechnology, Santa Cruz, CA, USA), after permeabilization
with Triton X-100, followed by addition of the TRITC-conjugated
secondary antibody and visualized with a confocal microscope (Carl
Zeiss).
Plasmids, siRNA and transfections
MCF-7-Dn-p53 was derived from MCF-7 cells through an
adenoviral vector expression system expressing dominant-negative
p53 (Dn-p53) under the control of a cytomegalovirus promoter
(Clontech). Similarly, p53 expression was stably knocked down in
MCF-7 cells using p53-shRNA (Santa Cruz Biotechnology). These
clones (2 μg each/million cells) were introduced into MCF-7 cells
using Lipofectamine 2000 (Invitrogen). Stably expressing clones
were isolated by limiting dilution and selection with G418 sulphate
(Cellgro) at a concentration of 400 μg/ml, and cells surviving this
treatment were cloned and screened by western blot analysis with
the specific antibody. MCF-7 cells were transfected with 300 pmol
of p38-MAPK-/Bax-/control-ds-siRNA (Santa Cruz Biotechnology) and
Lipofectamine 2000 separately for 12 h. The protein levels of
p53/p38MAPK/Bax were estimated by western blotting.
Western blot analysis
To obtain whole cell lysates, cells were homogenized
in lysis buffer (20 mM HEPES, pH 7.5, 10 mM KCl, 1.5 mM
MgCl2, 1 mM Na-EDTA, 1 mM Na-EGTA and 1 mM DTT)
supplemented with protease and phosphatase inhibitor cocktails.
Mitochondrial and cytosolic fractions were prepared (21). For direct western blot analysis, a
total of 50 μg of protein was resolved using SDS-PAGE and
transferred to nitrocellulose membranes for western blotting using
the relevant antibodies e.g., anti-caspase-6, anti-caspase-7,
anti-caspase-9, anti-p53 (DO-1), anti-p-ser-46-p53, anti-Bax
(N-20), anti-p38MAPK, anti-p-p38MAPK (pTGpY; Promega),
anti-cytochrome-c and anti-PUMA. The blots were developed
with NBT/BCIP (1:1). Equivalent protein loading in cytosolic,
nuclear and mitochondrial fractions was verified using
anti-α-actin/histone H1/MnSOD antibodies (Santa Cruz
Biotechnology), respectively.
Statistical analysis
Values are shown as standard error of the mean,
except when indicated otherwise. Data were analyzed, and
appropriate significance (P<0.05) of the differences between
mean values was determined by a Student’s t-test.
Results
Thuja induces mammary epithelial
carcinoma cell apoptosis
The effect of thuja on the viability of human breast
cancer cell line MCF-7 and normal peripheral blood mononuclear
cells (PBMCs) was examined at different potencies and placebos,
i.e., 6C, 30C, 200C, where for each potency a differential dose of
0–30 μl/ml was applied (Fig. 1A).
Total viable cells were scored by trypan blue dye exclusion assay.
It was observed that among all the potencies of thuja, a 30C
potency resulted in the most significant decrease (P<0.001) in
cell viability as compared to its placebo treatment (Fig. 1A). Moreover, at a 20 μl/ml dose of
30C, the percent MCF-7 cell death reached a plateau (Fig. 1A) while under the same conditions
the PBMC viability was found to be >90% (Fig. 1A). These results indicated better
efficacy of a 20 μl/ml dose of 30C thuja in MCF-7 cell killing with
minimum toxicity leading us to perform all further experiments
using this potency and dose of thuja. The time-dependent effect
(0–48 h) of thuja at 30C (20 μl/ml) in comparison to the placebo on
MCF-7 and PBMCs was examined, and the percentage of cell death was
assessed. Thuja at 30C at a concentration of 20 μl/ml exerted
significant cell death in MCF-7 cells in a time-dependent manner.
However, significant cell death in PBMCs was noted from 24 h
onwards following thuja treatment (Fig.
1B).
 | Figure 1Thuja induces apoptosis in breast
cancer cells. (A) The number of viable MCF-7 cells (upper panel)
and PBMCs (lower panel) following exposure to different potencies
(6C, 30C and 200C) of placebo and thuja at different concentrations
(10, 15, 20 and 30 μl/ml) was determined by trypan dye exclusion
assay, and the data are represented graphically
(*P<0.05, ***P<0.001 when compared with
the respective placebo-treated group). (B) Percentage of cell death
in MCF-7 cells (upper panel) and PBMCs (lower panel) was determined
following exposure to placebo and thuja at 30C (20 μl/ml) for
different time intervals (0, 6, 12, 24, 36 and 48 h) by trypan blue
positivity, and the data are represented graphically
(**P<0.01, ***P<0.001 when compared
with the respective placebo-treated group). (C) MCF-7 cells treated
with placebo and thuja at 30C (20 μl/ml) for 24 h were subjected to
Annexin V-PE/7AAD staining, and Annexin V-PE/7AAD-positive MCF-7
cells (regarded as apoptotic cells) were analyzed by flow
cytometry. (D) DAPI staining showed nuclear blebbing (arrows) and
nuclear fragmentation in the MCF-7 cells treated with thuja at 30C
(20 μl/ml) when visualized under a fluorescence microscope. Values
are the mean ± SEM of three independent experiments in each case or
representative of typical experiment. |
To confirm the nature of cell death as apoptosis,
Annexin V-PE/7AAD binding assay was utilized. Our flow cytometric
data demonstrated that, in comparison to placebo-treated MCF-7
cells, thuja-treated unfixed MCF-7 cells showed Annexin
V-PE-binding with minimum 7AAD binding (Fig. 1C) indicating that the mode of cell
death was apoptosis but not necrosis. These findings were confirmed
by the presence of nuclear fragmentation as evidenced by
DAPI-stained fluorescent images of the thuja-treated MCF-7 cells
(Fig. 1D). Collectivelly, these
data revealed that thuja at 30C asserts an apoptogenic effect on
mammary epithelial cancer MCF-7 cells.
Thuja-induced apoptosis is favored in
functional p53-expressing cells
Since tumor-suppressor protein p53 plays an
important role in the canonical apoptotic pathway, we explored the
changes in the protein profile of p53 after thuja administration in
wild-type p53-expressing MCF-7 cells. Our results depicted a
significant increase in the levels of p53 in MCF-7 cells after
thuja treatment (Fig. 2A). To
confirm whether or not thuja-induced cancer cell apoptosis is
p53-dependent, the percentage of apoptosis as detected by Annexin
binding was assessed in wild-type p53-expressing MCF-7 cells,
p53-mutated MDA-MB-231 cells and p53-shRNA-transfected MCF-7 cells
following thuja treatment (Fig.
2B). Notably, thuja at 30C at a 20 μl/ml dose significantly
(P<0.001) induced apoptosis in the wild-type p53-expressing
MCF-7 cells. The apoptogenic insult asserted by thuja was ~38% as
compared to the placebo effect which was only 10%, while
p53-mutated MDA-MB-231 and p53-knockdown MCF-7 cells were resistant
to the thuja-induced insult (Fig.
2B). To understand the mechanism of thuja-induced apoptosis,
the p53 cellular localization of p53 in MCF-7 cells following thuja
administration was examined by confocal microscopy. Translocation
of the p53 protein from the cytosol to the nucleus was noted in the
thuja-exposed MCF-7 cells (Fig.
2C). It is known that p53, being a transcription factor, is
activated when localized in the nucleus and transactivates its
downstream target genes. Therefore, the time-dependent accumulation
of p53 both in the nuclear and cytosolic fractions and the status
of its transactivated gene products, i.e., Bax and PUMA, in
thuja-treated and untreated MCF-7 cells were examined by western
blot analysis. Our results revealed that, in comparison to the
untreated and placebo-treated tumor cells, thuja-exposed cells
showed increased nuclear p53 expression with concomitant increase
in the levels of p53 transactivated gene products, Bax and PUMA, in
a time-dependent manner from 4 h onwards following thuja
administration as detected by western blot analysis (Fig. 2D and E). These results indicated
that p53 is imperative for thuja-induced functional p53-expressing
MCF-7 cell apoptosis.
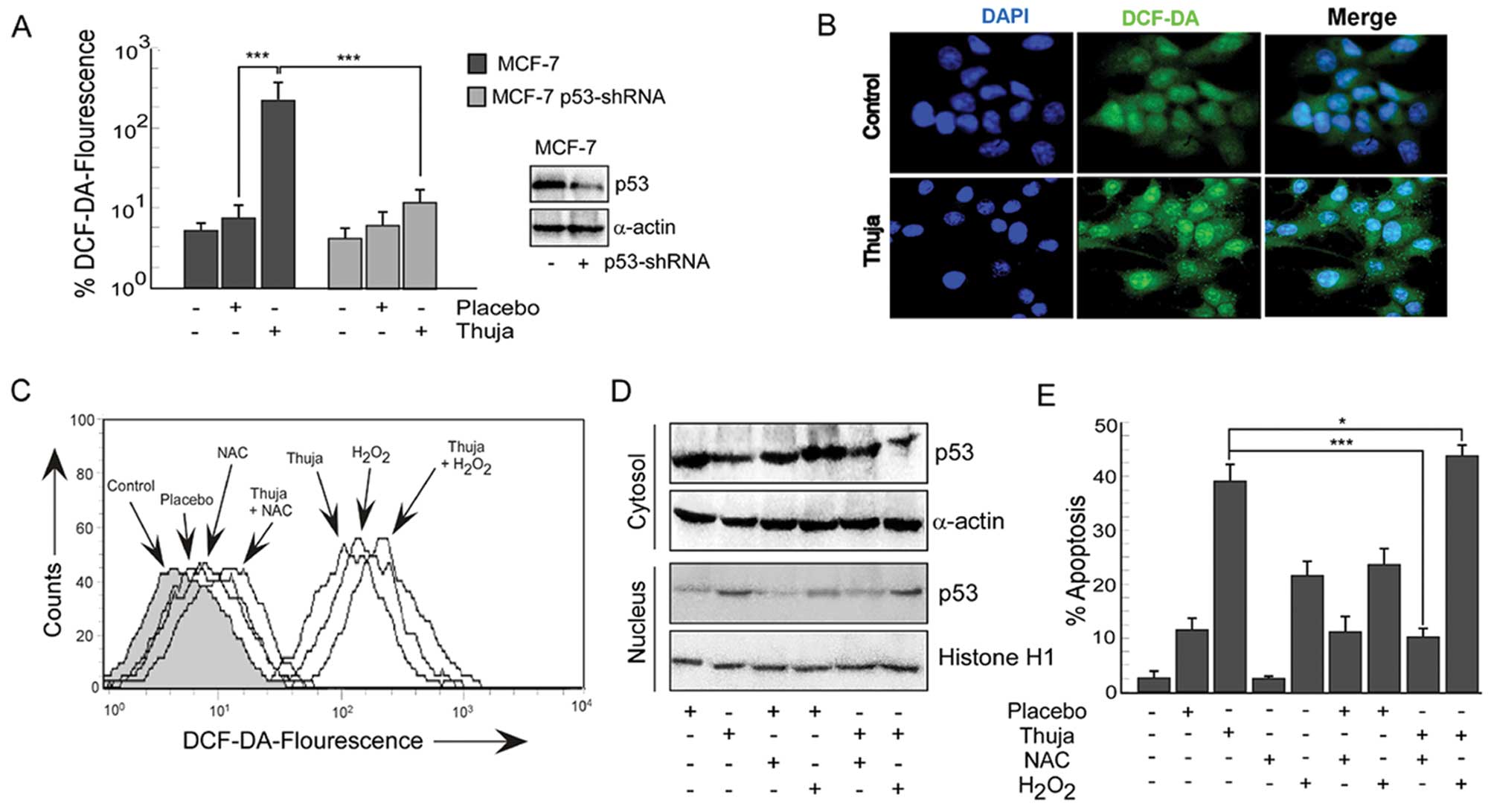
Thuja provokes ROS-p53 crosstalk in
mammary cancer cells
Since reactive oxygen species (ROS) have been
implicated as potential modulators of p53-dependent apoptosis, we
explored the role of ROS in thuja-induced p53-dependent MCF-7 cell
apoptosis. Our results revealed that, in comparison to the
untreated and placebo-treated MCF-7 cells, exposure to thuja caused
a significant increase in the levels of intracellular ROS in these
functional p53-expressing cells (Fig.
3A and B). Notably, thuja-treated p53-shRNA-transfected MCF-7
cells showed a much significant reduction in the ROS level as
compared to the functional p53-expressing MCF-7 cells although
complete abrogation of ROS was not observed (Fig. 3A). Efficiency of p53-shRNA
transfection was confirmed by western blot analysis (Fig. 3A, western blot). These findings
indicated that thuja-induced ROS production was significantly and
predominantly p53-dependent. These data suggest that p53 might
regulate the intracellular redox status in these cancer cells and
induce apoptosis by an ROS-dependent pathway. In addition, these
results also indicated the possibility of p53-independent ROS
production in the thuja-treated MCF-7 cells.
To further validate the role of ROS in thuja-induced
p53-dependent apoptosis, two approaches were employed. Firstly,
MCF-7 cells were pretreated with a pharmacological inhibitor of
ROS, i.e., N-acetyl-L-cysteine (NAC), before thuja administration
and secondly, H2O2, a pharmacological ROS
inducer, was administered in the presence and absence of thuja.
Thereafter, both the experimental sets were scored for the levels
of intracellular ROS, levels of p53 in nuclear and cytosolic
fractions and percent apoptosis. Notably, while NAC pretreatment
resulted in loss of DCF-DA fluorescence (Fig. 3C), reduction in nuclear p53 levels
(Fig. 3D) and significant decrease
in percent apoptosis (Fig. 3E) in
contrast to thuja administration alone, H2O2
treatment significantly upregulated intracellular ROS (Fig. 3C), increased nuclear p53 (Fig. 3D) and increased thuja-induced
apoptosis (Fig. 3E). Since the
silencing of p53 abrogates ROS production and inhibition of ROS
decreases p53 expression, there is the inter-dependency and
crosstalk between p53 and ROS in executing thuja-induced
apoptosis.
Thuja-induced ROS activate p53 in a
p38MAPK-dependent manner to generate a feedback loop
It is known that p53 is a redox-regulated
transcription factor. Thus, to delineate the functional impact of
thuja-induced ROS on p53 activation, several experiments were
designed. Our earlier findings implicated the role of thuja-induced
ROS in nuclear translocation of p53, a prerequisite for its
transcriptional activation (Fig.
3D). It is known that to circumvent diverse activities in
response to varied stress stimuli, p53 protein is under tight
regulatory control of post-translational modifications where
phosphorylation is one of the key events modulating p53 functions
(22). Our search for the factor(s)
responsible for functional activation of p53 revealed that, in
response to thuja, p53 manifested phosphorylation at serine 46
residues (Fig. 4A). Based on
previous research which found that p38MAPK regulates p53 function
in response to ROS-induced DNA damage (23), we evaluated the role, if any, of
p38MAPK in thuja-induced p53 activation. Our results indicated
upregulation of the phosphorylated form of p38MAPK without
intervention of the total p38MAPK status in MCF-7 cells following
thuja treatment with simultaneous phosphorylation of p53 at Ser46
and an increase in p53 transactivated gene product, Bax, while the
placebo failed to display any such effect (Fig. 4A).
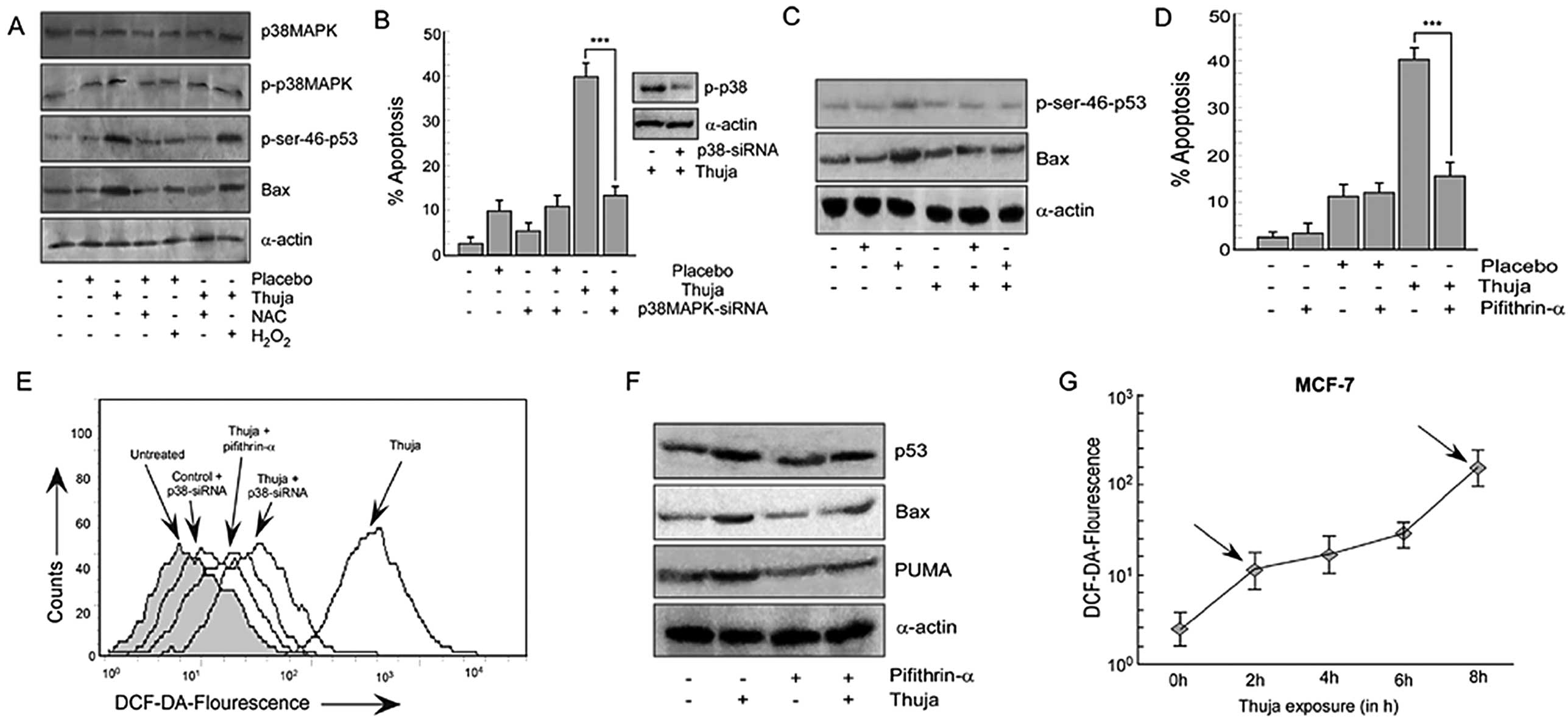 | Figure 4Thuja-induced ROS generation in MCF-7
cells is through activation of the p38MAPK pathway. (A) MCF-7 cells
were treated with thuja at 30C (20 μl/ml for 24 h) in the presence
or absence of NAC or H2O2 and the protein
expression profile of p38MAPK, p-p38MAPK, p-Ser-46-p53 and Bax was
determined by western blot analysis. (B) MCF-7 cells transfected
with p38MAPK-siRNA were subjected to thuja (20 μl/ml for 24 h)
treatment and were scored for the percentage of apoptosis by
Annexin V-PE/7AAD positivity, and data are represented graphically
(***P<0.001 when compared with the thuja-treated
group). The efficiency of p38MAPK-siRNA transfection was also
verified by western blot analysis (right panel). (C) MCF-7 cells
transfected with p38MAPK-siRNA followed by thuja (20 μl/ml)
exposure were analyzed by western blot analysis to determine the
protein levels of p-Ser-46-p53 and Bax. (D) MCF-7 cells pre-exposed
to pifithrin-α were treated with thuja at 30C (20 μl/ml for 24 h),
and the percentage of apoptosis was scored using flow cytometry by
Annexin V-PE/7AAD positivity, and data are represented graphically
(***P<0.001 compared to thuja treatment). (E) ROS
production was determined by flow cytometry by measuring
DCF-DA-fluorescence in p38MAPK-siRNA transfected and pifithrin-α (a
p53 transcriptional blocker)-treated MCF-7 cells in the presence or
absence of thuja at 30C (20 μl/ml). (F) In the same set of
experiments, the protein expression of p53, Bax and PUMA was
evaluated by western blot analysis. α-actin was used as an internal
loading control. (G) ROS production at different time points (2, 4,
6 and 8 h) in the MCF-7 cells treated with thuja at 30C (20 μl/ml)
was determined using flow cytometry by measuring
DCF-DA-fluorescence, and data are represented in a line diagram;
arrows indicate the biphasic ROS generation. |
Based on earlier observations which confirmed the
critical role of ROS in manipulating the levels of nuclear p53, we
attempted to clarify the mechanistic link between thuja-induced
ROS, p38MAPK and p53 phosphorylation. Thus, we analyzed the level
of total p38MAPK, p-p38MAPK and p-Ser46-p53 in thuja-induced MCF-7
cells upon administration of either NAC or
H2O2. As shown in Fig. 4A, NAC pretreatment abolished
phosphorylation of p38MAPK without affecting total p38MAPK and
simultaneously significantly decreased the p-Ser46-p53 level.
Reduction in intracellular ROS levels in NAC-treated cells further
confirmed the inhibitor efficiency (Fig. 3C). In contrast, treatment with
H2O2 resulted in an increase in p-p38MAPK and
phosphorylated-p53 levels. An increase in DCF-DA fluorescence in
the H2O2-treated cells confirmed an increase
in intracellular ROS levels (Fig.
3C). All of these results together underscore the role of ROS
in sustaining the activity of p38MAPK and p53.
p38MAPK-dependent p53 transactivation
causes thuja induced apoptosis
To ascertain the contribution of p38MAPK in
thuja-mediated apoptosis through post-translational modification of
p53, transiently silenced p38MAPK MCF-7 cells were used and
analyzed for the status of p-Ser46-p53 and Bax. The percentage of
apoptosis was also determined in these engineered as well as
control MCF-7 cells with and without thuja administration. The
silencing of p38MAPK downregulated the p-p38MAPK level in the
transfectants thereby confirming the transfection efficiency
(Fig. 4B, western blot) and this
decrease in p-p38MAPK significantly decreased thuja-induced
apoptosis (Fig. 4B). It was
observed that in contrast to the thuja-treated wild-type MCF-7
cells, p38MAPK-silenced MCF-7 cells exhibited decreased p-Ser46-p53
(Fig. 4C). Consequently, our
results confirmed that p38MAPK plays a crucial role in functionally
triggering p53 to execute its transcriptional functions and finally
to execute apoptosis. To further validate the sequence of events,
p38MAPK-silenced MCF-7 cells with and without thuja administration
were analyzed for intracellular ROS levels employing DCF-DA
staining. Notably, the silencing of p38MAPK in MCF-7 cells resulted
in a significant decrease in DCF-DA fluorescence signifying the
role of p38MAPK in ROS production following thuja administration
(Fig. 4E). Additionally, similar to
p53-knockout cells, p38MAPK-silenced MCF-7 cell also could not
neutralize the ROS level completely while inhibiting ROS
downregulated p-p38MAPK.
Thuja-induced ROS-p38MAPK-p53 feedback
loop is maintained through p53 transactivation
Results of the above experiments indicated the
potential role of ROS in p53 nuclear translocation. As shown in
Fig. 2E, an increase in Bax and
PUMA protein levels in MCF-7 cells following thuja exposure
confirmed that nuclear p53 is transcriptionally active. Next, to
ensure the involvement of p53-transactivated Bax in thuja-induced
apoptosis, MCF-7 cells were pretreated with pifithrin-α (24) before evaluating thuja-induced
apoptosis. The results demonstrated that blocking p53-mediated
transactivation inhibited thuja-induced cancer cell death, thereby
confirming the involvement of the p53-mediated transactivation
pathway in thuja-induced apoptosis (Fig. 4D). While examining the p53
transactivated product, it was observed that pifithrin-α-exposed
cells, although exhibiting an increased p53 level, however, showed
a decrease in the expression levels of Bax and PUMA following thuja
administration (Fig. 4F).
In a parallel experiment, the role of ROS on the p53
transactivated gene products, ie., Bax, was analyzed in NAC- or
H2O2-pretreated thuja-exposed MCF-7 cells. It
was observed that while the quenching of ROS by NAC diminished the
levels of Bax, H2O2 exposure upregulated this
p53-transactivated product (Fig.
4A). Additionally, to investigate the contribution of p53
transactivation in thuja-mediated ROS generation and subsequent
apoptosis, the transactivation of p53 was blocked by pre-exposing
MCF-7 cells to pifithrin-α and then intracellular ROS levels and
apoptosis were assessed. Abrogating p53 transcriptional functions
in MCF-7 cells resulted in significant downregulation of ROS
production even upon thuja administration (Fig. 4E). Remarkably, it was found that
these pifithrin-α-treated cells, similar to p53-knockout or
p38MAPK-silenced MCF-7 cells, also failed to neutralize the
intracellular ROS level completely, thereby pointing towards the
possibility of p38MAPK-p53 crosstalk independent of ROS generation.
These apparently ‘contradictory’ findings point towards the
presence of an ROS-p-p38MAPK-p53 feedback loop that executes
apoptosis in thuja-treated MCF-7 cells.
Thuja-induced bi-phasic ROS generation
via p53-transactivated Bax maintains a feedback loop
The obtained results portrayed the potential role of
ROS in thuja-induced apoptosis. It was also revealed that the
ROS-induced apoptosis was not only p53- and p38MAPK-dependent but
could also act independently. In these circumstances the
possibility for the production of early ROS was assumed which may
be produced before p38MAPK and p53 activation. The above assumption
was confirmed when the time frame of ROS production was determined.
Our effort to unveil this ‘mystery’ divulged a bi-phasic increase
in ROS, with the first peak being at 2 h (Fig. 4G), even before p53 nuclear
translocation that had an onset from 4 h following thuja exposure
(Fig. 2D). This was followed by a
second higher peak of ROS production at 8 h following thuja
exposure (Fig. 4G) the timing of
which matched with the p53 transactivated Bax expression following
thuja exposure (Fig. 2E). Our
earlier results revealed that the knockout of p53 (Fig. 3A) or the blocking of p53
transcriptional activity (Fig. 4E)
resulted in a significant decrease in ROS levels. Therefore it was
hypothesized that the second peak of ROS was induced by activated
p53. To further confirm this crosstalk, Bax-deprived MCF-7 cells
with or without thuja administration were analyzed for
intracellular ROS levels. These findings confirmed the role of
thuja in the generation of p53-independent early phase ROS in p53
activation via p38MAPK that finally potentiated p53 nuclear
translocation and Bax transactivation which directed the second
peak of ROS. Additionally, these findings allowed us to hypothesize
that the p53-dependent second phase of ROS is instrumental in
maintaining p53 activation through the ROS-p38MAPK-p53 feedback
loop while executing thuja-induced apoptosis. Subsequent
experiments were designed to identify the source of ROS generation
after 8 h of thuja exposure in MCF-7 cells. Notably, blocking of
mitochondrial disruption abrogated an increase in ROS levels at 8 h
(Fig. 5A) thereby indicating the
contribution of mitochondrial membrane disruption in thuja-induced
ROS generation at a late time period.
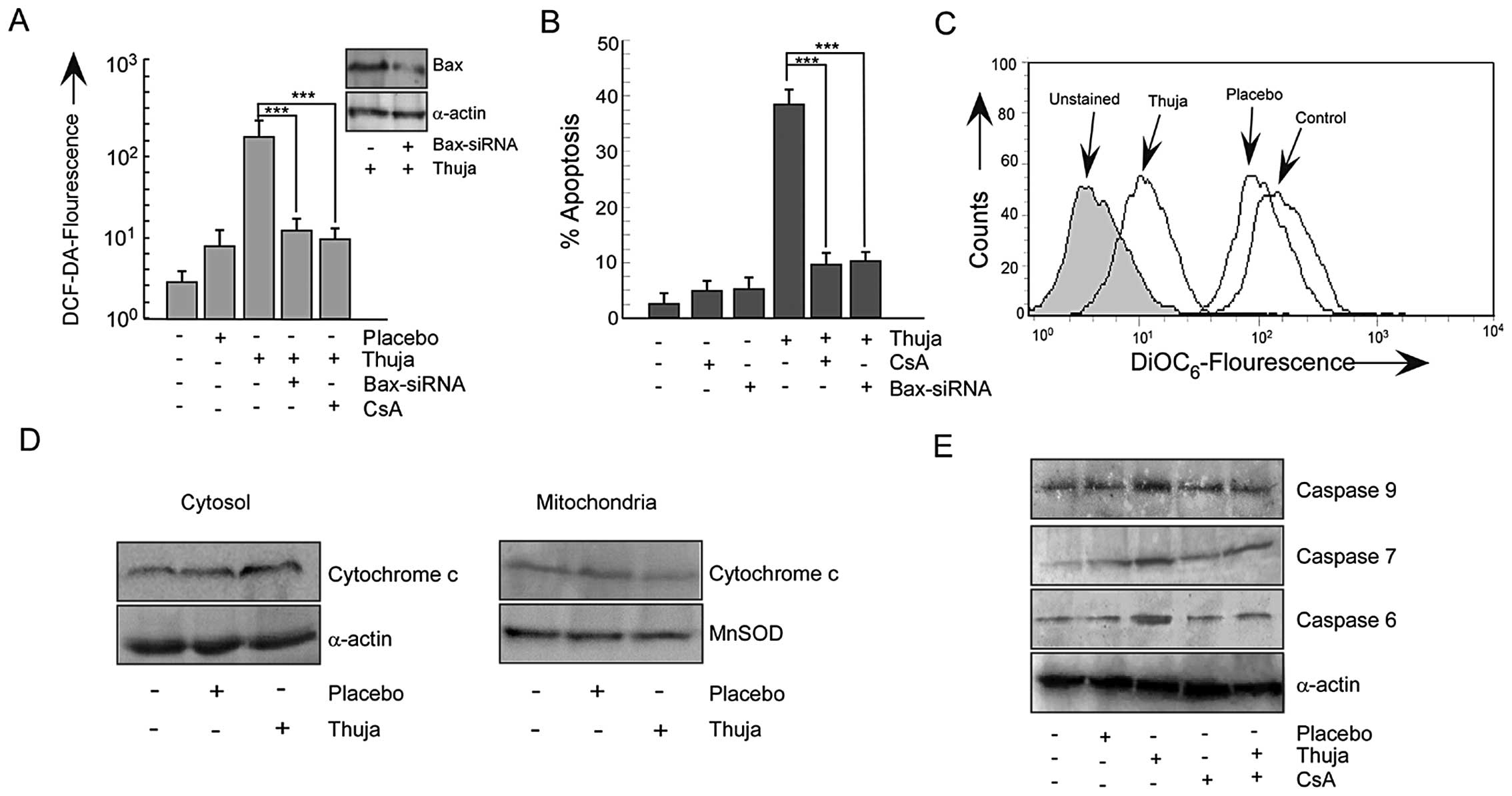
ROS-p53 feedback loop prepares the
mitochondrial death cascade in thuja-treated MCF-7 cells
An increase in Bax in apoptogenic cells tempted us
to explore the involvement of Bax in the mitochondrial
cascade-mediated apoptosis. For this two approaches were employed.
Firstly, MCF-7 cells were transfected with Bax-siRNA and secondly,
MCF-7 cells were pretreated with cyclosporine A (CsA), the
mitochondrial pore formation blocker, before thuja exposure. The
silencing of Bax or the inhibition of mitochondrial pore formation
resisted the thuja-induced ROS production (Fig. 5A) and significantly decreased the
percentage of apoptosis of MCF-7 cells (Fig. 5B). Gene manipulation or
pharmacological inhibition of p53-transactivation product Bax
failed to neutralize ROS completely reflecting the results of
p53-knockout or the p38MAPK-silencing. In the downstream of Bax,
the involvement of mitochondria was further confirmed by
DiOC6-florescent dye that demonstrated significant
mitochondrial transmembrane potential (MTP) loss in thuja-exposed
MCF-7 cells as compared to the control and placebo-treated MCF-7
cells (Fig. 5C). These findings
confirmed the role of mitochondria in executing the thuja-induced
p53-transactivated Bax-mediated apoptotic cascade. Detail
inspection determined that unlike the untreated and placebo-treated
cells, the thuja-treated MCF-7 cells showed a significant decrease
in cytochrome c level in mitochondria with a simultaneous
increase in the cytosol (Fig.
5D).
This ROS-p53 feedback loop activates the
mitochondrial death signal through cytochrome c release
finally culminating in apoptosis. Further search for the downstream
mechanism revealed that thuja influenced the execution phase of
apoptosis through activation of the initiator caspase-9 followed by
effector caspase-6 and -7 (Fig. 5E)
in these MCF-7 cells that lack functional caspase-3. Blocking
mitochondrial pore formation downregulated these caspases and
thereby implicated mitochondrial membrane disruption in
thuja-induced caspase activation and apoptosis (Fig. 5E). Mitochondrial membrane disruption
was downstream of Bax transactivation by p53. These results further
signify the existence of a feedback loop in thuja-treated MCF-7
cells in which ROS act both as an initiator of p53 activation as
well as plays a significant role in maintaining p53 in an activated
state via p38MAPK.
Discussion
Thuja, a bioactive derivative of Thuja
occidentalis, has been widely accredited for its
antitumorigenic potential (25,26).
Although reports have verified the anticancer effect of this remedy
(8–10), detailed reports elucidating the
molecular mechanisms underlying the anticancer effect of thuja are
needed. The present study demonstrated that the antitumorigenic
effect of thuja on breast cancer cells was not a ‘placebo effect’
as the placebo (potentized hydro-alcoholic solution)-treated cells
failed to induce significant cell death when compared to the
control cells. The present study further revealed that thuja
asserted its effects by re-orienting the molecular choreography of
cancer cells. Importantly, the preferential induction of the
cytotoxic effects in breast cancer cells, as compared to normal
cells, suggests a safe and non-toxic therapeutic opportunity.
It was shown that thuja is a potent inducer of
apoptosis in mammary epithelial carcinoma cells, with a more
pronounced effect in wild-type p53-expressing cells than in
functional p53-deficient cells; the contribution of p53-dependent
signaling being the underlying reason. However, two previous
independent reports from Thangapazham et al (27) and MacLaughlin et al (28) failed to confirm the inhibitory
effects of thuja in breast cancer cells and prostate cancer cells
in vitro, respectively. These apparently paradoxical results
were obtained since the studies tested the efficacy of Thuja
occidentalis on functional p53-deficient breast cancer
MDA-MB-231 cells and prostate cancer DU145 cells, as well as on
p53-null prostate cancer PC3 cells. Conversely, thuja efficiently
triggered the apoptotic cascade in the human melanoma A375 cell
line (9) and in lymphoma cells
(29), which express wild-type p53.
These findings corroborate the dependence of thuja-induced
apoptosis on the p53 status. Our study further points toward an
increase in expression as well as nuclear translocation of
wild-type p53 in thuja-exposed breast cancer cells. Moreover, the
differential susceptibility of these cell types also indicates that
the cytotoxic activities of thuja are dependent on the genetic
background of the cancer cells.
In the present study, it was found that
thuja-induced p53-dependent apoptosis in breast cancer cells was
mediated by oxidative stress. This is in accordance with recent
reports where induction of oxidative stress in response to
anticancer drugs derived from natural products has been implicated
in the activation of redox-regulated transcription factor
p53-dependent apoptosis in cervix carcinoma cells (30). Moreover, in this study, ROS
generation followed a bi-phasic pattern showing a peak at an early
time-point and another higher one at a late time-point. Although
the silencing of p53 reduced ROS production but failed to block it
completely, early ROS were generated even before p53 nuclear
translocation. Thus, it was hypothesized that ROS, generated at an
early time frame, were p53-independent and might be generated due
to initial DNA breakdown induced by thuja, similar to other
DNA-damaging anticancer agents, e.g., actinomycin D, cycloheximide
and vincristine, to activate p53 (31). This hypothesis was validated by the
experimental data which revealed that attenuated ROS production by
N-acetyl cysteine (NAC) inhibited p53 expression as well as
retarded its nuclear translocation in breast cancer cells. In
contrast, increasing ROS by H2O2 aided in p53
activation and translocation. Notably, in these cells activated p53
upregulated ROS in a feedback loop to trigger apoptosis. The
present study indicates the pivotal role of thuja in triggering
p53-dependent apoptosis through a bi-phasic increase in ROS
production.
Previous reports have shown that ROS direct
p53-mediated apoptosis through the aid of mitogen-activated protein
kinases (MAPKs) (32) that are
instrumental in p53 phosphorylation and required for its activation
(33). In fact, phosphorylation of
p53 at Ser15 and Ser20 due to DNA damage, at Ser15 and Ser37 by ATM
and ATR and at Ser20 by Chk2 and Chk1, enhances its stability and
nuclear activity (34).
Furthermore, it is proposed that p38 stress kinase also plays a
prominent role in genotoxic stress-induced activation of p53
through phosphorylation at Ser46 (33). In line with the general
understanding of ROS and p53 regulation, it was reported that
thuja-induced ROS generation triggered p38MAPK-mediated
phosphorylation of p53 at Ser46 residue. It is well appreciated
that phosphorylation of p53 at the Ser46 residue regulates the
transcriptional activation of the apoptosis-inducing gene (35). The attenuation of the apoptotic
extent in p53-silenced or pifithrin-α (a transcriptional inhibitor
of p53)-treated MCF-7 cells confirmed the transactivational role of
p53 in thuja-induced apoptosis. Moreover, the silencing of Bax, a
p53 transactivating product, reduced ROS generation as well as
apoptosis thereby authenticating that p53-mediated Bax
transactivation was required for ROS generation and apoptosis in
thuja-treated MCF-7 cells. It is also anticipated that, as the
half-life of p53 protein is very short (36), thuja-initiated cascade of
pro-apoptotic events resulting in the upsurge of mitochondrial ROS
may escalate DNA damage to maintain the p53 level in these cells.
Involvement of the mitochondria was further endorsed when ROS
generation and the percentage of apoptosis was reversed by CsA, a
mitochondrial pore formation blocker.
Collectively, these results point towards a ROS-p53
feedback loop in which, at an early phase, thuja generates ROS to
activate p53 via p38MAPK that in turn transactivates Bax to
initiate mitochondrial changes including the second phase of ROS
generation. This second phase of ROS not only helps maintain p53
through p38MAPK activation but also magnifies the thuja signal to
finally activate the caspase cascade (Fig. 6). Such a self-regulatory feedback
partnership, in fact, amplifies and accelerates the death of breast
cancer cells by thuja.
In conclusion, the present study demonstrated a dual
role of ROS, specifically, as an upstream signal that triggers p53
activation and as a downstream effector to amplify the ROS-p53
feedback loop to sustain p53 function in inducing apoptosis, in
thuja-mediated breast cancer cell apoptosis. Overall these findings
provide evidence for a molecular signature of the thuja effects in
human mammary epithelial carcinoma cells and lay the foundation for
the biological impact of the non-toxic phytochemical thuja in
clinical trials.
Acknowledgements
The authors are thankful to Mr. Uttam Ghosh and Mr.
Ranjan Dutta for their technical help. The present study was
supported by grants from the Central Council for Research in
Homeopathy (CCRH), Government of India.
Abbreviations:
|
Bax
|
Bcl-2 associated X protein
|
|
Bcl-2
|
B cell lymphoma-2
|
|
CAM
|
complementary and alternative
medicine
|
|
CsA
|
cyclosporine A
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
dihydrochloride
|
|
DiOC6
|
3,3-dihexyloxacarbocyanine iodide
|
|
MTP
|
mitochondrial membrane potential
|
|
H2O2
|
hydrogen peroxide
|
|
PBLs
|
peripheral blood lymphocytes
|
|
NAC
|
N-acetylcysteine
|
|
p38MAPK
|
p38 mitogen activated protein
kinase
|
|
ROS
|
reactive oxygen species
|
|
siRNA
|
short-interfering RNA
|
|
shRNA
|
short-hairpin RNA
|
References
|
1
|
Hanf V and Gonder U: Nutrition and primary
prevention of breast cancer: foods, nutrients and breast cancer
risk. Eur J Obstet Gynecol Reprod Biol. 123:139–149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cassileth BR and Vickers AJ: Complementary
and alternative therapies. Urol Clin North Am. 30:369–376. 2003.
View Article : Google Scholar
|
|
3
|
Flinn JE: Bromium in acute lymphatic
leukemia. J Am Inst Homeopath. 58:213–214. 1965.PubMed/NCBI
|
|
4
|
Gruchmann W: Arsenic: destroyer and
healer; a contribution to the management of carcinoma. Hippokrates.
27:444–445. 1956.(In German).
|
|
5
|
Pathak S, Multani AS and Banerji P and
Banerji P: Ruta 6 selectively induces cell death in brain cancer
cells but proliferation in normal peripheral blood lymphocytes: a
novel treatment for human brain cancer. Int J Oncol. 23:975–982.
2003.PubMed/NCBI
|
|
6
|
Pathak S, Kumar Das J, Jyoti Biswas S and
Khuda-Bukhsh AR: Protective potentials of a potentized homeopathic
drug, Lycopodium-30, in ameliorating azo dye induced
hepatocarcinogenesis in mice. Mol Cell Biochem. 285:121–131. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frenkel M, Mishra BM, Sen S, Yang P,
Pawlus A, Vence L, Leblanc A, Cohen L and Banerji P and Banerji P:
Cytotoxic effects of ultra-diluted remedies on breast cancer cells.
Int J Oncol. 36:395–403. 2010.PubMed/NCBI
|
|
8
|
Ojeswi BK, Khoobchandani M, Hazra DK and
Srivastava MM: Protective effect of Thuja occidentalis
against DMBA-induced breast cancer with reference to oxidative
stress. Hum Exp Toxicol. 29:369–375. 2010.
|
|
9
|
Biswas R, Mandal SK, Dutta S,
Bhattacharyya SS, Boujedaini N and Khuda-Bukhsh AR: Thujone-rich
fraction of Thuja occidentalis demonstrates major
anti-cancer potentials: evidences from in vitro studies on
A375 cells. Evid Based Complement Alternat Med.
2011:5681482011.PubMed/NCBI
|
|
10
|
Naser B, Bodinet C, Tegtmeier M and
Lindequist U: Thuja occidentalis (Arbor vitae): a review of
its pharmaceutical, pharmacological and clinical properties. Evid
Based Complement Alternat Med. 2:69–78. 2005. View Article : Google Scholar
|
|
11
|
Sunila ES and Kuttan G: Protective effect
of Thuja occidentalis against radiation-induced toxicity in
mice. Integr Cancer Ther. 4:322–328. 2005.
|
|
12
|
Sunila ES and Kuttan G: A preliminary
study on antimetastatic activity of Thuja occidentalis L. in
mice model. Immunopharmacol Immunotoxicol. 28:269–280. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dubey SK and Batra A: Hepatoprotective
activity from ethanol fraction of Thuja occidentalis Linn.
Asian J Res Chem. 1:32–35. 2008.
|
|
14
|
Dubey SK and Batra A: Antioxidant activity
of Thuja occidentalis Linn. Asian J Pharm Clin Res. 2:73–76.
2009.
|
|
15
|
Efeyan A and Serrano M: p53: guardian of
the genome and policeman of the oncogenes. Cell Cycle. 6:1006–1010.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu B, Chen Y and St Clair DK: ROS and
p53: a versatile partnership. Free Radic Biol Med. 44:1529–1535.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pervaiz S and Clement MV: Tumor
intracellular redox status and drug resistance - serendipity or a
causal relationship? Curr Pharm Des. 10:1969–1977. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mandal D, Lahiry L, Bhattacharyya A,
Chattopadhyay S, Siddiqi M, Sa G and Das T: Black tea protects
thymocytes in tumor-bearing animals by differential regulation of
intracellular ROS in tumor cells and thymocytes. J Environ Pathol
Toxicol Oncol. 24:91–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adhikary A, Mohanty S, Lahiry L, Hossain
DM, Chakraborty S and Das T: Theaflavins retard human breast cancer
cell migration by inhibiting NF-κB via p53-ROS cross-talk. FEBS
Lett. 584:7–14. 2010.PubMed/NCBI
|
|
20
|
Mazumdar M, Adhikary A, Chakraborty S,
Mukherjee S, Manna A, Saha S, Mohanty S, Dutta A, Bhattacharjee P,
Ray P, Chattopadhyay S, Banerjee S, Chakraborty J, Ray AK, Sa G and
Das T: Targeting RET to induce medullary thyroid cancer cell
apoptosis: an antagonistic interplay between PI3K/Akt and
p38MAPK/caspase-8 pathways. Apoptosis. 18:589–604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lahiry L, Saha B, Chakraborty J,
Bhattacharyya S, Chattopadhyay S, Banerjee S, Choudhuri T, Mandal
D, Bhattacharyya A, Sa G and Das T: Contribution of p53-mediated
Bax transactivation in theaflavin-induced mammary epithelial
carcinoma cell apoptosis. Apoptosis. 13:771–781. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saito S, Yamaguchi H, Higashimoto Y, Chao
C, Xu Y, Fornace AJ Jr, Appella E and Anderson CW: Phosphorylation
site interdependence of human p53 post-translational modifications
in response to stress. J Biol Chem. 278:37536–37544. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Freund A, Patil CK and Campisi J: p38MAPK
is a novel DNA damage response-independent regulator of the
senescence-associated secretory phenotype. EMBO J. 30:1536–1548.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murphy PJ, Galigniana MD, Morishima Y,
Harrell JM, Kwok RP, Ljungman M and Pratt WB: Pifithrin-α inhibits
p53 signaling after interaction of the tumor suppressor protein
with hsp90 and its nuclear translocation. J Biol Chem.
279:30195–30201. 2004.
|
|
25
|
Chang LC, Song LL, Park EJ, Luyengi L, Lee
KJ, Farnsworth NR, Pezzuto JM and Kinghorn AD: Bioactive
constituents of Thuja occidentalis. J Nat Prod.
63:1235–1238. 2000. View Article : Google Scholar
|
|
26
|
British Herbal Pharmacopoeia. Thuja,
British HerbalMedicine Association; West Yorks, UK: 1983
|
|
27
|
Thangapazham RL, Gaddipati JP, Rajeshkumar
NV, Sharma A, Singh AK, Ives JA, Maheshwari RK and Jonas WB:
Homeopathic medicines do not alter growth and gene expression in
prostate and breast cancer cells in vitro. Integr Cancer Ther.
5:356–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
MacLaughlin BW, Gutsmuths B, Pretner E,
Jonas WB, Ives J, Kulawardane DV and Amri H: Effects of homeopathic
preparations on human prostate cancer growth in cellular and animal
models. Integr Cancer Ther. 5:362–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Preethi K, Ellanghiyil S, Kuttan G and
Kuttan R: Induction of apoptosis of tumor cells by some potentiated
homeopathic drugs: implications on mechanism of action. Integr
Cancer Ther. 11:172–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bishayee K, Paul A, Ghosh S, Sikdar S,
Mukherjee A, Biswas R, Boujedaini N and Khuda-Bukhsh AR:
Condurango-glycoside-A fraction of Gonolobus condurango
induces DNA damage associated senescence and apoptosis via
ROS-dependent p53 signalling pathway in HeLa cells. Mol Cell
Biochem. 82:173–183. 2013.
|
|
31
|
Huschtscha L, Bartier W, Malmstrom A and
Tattersall M: Cell-death by apoptosis following anticancer
drug-treatment in vitro. Int J Oncol. 6:585–593.
1995.PubMed/NCBI
|
|
32
|
Gu B and Zhu WG: Surf the
post-translational modification network of p53 regulation. Int J
Biol Sci. 8:672–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bulavin DV, Saito S, Hollander MC,
Sakaguchi K, Anderson CW, Appella E and Fornace AJ Jr:
Phosphorylation of human p53 by p38 kinase coordinates N-terminal
phosphorylation and apoptosis in response to UV radiation. EMBO J.
18:6845–6854. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sanchez-Prieto R, Rojas JM, Taya Y and
Gutkind JS: A role for the p38 mitogen-activated protein kinase
pathway in the transcriptional activation of p53 on genotoxic
stress by chemotherapeutic agents. Cancer Res. 60:2464–2472.
2000.PubMed/NCBI
|
|
35
|
Oda K, Arakawa H, Tanaka T, Matsuda K,
Tanikawa C, Mori T, Nishimori H, Tamai K, Tokino T, Nakamura Y and
Taya Y: p53AIP1, a potential mediator of p53-dependent apoptosis,
and its regulation by Ser-46-phosphorylated p53. Cell. 102:849–862.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim H, You S, Farris J, Foster LK and
Foster DN: Post-transcriptional inactivation of p53 in immortalized
murine embryo fibroblast cells. Oncogene. 20:3306–3310. 2001.
View Article : Google Scholar : PubMed/NCBI
|