Introduction
Autophagy is a conserved catabolic process that
includes the sequestration of organelles and long-lived proteins
residing in the cytoplasm into the autophagosome. Accompanied by a
maturation process, the engulfed components are degraded by
lysosomal hydrolases. Autophagic processes have been well
characterized in yeast, and >30 autophagy-related genes have
been identified in studies of yeast genetics (1). In addition to its role in the
degradation of proteins and organelles, autophagy plays a critical
role in cellular survival by providing energy during periods of
metabolic stress. Nevertheless, depending on the cellular context
and stimulus, autophagy may be indispensable for apoptosis by
preceding and further turning on the apoptosis process [programmed
cell death (PCD) type I] (2).
Moreover, when stress conditions are excessive, autophagy becomes a
cellular suicide pathway that works by digesting essential cellular
proteins and structures. Autophagic cell death is also known as PCD
type II (3).
As the mammalian ortholog of the yeast Atg6 gene,
Beclin 1 is an essential mediator of autophagy (4–7).
Beclin 1 forms a multimeric complex with vacuolar protein sorting
34 (Vps34) and class 3 phosphatidylinositol 3-kinase (PI3k), which
is necessary for the formation of autophagosome. Accumulating
evidence has shown that Beclin 1 plays a role as a tumor
suppressor. Augmentation of Beclin 1 expression in MCF7 cells
decreased their proliferation, clonogenicity in soft agar, and
tumorigenicity in nude or severe combined immunodeficient mice
(4,8). Mice with heterozygous disruption of
Beclin 1 suffer from a high incidence of spontaneous tumors
(5,9). Studies have also shown that the
tumor-suppressing effects of Beclin 1 might also function by
regulating autophagic cell death.
Members of the Bcl-2 family are important regulators
of apoptosis (10) and include both
anti- and pro-apoptotic members. Beclin 1, has been identified as a
Bcl-2-interacting protein; this protein contains a BH3-like domain,
which binds to the anti-apoptotic Bcl-2 family of proteins such as
Bcl-2 and Bcl-xL, but Beclin 1 does not bind to the pro-apoptotic
proteins Bax and Bak (11). The
Bcl-2 protein modulates either apoptosis or autophagy through its
interaction with Beclin 1 (12,13).
The levels of the Beclin 1 and Bcl-2 proteins in human pathogenic
cells suggest the existence of an autophagy/apoptosis system
dysfunction (14). Overexpression
of Beclin 1 in MKN28 human gastric cancer cells augmented
cis-diamminedichloroplatinum (CDDP)-induced apoptosis
through an enhancement of the activity of caspase-3/-7/-9. These
studies also suggest that the pro-apoptotic function of Beclin 1
may be through the inhibition of the anti-apoptotic function of
Bcl-2 and Bcl-xL (8). Furthermore,
a positive regulation of caspase-9 by Beclin 1 has been observed in
HeLa cells. In this system, the autophagy-promoting activity of
Beclin 1 was associated with the inhibition of cellular
proliferation in the in vivo nude mouse model of
tumorigenesis (15). Research
examining the dietary bioflavonoid resveratrol (RV), demonstrated
that RV-induced apoptosis was prevented by RNA interference
knockdown of Beclin 1 in human colorectal cancer cells. The authors
conjectured that the autophagy and apoptosis pathways were strictly
linked and merged at the execution point, with the caspases acting
as death executioners downstream of autophagy (16). Beclin 1 may be the critical
‘molecular switch’ and may play an important role in regulating
autophagy and apoptosis (15).
Malignant gliomas are the most frequent and lethal
types of cancer originating in the central nervous system.
Glioblastoma (GBM) accounts for ~60–70% of malignant gliomas
(17) and is the most biologically
aggressive malignant glioma subtype. There has been marked progress
in the understanding of the molecular pathogenesis of malignant
gliomas (17,18). A previous study demonstrated the low
expression of Beclin 1 mRNA and protein in GBMs (19). We also confirmed Beclin 1 loss in
GBMs using clinical specimens (20). However, the effect of Beclin 1 loss
on the molecular genetics of the interaction between autophagy and
apoptosis in GBM cells remains unclear. The present study explored
the functioning of Beclin 1 in the induction of autophagy and the
alteration of apoptosis-related proteins. Beclin 1 was both
silenced and overexpressed in U87 GBM cells to determine if such
major changes in the activity of Beclin 1 could revert the tumor
phenotype of these cells.
Materials and methods
Cell lines and culture
Human GBM cell lines U87 and U251 were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA); these cell lines were grown and maintained in DMEM containing
10% fetal bovine serum (HyClone, Logan, UT, USA) on 2%
agarose-coated slides (Nunc, Roskilde, Denmark). Human
astrocytes-cerebellar (HAc) astroglial cells were used as controls
for these cells.
Real-time PCR
RNA extraction and RT-PCR analysis of the expression
of Beclin 1 mRNA were performed as follows; cells were collected
and total RNA was extracted with a spin column (Qiagen, Hilden,
Germany) following the manufacturer’s instructions. The first
strand of cDNA was synthesized using a cDNA synthesis kit (Promega,
Madison, WI, USA). The gene expression levels were analyzed by
quantitative real-time PCR conducted on an ABI 7500 Real-Time PCR
system (Applied Biosystems, Carlsbad, CA, USA). The primers used in
the experiments were: forward, 5′-AGCTGGATGATGAGCTGAAGAG-3′ and
reverse, 5′-GATTGTGCCAAACTGTCCACTG-3′. After an initial incubation
for 2 min at 50°C, the cDNA was denatured at 95°C for 10 min,
followed by 40 cycles of PCR (95°C, 15 sec; 60°C, 60 sec). All
results were obtained from at least three independent experiments.
Using β-actin as an internal control, the mRNA levels of all genes
were normalized.
Western blotting
Cellular protein preparations were prepared from the
cell lines. Anti-Beclin 1 (Santa Cruz Biotechnology, Santa Cruz,
CA, USA) and β-actin antibodies (Sigma, Milpitas, CA, USA) were
incubated with the cellular protein preparations for 1 h. The
secondary antibody was added to these samples, and the resulting
solutions were incubated for 30 min at room temperature in PBS-T
containing 2% BSA. Signals were detected by the ECL Plus Western
Blotting Detection System according to the manufacturer’s
specifications (Amersham, Buckinghamshire, UK).
RNA interference
siRNA was designed using an siRNA Target Finder
program (Ambion, Austin, TX, USA). The oligonucleotides that
encoded the chosen siRNA sequences were designed for insertion into
the pSUPER plasmid (OligoEngine, Seattle, WA, USA) following the
published protocols (21). The
candidate sequence-specific siRNA expression vectors were termed
Beclin 1/siRNA1, Beclin 1/siRNA2, and Beclin 1/siRNA3; the negative
control was abbreviated as pSUPER-non. Untransfected cells were
used as controls. Stable transfection of pSUPER/Beclin 1 siRNA was
performed in U87 cells using Lipofectamine 2000 (Invitrogen-Gibco,
Carlsbad, CA, USA) following the guidelines provided by the
manufacturer. The levels of knockdown of Beclin 1 mRNA and protein
were determined by western blotting and real-time PCR analysis. We
chose one of the siRNAs with the best inhibition efficiency for
further study.
Evaluation of proliferation of GBM cells
by the 3H-TdR incorporation test
Cells were incubated in 96-well microtiter plates.
Then, 1 μCi of 3H-TdR (GE Healthcare, Fairfield, CT,
USA) was added to each well and thoroughly mixed for 14 h before
harvest. [3H]Thymidine incorporation was measured as
counts per minute (cpm) as detected by a MicroBeta counter
(Perkin-Elmer, Lincoln, RI, USA).
Assessment of U87 cell apoptosis by flow
cytometry and Hoechst staining
Preparation of single U87 cells was performed as
follows; apoptosis was induced in a 1×106 cells/ml
suspension by the addition of 1 mg/ml staurosporine. Cells were
incubated in a 37°C, 5% CO2 incubator for 1 h. Next, 500
μl of non-induced cell suspension was added to a second plastic
12×75 mm test tube, followed by the addition of 5 μl of Annexin V
FITC conjugate and 10 μl propidium iodide solution. The
fluorescence of the cells was determined immediately (BD
Biosciences, Franklin Lakes, NJ, USA). At the indicated times
during treatment, cells were fixed on the dishes with methanol and
stained for 10 min with Hoechst 33258 (Beyotime Institute of
Biotechnology, Jiangsu, China) at 0.5 pg/ml. The percentage of
cells containing apoptotic nuclei was determined using fluorescence
microscopy.
Immunofluorescence
U87 cells were incubated with a cyto C-specific
(Epitomics) antibody. The cells were subsequently incubated with an
FITC-conjugated anti-rabbit secondary antibody (Jackson
ImmunoResearch Inc., West Grove, PA, USA). The mitochondria were
counterstained with MitoTracker (Invitrogen, Carlsbad, CA, USA).
Fluorescence microscopy was performed using a confocal microscope
system (Leica Microsystems, Wetzlar, Germany).
Co-immunoprecipitation
Transfected cells were lysed in cell lysis buffer
(50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40,
10% glycerol with a mixture of protease inhibitors) for 1 h. Whole
cell extracts were collected and precleared. Beads coated with
Beclin 1 antibodies were incubated with the precleared whole cell
extracts at 4°C overnight. The beads were washed with cell lysis
buffer four times. Finally, the beads were incubated in FLAG
peptides or were boiled for 10 min. The eluents were analyzed by
western blotting to test for the levels of Bcl-2, Bcl-XL, Bax and
Bid.
Statistical analysis
All data are presented as the means ± standard error
of the mean. For comparisons of the relative intensities of the
western blot bands representing Beclin 1, Bcl-2, Bcl-xL, Bax, Bak,
LC3, p62, cytochrome c and caspase-3/-7/-8/-9 normalized to
β-actin, an unpaired Student’s t-test was used to compare
differences between the paired transfectants (pcDNA3.1-Bec
transfectants vs. PcDNA3 transfectants, pSUPER-Bec transfectants
vs. pSUPER-non transfectants). The results were considered
statistically significant when P-values were <0.05.
Results
Expression of Beclin 1 and Bcl-2 family
proteins in human glial cell lines
We initially examined the expression levels of
Beclin 1 and Bcl-2 family proteins in U87, U251 and HEB cells (a
human glial cell line) by western blotting. Next, we compared the
expression levels of Beclin 1 in these cell lines. As shown in
Fig. 1, the expression of Beclin 1
was significantly lower in U87 cells. Therefore, we chose the U87
cells as a model to investigate further the function of Beclin 1.
In accordance with previous reports (22), the Bcl-2 family protein was
expressed at higher levels in the U87 cells than in normal
astrocytes (Fig. 1).
Autophagic capacity is regulated by
Beclin 1 in U87 cells
To evaluate the function of Beclin 1 in autophagy,
we altered Beclin 1 expression in U87 cells by introducing a Beclin
1 expression vector (pcDNA3.1-Bec) or an siRNA targeted to the
Beclin 1 gene (pSUPER-Bec). As shown in Fig. 2, pcDNA3.1-Bec transfectants showed
greater Beclin 1 mRNA and protein expression than pcDNA3.1
transfectants (vector transfectants). Either the mRNA or the
protein of Beclin 1 was significantly decreased in the pSUPER-Bec
transfectants compared with the pSUPER-non transfectants (scramble
RNA transfectants).
Autophagic capacity was monitored by LC3 and p62
evaluation by western blotting (23). The outcomes of western blotting
showed that the expression of LC3-II was increased in pcDNA3.1-Bec
transfectants compared with vector transfectants or untreated
cells. However, LC3-II expression was markedly decreased in the
pSUPER-Bec transfectants (Fig. 2B).
Furthermore, the level of p62 was lower in pcDNA3.1-Bec
transfectants but higher in pSUPER-Bec transfectants than either
the vector transfectants or the scramble RNA transfectants
(Fig. 2B). These results indicated
that the autophagic capacity was upregulated in pcDNA3.1-Bec
transfectants but downregulated in pSUPER-Bec transfectants. These
results indicate that Beclin 1 affects the capacity of autophagy in
U87 cells.
Beclin 1 augments apoptosis and reduces
cell proliferation in U87 cells
We then determined the effect of altered expression
of Beclin 1 on the apoptosis of U87 cells. In pSUPER-Bec
transfectants, there was no significant change in apoptosis
compared with scramble RNA transfectants (Fig. 3A). Nonetheless, an obviously
increased rate of apoptosis was observed in the flow cytometry
assay in pcDNA3.1-Bec transfectants compared with other
transfectants or untreated cells (Fig.
3A and Table I). The
proliferation of each of the U87 cell transfectants and the
untreated cells was measured. Notably, overexpression of Beclin 1
significantly suppressed the proliferation of the U87 cells,
whereas silencing Beclin 1 had no effect on cell proliferation
(Fig. 3B and C).
 | Figure 3(A) Apoptosis, as measured by flow
cytometry (FCM), of cells transfected for 48 h. In pSUPER-Bec
transfectants, there was no significant change in apoptosis
compared with scramble RNA transfectants. The rate of apoptosis was
increased during the FCM assay in pcDNA3.1-Bec transfectants
compared with other transfectants or untreated cells. (B,C)
Overexpression of Beclin 1 inhibited cellular proliferation. The
proliferation of U87 cells with each transfectant or with untreated
cells was measured. Overexpression of Beclin 1 significantly
suppressed the proliferation of U87 cells, whereas silencing Beclin
1 had no effect on cell proliferation. P<0.05. (D)
Immunodetection revealed pro-caspase-3, pro-caspase-8 and
pro-caspase-9 expression in the cells following transfection for 48
h. No significant difference in the increase of caspase-8 activity
was seen between the pcDNA3.1-Bec transfectants and the other
transfectants. However, not only was there an increase in
caspase-3/-7 activity, but there was also an increase in caspase-9
activity that was greater in pcDNA3.1-Bec transfectants than in
other transfectants. (E) Immunofluorescence and immunoblotting
detection of transfected pcDNA3.1-Bec. Cytochrome c was
released into the cytoplasm at a different time in the U87 cells.
Cytochrome c was visualized with FITC-conjugated anti-rabbit
secondary antibody (green). Mitochondria were labeled with
MitoTracker (red). Magnification, ×1,000. The expression of
cytochrome c increased over time. |
 | Table IApoptosis of transfected cells as
revealed by flow cytometric sorting. |
Table I
Apoptosis of transfected cells as
revealed by flow cytometric sorting.
| Group | Q4 Proportion of
cells (%) | Q2 Proportion of
cells (%) |
|---|
| Control | 0.67±0.25 | 0.93±0.25 |
| pSUPER-non | 1.10±0.36 | 1.63±0.40 |
| pSUPER-Bec | 0.97±0.32 | 1.50±0.60 |
| pcDNA3.1-non | 1.37±0.55 | 1.37±0.31 |
| pcDNA3.1-Bec | 6.43±0.80a,b | 6.80±1.05a,b |
Previous studies have reported that the formation of
the Beclin 1-Bcl-2 or the Beclin 1-Bcl-xL complex may set Bax and
Bak free, which could possibly trigger mitochondrial membrane
disruption and the release of apoptogenic factors, such as
cytochrome c (24). We
further analyzed the anatomical distribution of cytosolic
cytochrome c using western blotting immunofluorescence as
previously described. This analysis revealed that cytosolic
localization of cytochrome c was hardly detected in normal
U87 cells and pcDNA3.1 transfectants but flourished in the
pcDNA3.1-Bec transfectants (Fig. 3D and
E).
The activation of caspases, especially caspase-3/-9,
is critical for the apoptotic pathway, as their activation leads to
the release of cytochrome c from the mitochondria and
activation of the death signal (25,26).
We also measured caspase-8, caspase-9 and caspase-3/-7 activity in
different transfectants and untreated cells. As shown in Fig. 3D, no difference in the increase of
caspase-8 activity was observed between the pcDNA3.1-Bec
transfectants and other transfectants. However, not only was there
an increase of caspase-3/-7 activity but there was also an increase
in caspase-9 activity that was greater in the pcDNA3.1-Bec
transfectants than in the other transfectants. These results
indicated that Beclin 1 enhanced apoptosis in pcDNA3.1-Bec
transfectants by enhancing caspase-3/-9 activity.
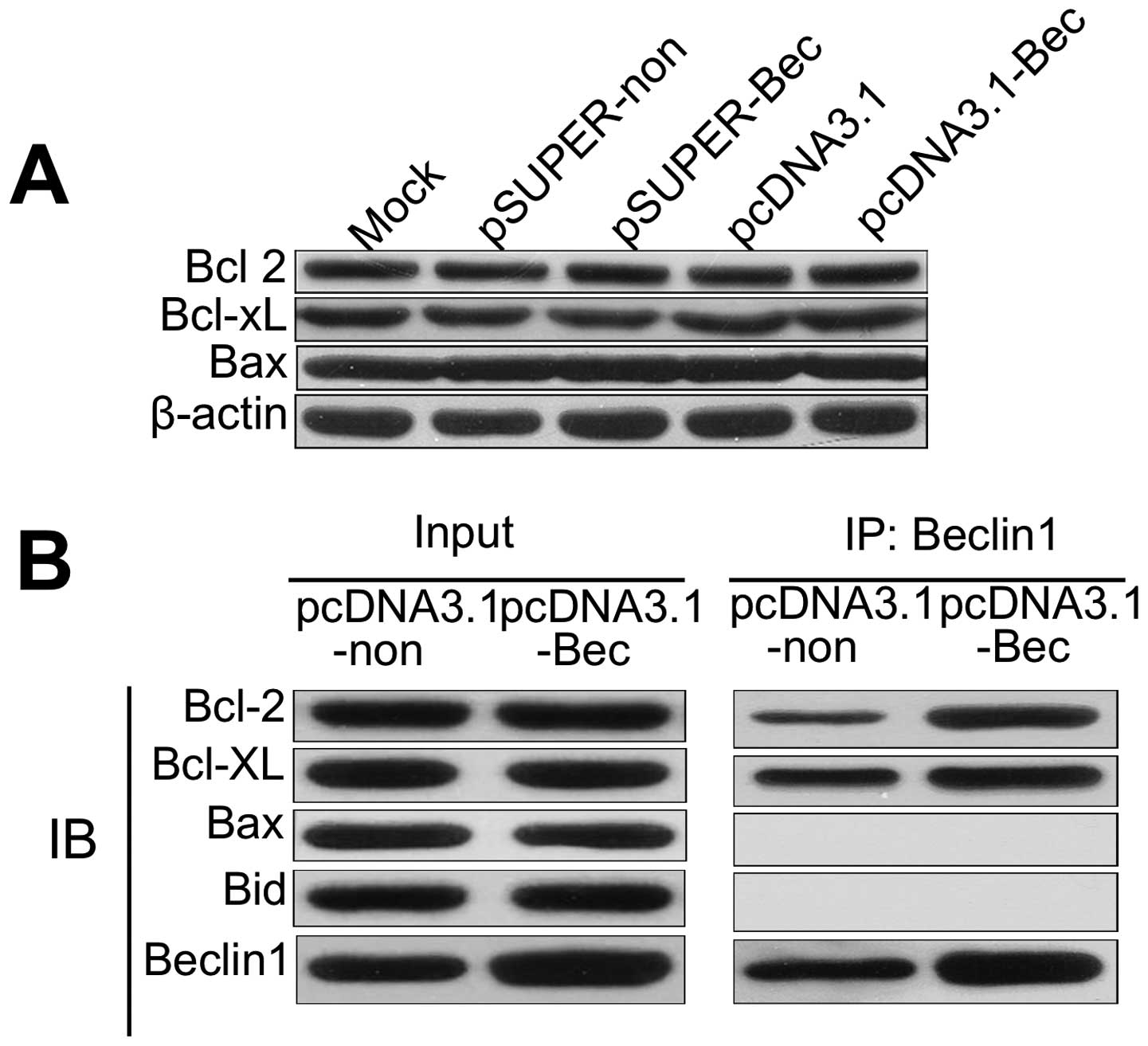
Overexpressed Beclin 1 can be combined
with higher amounts of Bcl-2
We first examined the influence of altered
expression of Beclin 1 on the expression of Bcl-2, Bcl-xl and Bax.
As shown in Fig. 4A, the expression
of the apoptosis-related proteins was not affected in pSUPER-Bec or
pcDNA3.1-Bec cells compared with controls. It can be inferred that
the overexpression of Beclin 1 increased apoptosis since Beclin 1
sequestrated Bcl-2. Overexpressed Beclin 1 binds larger amounts of
Bcl-2, which inhibited the pro-survival function of Bcl-2.
Furthermore, apoptosis may be induced by the loss of Bcl-2 and its
pro-survival functioning.
Immunoprecipitation (IP) was used to detect the
binding of Bcl-2 to Beclin 1. We examined whether Beclin 1 protein
expressed in the Beclin 1 gene transfectants bound to Bcl-2 family
proteins. In pcDNA3.1-Bec transfectants, Beclin 1 was detected in
the immunoprecipitates prepared with either the anti-Bcl-2 antibody
or the anti-Bcl-xL antibody by IP (Fig.
4B). Bcl-2 and Bcl-xL were detected in immunoprecipitates
prepared with the anti-Beclin 1 antibody (Fig. 4B). In addition, neither the Beclin
1-Bax complex nor the Beclin 1-Bak complex was detected (Fig. 4B). However, a complex of Beclin 1
and the Bcl-2 family proteins was hardly detected in pcDNA3.1
transfectants (Fig. 4B).
Overexpressed Beclin 1 cell lines can combine with more Bcl-2,
which blocks Bcl-2 cells stimulated cells for survival.
Discussion
The tumor-suppression activity of Beclin 1 has been
shown in several solid tumors including breast (4) and cervical cancer (15). Furthermore, Beclin 1 displays
growth-inhibitory activity, regulation of autophagic cell death and
induction of apoptosis. We found that enforced expression of Beclin
1 not only promoted autophagy in U87 cells but also induced
apoptosis via binding to anti-apoptotic Bcl-2 family proteins such
as Bcl-2 and Bcl-xL.
The interaction between Beclin 1 and its binding
partners regulates the initial steps of autophagy (27). The association of Beclin 1 with
hVps34 and PI3k is essential for the induction of autophagy, and
the autophagy-inducing activity of Beclin 1 is inhibited by the
overexpression of Bcl-2 family members including Bcl-2, Bcl-xL and
Mcl-1 (28,29). There is evidence that the orthologs
of Beclin 1, hVps34 and Bcl-2 do not form a trimolecular complex
(30), suggesting that Beclin 1 can
be present in only two different complexes, one that stimulates
autophagy and involves an interaction with hVps34 and another that
inhibits autophagy and involves an interaction with Bcl-2. Although
it is clear that overexpression of Bcl-2 can inhibit Beclin
1-mediated autophagy, it remains unclear whether the
autophagy-inhibitory Beclin 1-Bcl-2 or Beclin 1-Bcl-xL interactions
play any role in apoptosis.
Overexpression of Beclin 1 in U87 cells showed a
reduction of cellular proliferation but also an augmentation of
apoptosis. Therefore, apoptosis was crucial for cell death and
proliferation in our experiments. We also demonstrated that Beclin
1 induced apoptosis in U87 cells by binding to Bcl-2 and Bcl-xL,
leading to the release of cytochrome c into the cytosol and
subsequently activating caspases-3/-9. This result is in accordance
with a study by Furuya et al (8). The interaction between Beclin 1 and
the Bcl-2 family was first proposed following the observation that
Beclin 1 binds to Bcl-2 and Bcl-xL but not to Bax.
Moreover, this model was proposed after observing
that the interplay between Beclin 1 and Bcl-2 family members
indicated that Beclin 1 binds to Bcl-2 and Bcl-xL instead of Bax
(31). Analyses of the
Bcl-xL-binding domain of Beclin 1 support the postulate that Beclin
1 is a new member of the BH3-only family proteins. The finding that
Beclin 1 is a newly discovered BH3-only protein immediately raises
the possibility that Beclin 1 might have a proapoptotic role
(32). BH3-only proteins can induce
apoptosis by directly or indirectly stimulating the
mitochondrion-permeabilizing activity of the pro-apoptotic
multidomain proteins from the Bcl-2 family such as Bax and Bak
(33). The formation of the Beclin
1-Bcl-2 or the Beclin 1-Bcl-xL complex may release Bax and Bak,
which trigger mitochondrial membrane disruption and release of
apoptogenic factors, such as cytochrome c (24). It has been shown that in cells
triggered to undergo apoptosis, mitochondrial cytochrome c
is released. Under these conditions and in the presence of dATP and
Apaf-1, caspase-9 is activated, which in turn activates other
caspases, such as caspase-3 (34,35).
Some authors have suggested that Beclin 1 is not
involved in apoptotic cell death. In previous studies, Yue et
al (5) found no difference in
apoptosis induced by ultraviolet light between wild-type mouse
embryonic stem (ES) cells and Beclin 1-deficient ES cells. In a
second set of studies, Qu et al (9) reported that the proportion of
apoptotic cells in developing mammary glands of Beclin 1+/− mice
did not differ from that in Beclin 1+/+ mice; it is worth noting
that Bcl-2 and Bcl-xL are expressed less in normal cells, including
ES cells, than in cancer cells (22,36,37).
In the present study, we found that U87 cells showed less
expression of Beclin 1 but more expression of the Bcl-2 family
members than normal astrocytes. Therefore, these authors may have
failed to find the pro-apoptotic function of Beclin 1, which
involves inhibition of the anti-apoptotic function of Bcl-2 and
Bcl-xL in non-transformed cells expressing low levels of these
proteins (8).
Previous studies have shown that binding of Bcl-2 to
Beclin 1 inhibits Beclin 1-mediated autophagy (28). Thus, it is possible that the
interaction between Beclin 1 and Bcl-2 has dual effects: one is to
modulate the apoptotic pathway and the second is to inhibit
autophagy. These two effects may be mutually exclusive or may act
together. Accordingly, upon complexation, Bcl-2 might inhibit
Beclin 1’s ability to participate in the autophagic process,
whereas Bcl-2 will be concomitantly neutralized, thereby
sensitizing the cells to apoptosis. In this way, the interaction
between Beclin 1 and anti-apoptotic Bcl-2 family members may affect
cell fate (11). These observations
indicate that Beclin 1, in addition to its role in autophagy, may
play a role in apoptosis by neutralizing the anti-apoptotic
proteins in the Bcl-2 family.
In mammalian cells, knockdown of Beclin 1 sensitized
cells to apoptosis induction by starvation (38). Nevertheless, Beclin 1 downregulation
also inhibited cell death induction by conditions in which
essential pro-apoptotic mitochondrial outer membrane
permeabilization (MOMP) or caspase activation was blocked (39,40),
and restoration of normal Beclin 1 levels in Beclin 1-deficient
tumor cells facilitated the induction of cell death by vitamin D
analogues (41). In these studies,
the Beclin 1-mediated autophagy played key roles in cellular
survival or death. However, in the present study, without metabolic
stress or drugs, the levels of autophagic capacity were relatively
low. When U87 cells were cultured in full nutrient and oxygen-rich
conditions, the protective effect of energy-provision and
nutrient-supply from autophagy was useless. Reduction of Beclin 1
levels by siRNA resulted in defective autophagy, but pSUPER-Bec
transfectants had no significant difference from pSUPER-non
transfectants in apoptosis and proliferation. We suggest that the
low level of autophagic capacity in our experiment had little
influence on cell proliferation.
Cancer often expresses high levels of Bcl-2-like
anti-apoptotic proteins to evade the apoptotic fate imposed by
unscheduled cell proliferation, activation of oncogenes or DNA
damage (42,43). The downregulation of Beclin 1 in
cancer (4,44,45)
may aggravate the anti-apoptotic effect of Bcl-2-like proteins.
In conclusion, our findings indicate that Beclin 1
not only enhanced autophagy and promoted apoptosis but also reduced
cell proliferation. The above results suggested that the autophagy
gene Beclin 1 plays an important role in fine tuning autophagy and
apoptosis through interactions with Bcl-2 family members. These
proteins may be suitable molecular targets for cancer
treatment.
Acknowledgements
The authors thank Dr Chongli Yan and Dr Weiwei Guo
for their assistance and critical appraisal of the manuscript. The
present study was supported by grants from the China Postdoctoral
Science Foundation (no. 20100480568).
Abbreviations:
|
Atg
|
autophagy-related gene
|
|
Bcl-2
|
B-cell CLL/lymphoma 2
|
|
Vps34
|
vacuolar protein sorting 34
|
|
hVps34
|
human Vps34
|
|
PI3k
|
class 3 phosphatidylinositol
3-kinase
|
|
LC3
|
microtubule-associated protein 1 light
chain 3
|
References
|
1
|
Klionsky DJ, Cregg JM, Dunn WA Jr, et al:
A unified nomenclature for yeast autophagy-related genes. Dev Cell.
5:539–545. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levine B and Yuan J: Autophagy in cell
death: an innocent convict? J Clin Invest. 115:2679–2688. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clarke PG: Developmental cell death:
morphological diversity and multiple mechanisms. Anat Embryol
(Berl). 181:195–213. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang XH, Jackson S, Seaman M, et al:
Induction of autophagy and inhibition of tumorigenesis by beclin 1.
Nature. 402:672–676. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yue Z, Jin S, Yang C, Levine AJ and Heintz
N: Beclin 1, an autophagy gene essential for early embryonic
development, is a haploinsufficient tumor suppressor. Proc Natl
Acad Sci USA. 100:15077–15082. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeng X, Overmeyer JH and Maltese WA:
Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase
complex in macroautophagy versus endocytosis and lysosomal enzyme
trafficking. J Cell Sci. 119:259–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Furuya D, Tsuji N, Yagihashi A and
Watanabe N: Beclin 1 augmented cis-diamminedichloroplatinum
induced apoptosis via enhancing caspase-9 activity. Exp Cell Res.
307:26–40. 2005.PubMed/NCBI
|
|
9
|
Qu X, Yu J, Bhagat G, et al: Promotion of
tumorigenesis by heterozygous disruption of the beclin 1 autophagy
gene. J Clin Invest. 112:1809–1820. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Erlich S, Mizrachy L, Segev O, et al:
Differential interactions between Beclin 1 and Bcl-2 family
members. Autophagy. 3:561–568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trisciuoglio D, De Luca T, Desideri M, et
al: Removal of the BH4 domain from Bcl-2 protein triggers an
autophagic process that impairs tumor growth. Neoplasia.
15:315–327. 2013.PubMed/NCBI
|
|
13
|
Oh S, Xiaofei E, Ni D, et al:
Downregulation of autophagy by Bcl-2 promotes MCF7 breast cancer
cell growth independent of its inhibition of apoptosis. Cell Death
Differ. 18:452–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ricci A, Cherubini E, Scozzi D, et al:
Decreased expression of autophagic beclin 1 protein in idiopathic
pulmonary fibrosis fibroblasts. J Cell Physiol. 28:1516–1524.
2012.
|
|
15
|
Wang ZH, Xu L, Duan ZL, Zeng LQ, Yan NH
and Peng ZL: Beclin 1-mediated macroautophagy involves regulation
of caspase-9 expression in cervical cancer HeLa cells. Gynecol
Oncol. 107:107–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trincheri NF, Follo C, Nicotra G,
Peracchio C, Castino R and Isidoro C: Resveratrol-induced apoptosis
depends on the lipid kinase activity of Vps34 and on the formation
of autophagolysosomes. Carcinogenesis. 29:381–389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Furnari FB, Fenton T, Bachoo RM, et al:
Malignant astrocytic glioma: genetics, biology, and paths to
treatment. Genes Dev. 21:2683–2710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miracco C, Cosci E, Oliveri G, et al:
Protein and mRNA expression of autophagy gene Beclin 1 in
human brain tumours. Int J Oncol. 30:429–436. 2007.
|
|
20
|
Huang X, Bai HM, Chen L, Li B and Lu YC:
Reduced expression of LC3B-II and Beclin 1 in glioblastoma
multiforme indicates a down-regulated autophagic capacity that
relates to the progression of astrocytic tumors. J Clin Neurosci.
17:1515–1519. 2010. View Article : Google Scholar
|
|
21
|
Brummelkamp TR, Bernards R and Agami R: A
system for stable expression of short interfering RNAs in mammalian
cells. Science. 296:550–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rieger L, Weller M, Bornemann A, Schabet
M, Dichgans J and Meyermann R: BCL-2 family protein expression in
human malignant glioma: a clinical-pathological correlative study.
J Neurol Sci. 155:68–75. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klionsky DJ, Abeliovich H, Agostinis P, et
al: Guidelines for the use and interpretation of assays for
monitoring autophagy in higher eukaryotes. Autophagy. 4:151–175.
2008. View Article : Google Scholar
|
|
24
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hausmann G, O’Reilly LA, van Driel R, et
al: Pro-apoptotic apoptosis protease-activating factor 1 (Apaf-1)
has a cytoplasmic localization distinct from Bcl-2 or
Bcl-xL. J Cell Biol. 149:623–634. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: a double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pattingre S, Tassa A, Qu X, et al: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maiuri MC, Le Toumelin G, Criollo A, et
al: Functional and physical interaction between Bcl-XL
and a BH3-like domain in Beclin-1. EMBO J. 26:2527–2539. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takacs-Vellai K, Vellai T, Puoti A, et al:
Inactivation of the autophagy gene bec-1 triggers apoptotic
cell death in C. elegans. Curr Biol. 15:1513–1517.
2005.PubMed/NCBI
|
|
31
|
Liang XH, Kleeman LK, Jiang HH, et al:
Protection against fatal Sindbis virus encephalitis by beclin, a
novel Bcl-2-interacting protein. J Virol. 72:8586–8596.
1998.PubMed/NCBI
|
|
32
|
Feng W, Huang S, Wu H and Zhang M:
Molecular basis of Bcl-xL’s target recognition versatility revealed
by the structure of Bcl-xL in complex with the BH3 domain of
Beclin-1. J Mol Biol. 372:223–235. 2007.
|
|
33
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zou H, Henzel WJ, Liu X, Lutschg A and
Wang X: Apaf-1, a human protein homologous to C. elegans
CED-4, participates in cytochrome c-dependent activation of
caspase-3. Cell. 90:405–413. 1997.PubMed/NCBI
|
|
35
|
Li P, Nijhawan D, Budihardjo I, et al:
Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9
complex initiates an apoptotic protease cascade. Cell. 91:479–489.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Okazawa H, Shimizu J, Kamei M, Imafuku I,
Hamada H and Kanazawa I: Bcl-2 inhibits retinoic acid-induced
apoptosis during the neural differentiation of embryonal stem
cells. J Cell Biol. 132:955–968. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Orelio C, Harvey KN, Miles C, Oostendorp
RA, van der Horn K and Dzierzak E: The role of apoptosis in the
development of AGM hematopoietic stem cells revealed by Bcl-2
overexpression. Blood. 103:4084–4092. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lum JJ, Bauer DE, Kong M, et al: Growth
factor regulation of autophagy and cell survival in the absence of
apoptosis. Cell. 120:237–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shimizu S, Kanaseki T, Mizushima N, et al:
Role of Bcl-2 family proteins in a non-apoptotic programmed cell
death dependent on autophagy genes. Nat Cell Biol. 6:1221–1228.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu L, Alva A, Su H, et al: Regulation of
an ATG7-beclin 1 program of autophagic cell death by caspase-8.
Science. 304:1500–1502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hoyer-Hansen M, Bastholm L, Mathiasen IS,
Elling F and Jaattela M: Vitamin D analog EB1089 triggers dramatic
lysosomal changes and Beclin 1-mediated autophagic cell death. Cell
Death Differ. 12:1297–1309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Amundson SA, Myers TG, Scudiero D, Kitada
S, Reed JC and Fornace AJ Jr: An informatics approach identifying
markers of chemosensitivity in human cancer cell lines. Cancer Res.
60:6101–6110. 2000.PubMed/NCBI
|
|
43
|
Kirkin V, Joos S and Zornig M: The role of
Bcl-2 family members in tumorigenesis. Biochim Biophys Acta.
1644:229–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Paglin S, Hollister T, Delohery T, et al:
A novel response of cancer cells to radiation involves autophagy
and formation of acidic vesicles. Cancer Res. 61:439–444.
2001.PubMed/NCBI
|
|
45
|
Shen Y, Li DD, Wang LL, Deng R and Zhu XF:
Decreased expression of autophagy-related proteins in malignant
epithelial ovarian cancer. Autophagy. 4:1067–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|