Introduction
Breast conservation surgery is initially performed
on patients suffering from breast cancer. Thereafter, radiotherapy
of the whole breast is performed on these patients as it is the
standard mode of treatment. Treatment is mostly executed using a
wedge-based 3-dimensional conformal technique. In this case, 2
opposing tangential fields are chosen to target the entire breast.
However, care is taken to minimize the exposure to lung tissues
within the treatment fields. In clinical practice, wedges are
frequently employed to determine the differential thickness across
the breast, while blocks or static multileaf collimators (MLCs) are
strategically placed to shield the heart and lung as much as
possible. The conventional 3-dimensional conformal radiotherapy
(3D-CRT) has been successful in improving local control (1,2).
However, wedges can only provide 2-dimensional compensation of
missing tissue, which could be suboptimal and result in an
inhomogeneous dose distribution, particularly in the case of women
with large breasts (3). Large
breast size often deters homogeneous results in increased hot
spots: these are located within both the target and the surrounding
normal tissues. Previous studies have suggested that dose
variations >5–10% of the prescribed dose within the target
breast (hot spots) correlated with the occurrence of soft-tissue
toxicity. However, these measures were associated with poor
cosmetic outcomes (4). Moreover,
the concave shape of the chest wall and overlying breast results in
unavoidable irradiation to portions of the underlying lung and
heart with 3D-CRT. This is particularly true while treating
patients diagnosed with cancer in the left breast (5). In addition, physical compensators
significantly scatter the dose to the contralateral breast
(6). In this case, the patient
becomes more vulnerable to developing radiation-induced
contralateral breast cancer (7).
Thus, normal tissue toxicities remain an area of critical concern
(1,8,9).
Intensity-modulated radiation therapy (IMRT) has
been used in breast cancer treatment. IMRT boosts improvements in
dose distribution to the target volume, while minimizing the
exposure of high volume doses to heart and lung tissues (12). In other words, it helps in achieving
the objective of reducing acute and late radiation toxicity
(10,11). Thus, IMRT has emerged as a standard
treatment of several inflicted sites (13). Delivery of irradiation to an
irregular shape can be optimized with IMRT. In addition, the
technology offers the ability to produce concavities in the
treatment volume so as to improve conformality. Studies in breast
cancer patients have shown that better dose uniformity is achieved
throughout the breast with IMRT, wherein a median of 0.1% of the
treatment volume receives ≥110% of the prescribed dose vs. 10% with
conventional wedges (14). However,
IMRT has an important shortcoming; it increases the volume of
low-dose exposure for major normal organs due to its dose
distribution characteristics. It is important to reiterate that
this fact has already been confirmed in previous studies, which
investigated the dose distribution of IMRT used in the treatment of
other diseases (15–18). Furthermore, this technique requires
longer planning and treatment times. Apart from this, an additional
time is required for processing the quality assurance (QA) of the
IMRT beams. A significant workload of the radiotherapy department
has been associated with the execution of adjuvant breast cancer
radiotherapy. Thus, a slight increase in the treatment complexity
will have serious implications for resource allocations. IMRT has
been associated with substantially greater costs. Thus, it is
financially cumbersome for the patient (or insurance company).
Hence, while determining the most appropriate RT for a patient, one
must also consider resource limitations (19).
Mayo et al (20) found that the 4-field hybrid IMRT
plan (combining 2 open tangents with tangential IMRT beams), with a
quality comparable to that of forward-planned IMRT (FP-IMRT), could
be achieved in substantially less planning time. Moreover,
improvements in the uniformity of dose to the target volume and
conformality may be achieved by adding 2 anterior oblique IMRT
beams to the 4-field hybrid technique (6-field hybrid IMRT). In a
clinical trial conducted by Farace et al (21), hybrid IMRT was performed by direct
aperture optimization. However, when treatment goals were not
achieved by using a 4-field technique, a 6-field technique was
applied. Their results proved that hybrid IMRT can be planned for a
large number of patients with little impact on human or
departmental resources. Here, hybrid IMRT is an improvised version
that is executed with the help of semi-automated tools. Mayo et
al (20) compared the dose
distribution of 2-field tangent-only IMRT plans with hybrid IMRT.
They propounded that the 2-field tangent-only IMRT plans were more
effective in reducing the high-dose exposure to lungs and heart.
However, these plans worsened dose homogeneity within the breast.
Moreover, they also worsened the maximal dose outside the target.
Under such circumstances, 2 open fields are essential to achieve
requisite dose uniformity for treating breasts afflicted with
cancer. Multi-beam IMRT can reduce the high-dose region outside the
planning target volume (PTV) and reach homogeneity dose
distribution inside the PTV simultaneously. Under such conditions,
open fields are not necessary (22). However, we need to determine whether
a hybrid IMRT planning technique can achieve dosimetric equivalence
comparable to that of multi-beam IMRT, particularly in cases of
patients with cancer detected in the left breast. However, the
comparison between hybrid IMRT and multi-beam IMRT has rarely been
reported in medical literature.
In this study, we compared the efficacy of different
oncology techniques, such as 3D-CRT, 4-field IP-IMRT and hybrid
IMRT (combining 3D-CRT and 4-field IP-IMRT). We examined these
techniques based on 2 important parameters: dose to the PTV and
organs at risk (OAR). In this study, we deliberately restricted our
analysis to the IMRT and hybrid plans as they employ the same
tangential beam angles that are used for the 3D-CRT plan. This
analysis explores the dose directed to the normal tissue and the
associated exit dose through heart and lungs (23).
Materials and methods
Patient preparation
Eight patients were randomly selected for this
dosimetric study. These patients were diagnosed with left-sided
breast cancer. They had previously been treated with breast
conserving surgery by a single oncologist at Xiangya Hospital. The
breast volumes for these selected patients varied between 304 to
1633 cc, with an average breast volume of 812.75±444.93 cc. These
values were generally encountered in breast cancer patients. The
patients were immobilized in a vacuum pad; CT scans were performed
on these patients in the treatment position, marking the range of
breast with lead wires. Scans were transferred to the treatment
planning computer (Varian Eclipse version 6.7; Varian Medical
Systems, Palo Alto, CA, USA). The breast tissue [clinical target
volume, (CTV)] was defined by a radiation oncologist. The
contralateral breast, right lung, left lung, and heart tissues were
delineated in the CT scans.
Volume delineation
The breast CTV was delineated by the radiation
oncologist with the following considerations. According to anatomic
references, the CTV is generally defined superiorly by the inferior
aspect of the clavicular head and inferiorly by the inframammary
fold. This is identified through skin reconstruction and physical
examination. Medially, the CTV is limited by the sternum and is
generally delineated by 2 cm, which is medial to the edge of the
sternum. Laterally, the breast tissue is identified in the
mid-axillary line. While dealing with different cases of breast
cancer, physicians can make exceptions to these anatomic references
after performing a physical examination and image assessment.
A planning volume, the PTV was defined to extend
beyond the CTV by as much as 1 cm in the superior and inferior
direction, 0.5 cm in other directions, and then modified to exclude
0.5 cm of the buildup region near the skin. This additional margin
pushes the high-dose gradient farther away from the edge of the
PTV. Thus, in actual PTV coverage, the effect of day-to-day
variability gets reduced in patient setup. Excluding the region
near the skin drives the optimization algorithm away from
attempting to achieve full dose in the buildup region. After
completion of optimization on the IMRT PTV, normalization of the
plan was performed by referring the coverage of breast PTV.
The body was delineated on the CT scans, and Boolean
operations were used to construct a modified body volume that
excluded breast PTV. Dose-volume histograms (DVHs) of this tissue
outside the breast PTV (VOB) were used to characterize
doses associated with non-target tissue within the radiation
fields.
Treatment planning techniques
Three treatment plans were developed for each
patient. These plans were executed, and the afflicted breast was
exposed to 50 Gy using 6MV photon beams. While executing the plan
on each patient, the same isocenter and tangential beams were
applied. All plans were developed to suit the requisites of a 6MV
beam on a 2100C/D accelerator. Millennium 80 MLC from Varian was
used as the instrument. In clinical practice, the following methods
are routinely used in treating breast cancer patients.
The 3D-CRT plan consisted of standard medial and
lateral tangent beams with wedges. MLCs were used to shield the
heart and lung tissues. According to the requirements, 15–30°
physical wedges and dynamic wedges were used to treat patients.
The IMRT plan consisted of 4-IMRT fields. These IMRT
beams were focused at angles, with an objective of reducing hot
spots created outside the breast tissue, especially in the entrance
regions of the tangent beams. IMRT beams were ~15° anterior from
the nearest tangent beams (Fig.
1A).
The hybrid IMRT plan combined 2 3D-conformal beams
and 4-IMRT beams. The standard medial and lateral primary beam MLCs
were designed for the 3D-CRT, but they were used without wedges.
These IMRT beams were focused at angles that aimed to reduce hot
spots created outside the breast tissue, especially in the entrance
regions of the tangent beams. Thus, these beams were ~45° anterior
from the nearest tangent beams (Fig.
1B). The relative weights of the 3D-conformal beams and IMRT
beams calculated by the optimization algorithm were acceptable; 40%
of the dose was delivered with IMRT beams.
The IMRT-involved plans were normalized, and the
prescribed dose (50 Gy) was received by at least 90% of the breast
PTV. As shown in Table I, these
normalized plans were developed using the Varian Medical Systems
Eclipse/Helios treatment planning system with the optimization
constraints. Optimization was stopped when these constraints were
forced beyond permissible limits; the PTV dose homogeneity was
compromised. After optimization, the intensity profiling was
carried out in the anterior direction such that it extended up to 2
cm beyond the skin surface; this provided adequate margin for the
patient’s breathing and accommodated set-up uncertainties.
 | Table IOptimization parameters used in
Eclipse/Helios in this study for all plans involving
intensity-modulated radiotherapy. |
Table I
Optimization parameters used in
Eclipse/Helios in this study for all plans involving
intensity-modulated radiotherapy.
| Tissue limit | Limit | Dose (Gy) |
|---|
| PTV | Max | <55 |
| Min | >45 |
|
D90% | >50 |
| Ipsilateral
lung | Mean | <15 |
|
D75% | <30 |
| Contralateral
lung | Mean | <2.5 |
| Heart |
D95% | <40 |
|
D90% | <30 |
| Contralateral
breast | Mean | <1.0 |
Plan evaluation criteria
Batho power law correction was used to evaluate
tissue heterogeneity during dose calculations. Isodose contour
distributions of different plans were evaluated and compared.
Cumulative DVHs were evaluated to assess target volumes and normal
structures. Quantitative data were extracted from the DVHs. Plans
were compared through 3 significant parameters: PTV dose
conformity, homogeneity, and the volumes of normal tissues
treated.
To evaluate the quality of plans implemented in the
treatment of tumors, the conformity index (CI) and heterogeneity
index (HI) were computed on the basis of DVHs of PTVs. CI was
defined as the product of the fraction of PTV receiving at least
Dmin and the ratio of the volume of PTV receiving at least Dmin to
the volume of tissue receiving at least Dmin (the treated volume,
Vt). Thus, CI = (VPTV95%/VPTV) ×
(VPTV95%/Vt). A larger (<1) CI indicated a
greater volume of overlap between PTV and treated volume,
suggesting that the plan was able to achieve better dose conformity
in the treatment. HI was defined using the equation HI = D5%/D95%,
where D5% and D95% correspond to the dose given to 5 and 95% of the
PTV, respectively. A smaller (>1) HI indicated that a smaller
dose exceeded the prescription dose, thereby suggesting the
prevalence of better dose homogeneity inside the PTV.
We compared the normal tissues treated on the basis
of the following parameters: heart max dose and volume receiving 30
Gy or greater (V30), ipsilateral (left) lung mean dose and volume
receiving 20 Gy or greater (V20), and contralateral (right) lung
mean dose and volume receiving 5 Gy or greater (V5). The primary
goal of the IMRT plans was to reduce the volume of exposure to
heart and lung, while the patient received a high RT dose. The
parameters selected for comparison of heart (V30) and ipsilateral
lung (V20, V13) were chosen, as there was evidence (42,48) to
prove that doses beyond these values could cause acute or late
clinical symptoms. To assist further analysis, V40, V20, V10 and V5
parameters were recorded for heart assessment, while V40, V30 and
V5 were recorded to assess ipsilateral lung. Soft tissue
surrounding the breast and contralateral breast comparison
parameters (mean doses and V5) were taken into consideration while
determining doses that may be associated with a carcinogenic risk.
A major goal of these plans was to reduce hot spots in the soft
tissue surrounding the breast. These regions were investigated by
evaluating the volume of tissue outside the breast PTV
(VOB) receiving 100 and 110% of the prescribed dose.
Total monitor units (MUs) were tabulated for each
plan. The ratio for each plan of total MUs to those for the 3D-CRT
plan was calculated for each patient.
Statistical analysis
A two-way analysis of variance (ANOVA) was performed
without replication in order to determine if significant
differences existed among the planning techniques. Thereafter,
paired t-tests were used to identify which techniques differed from
others for each dosimetric parameter. To compare the 3 techniques,
there were 3 comparisons of each parameter. To determine
statistical significance, a two-sided significance level of
P<0.017 was used by taking into account Bonferroni’s correction
(24).
Results
Fig. 2 displays the
typical results for the isodose distributions in each of the 3
plans analyzed in this study. The 3D-CRT technique reduced the
volume of tissue irradiated outside the breast by using MLCs to
conform the radiation fields. However, a large volume of tissue
outside the breast was still encompassed (shown as the green line
in Fig. 2), especially in patients
having a thorax with a larger curvature. These hot spots are
generally located where the physical thickness is less (shown as
the red line in Fig. 2), i.e., they
are usually located near the lungs (due to their low density
compared to surrounding tissue), the apex of the breast, and the
axilla. Here, the patient is radio-graphically ‘thinner’ than on
the central axis. The IMRT-involved plans (4-field IP-IMRT and
hybrid plans) markedly reduced the hot regions and ensured a more
conformal dose distribution around the breast tissue. However, the
IMRT plan increased the low-dose exposure on the volume of tissues
outside the breast (shown as the white line in Fig. 2).
Fig. 3 represents a
typical comparison of DVHs among the 3D-CRT, IP-IMRT and hybrid
plans. Compared with the IP-IMRT plans, the relative volume of
breast receiving >100% prescribed dose was considerably larger
when patients were treated with the 3D-CRT plans. By contrast,
compared with the IP-IMRT plans, the relative volumes of low-dose
region in vital organs (heart, lungs and contralateral breast) were
considerably smaller in the 3D-CRT plans. Compared with the 3D-CRT
and IP-IMRT plans, hybrid IMRT achieved a good balance between the
inner hot spots and low-dose region outside the breast PTV.
As shown in Fig. 4,
compared with the 3D-CRT plan, both IMRT and hybrid plans showed an
improvement in dose characteristics of the PTV. The relative volume
of PTV >110% dose (hot spots) was significantly reduced in the
IMRT-involved plans. On the other hand, the relative volume of PTV
>95% and PTV >90% dose were significantly greater in hybrid
IMRT plans (P<0.017). As shown in Table II, dose homogeneity was measured by
HI while dose conformity was measured by CI. Compared with 3D-CRT
plans, both these parameters showed significant improvements
through IMRT-involved plans. Improved dose homogeneity was achieved
through hybrid techniques. However, this improvement was
insignificant with that achieved by the 4-field IP-IMRT plan
(P=0.024).
 | Table IIDose characteristics of the PTV
(breast) associated with the 3 planning strategies, including the
mean dose and dose homogeneity for left-sided patients. |
Table II
Dose characteristics of the PTV
(breast) associated with the 3 planning strategies, including the
mean dose and dose homogeneity for left-sided patients.
| Technique | Mean PTV dose
(%) | V90
(%) | V95
(%) | V100
(%) | V110
(%) | HI | CI |
|---|
| 3D-CRT | 104.73±1.41 | 99.6±0.37 | 98.36±0.60 | 90.93±0.55 | 9.31±10.46 | 1.12±0.03 | 0.58±0.10 |
| IMRT | 103.88±0.70 | 99.56±0.30 | 97.92±0.94 | 90.82±0.35 | 0.18±0.03a | 1.09±0.02a | 0.68±0.10a |
| Hybrid | 103.43±0.56 | 99.87±0.20ab | 99.3±0.49ab | 92.51±1.80 | 0.16±0.41a | 1.07±0.01a | 0.66±1.42a |
The average total MUs for 3D-CRT was 319.88±36.08
MU. Our results illustrate that IP-IMRT plans and hybrid plans
require higher average MUs: their MUs were 2.2 and 1.75-fold
greater than the 3D-CRT plans, respectively (P≤0.017). This
observation of MUs was recorded while delivering the same
prescribed dose through different techniques.
Table III displays
the characteristics of the dose afflicted to the lungs in each of
the techniques. In the case of IMRT-involved plans, mean dose to
the bilateral lungs was significantly higher than that reported for
the 3D-CRT plan. In the case of the contralateral lung, the
IMRT-involved plans significantly increased the volume receiving
>5 Gy (V5) as compared to the 3D-CRT plan. In addition, the V5
for the IMRT plan was >3-fold higher than that for the hybrid
plan. In none of the plans did the contralateral lung receive
>20 Gy. In the case of ipsilateral lung, in the low-dose region,
the percentage of volume receiving >5 Gy was significantly
higher for the IMRT-involved plans than for the 3D-CRT plan.
Furthermore, the percentage of volume receiving >13 Gy was
higher in the case of the IMRT plan (P<0.017). Compared with the
other plans, in the high-dose region, the IMRT plan significantly
reduced the percentage of ipsilateral lung receiving >40 Gy. As
shown in Fig. 5, while analyzing
the entire lung, there was only a slight difference among the 3
techniques with respect to V20: all techniques reported <8%, but
V13 was significantly increased in IP-IMRT plans.
 | Table IIICharacteristics of dose directed to
the lungs in different treatment techniques. |
Table III
Characteristics of dose directed to
the lungs in different treatment techniques.
| Organ | Technique | Mean dose (%) | V >5 Gy (%) | V >13 Gy
(%) | V >20 Gy
(%) | V >30 Gy
(%) | V >40 Gy
(%) |
|---|
| Ipsilateral
lung | 3D-CRT | 20.06±1.93 | 28.82±2.98 | 19.39±1.54 | 17.26±1.53 | 14.5±2.04 | 12.34±2.04 |
| IMRT | 22.58±1.53a | 44.94±3.38a | 27.66±2.90a | 17.80±1.07 | 13.56±1.12 | 10.54±1.50a |
| Hybrid | 22.22±1.23a | 44.35±7.45a | 20.70±1.08b | 17.71±1.32 | 14.84±1.44 | 12.02±1.36b |
| Contralateral
lung | 3D-CRT | 1.77±0.44 | 0.45±1.01 | 0.27±0.06 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| IMRT | 2.80±1.17a | 8.01±8.33a | 0.01±0.01 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| Hybrid | 3.48±0.80a | 2.25±3.13b | 0.07±0.19 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
While treating patients diagnosed with cancer in the
left breast, the portions of the heart were treated to >30 Gy,
irrespective of the selected plan. However, as shown in Fig. 6, the 3 techniques do not show any
significant difference in the following parameters: maximum heart
doses and average volume of heart receiving >30 Gy. In the
low-dose region, the percentage of heart receiving >5 Gy was
significantly greater when patients were subjected to the
IMRT-involved plans. For the IMRT plan, the average volume of heart
receiving >10 Gy was higher, but the average volume of heart
receiving >40 Gy was significantly lower as compared to the
other 2 plans (P<0.017).
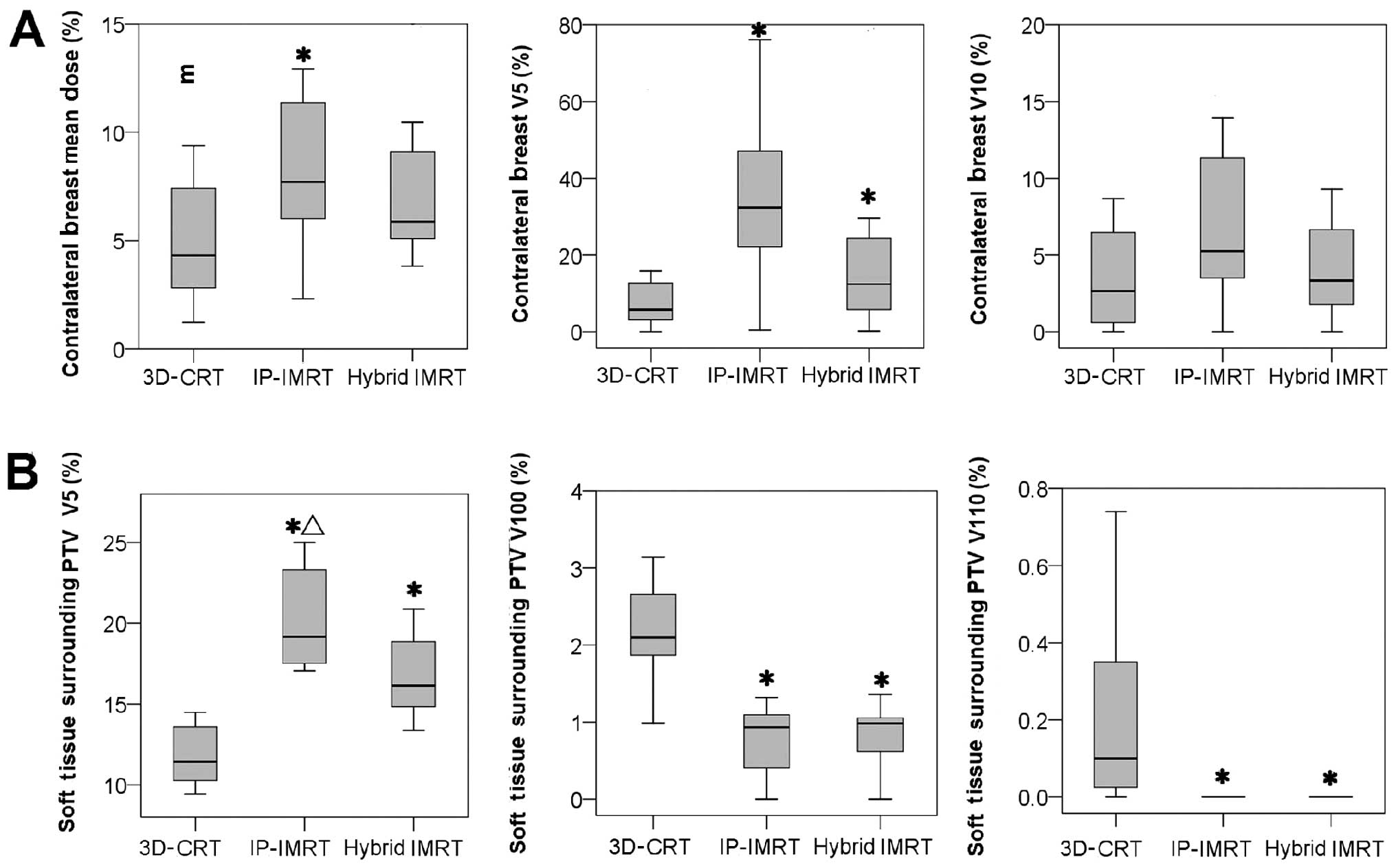
As presented in Fig.
7A, the average mean dose to the contralateral breast was
significantly greater for the IMRT plan compared to the other 2
plans. In the case of the IMRT and the hybrid plans, the percentage
of contralateral breast receiving >5 Gy was significantly
higher. Furthermore, the IMRT plans witnessed greater exposure
compared to the hybrid plans, but this was statistically
insignificant (P=0.025). The percentage of contralateral breast
receiving >10 Gy was reported to be the highest while executing
the IMRT plans. The exposure was comparatively less in the case of
the other treatment plans, including the hybrid IMRT plans
(P=0.018) and the 3D-CRT plans (P=0.018). However, this disparity
in the parameters of the three treatment plans was statistically
insignificant.
Fig. 7B summarizes
comprehensively the volume of soft tissue outside the PTV
(VOB), which received prescribed or higher dose.
Compared to the 3D-CRT technique, the IMRT-involved plans reduced
the volume of tissue that received prescribed dose
(V100%). Similar results were obtained for higher dose
(V110%), and there was 0 volume of tissue receiving
>110% in the IMRT-involved plans. In the low-dose region, the
percentage of VOB receiving >5 Gy was significantly greater for
the IMRT-involved plans than for the 3D-CRT plan. However, this
type of radiation exposure was compromised in the hybrid plan as
compared to the IMRT plan (P=0.012).
Discussion
In the present study, the selected patients were
representative of the different types of cases encountered in
clinical practice. In this case, all reported plans were calculated
by taking into account dose heterogeneity. The wedge-based 3D-CRT
plans were considered as the standard that optimized the dose
uniformity to the target tissue. The inverse-planned IMRT plans
were developed on the Varian Eclipse system with the help of Helios
optimization algorithm. As anticipated, the improvement in dose
distribution was greater in cases of patients with larger breasts,
whose hot spot regions encompassed larger areas while treating with
3D-CRT plans.
The dose uniformity to the PTV was comparable for
all techniques in our study, wherein the mean doses varied between
103.43 and 104.73%. However, compared with the 3D-CRT technique,
the PTV volume receiving high-dose (110%) witnessed a sharp decline
from 9.3 to 0.2% provided patients were treated with two
IMRT-involved techniques. Comparing HI and CI among three
techniques, the IMRT-involved techniques showed a significant
improvement over the 3D-CRT plans. Noticeably, for hybrid IMRT, the
PTV volume received 95 or 90% prescription dose: this was
significantly higher than for the other 2 techniques. Thus,
compared with the IP-IMRT technique, the hybrid IMRT technique
improved HI; however, the improvement was not statistically
significant (P=0.024). The hot spots outside the target were also
evaluated. The hybrid IMRT plans were comparable with the IP-IMRT
plans, but they were better than the 3D-CRT plans in restricting
the volumes of tissue outside the target from receiving ≥100 and
≥110%, prescribed dose.
These results indicate that greater dose homogeneity
and conformity were achieved by the IMRT-involved plans. While
performing IMRT of the breast (25–29),
clinical benefits associated with skin toxicity reduction have been
reported. Furthermore, it has also been reported that IMRT is
beneficial in reducing chronic breast edema (27) and the incidence of change in breast
appearance (30). Besides cosmetic
requirements, disease control is of paramount importance. However,
only 2 studies (31,32) reported on the following outcomes in
patients treated for breast cancer: there seemed to be no evidence
for differences in local recurrence rates, with less follow-up for
patients receiving IMRT-based treatment. This may be attributed to
the short follow-up period observed in some studies.
Both IMRT and the hybrid plan had total monitor
units (MUs) that were ~2.2-fold larger than those employed in the
3D-CRT plan. Moreover, the average ratio of total MUS for the IMRT
plan was significantly higher than for the hybrid plan. Increasing
the number of MUs for dynamic deliveries consequently increases the
peripheral dose and whole body dose. In fact, it may also increase
the probability of radiation-induced secondary malignancies.
When IMRT plans are employed with the field
directions that are the same as those encountered with standard
tangential irradiation, patients may experience several benefits in
the treatment procedures. These benefits are associated with dose
reduction to surrounding organs at risk. Nevertheless, additionally
improved dose homogeneity within the target volume can only be
achieved through more complex IMRT treatments (33–36).
In our study, we first proved that hybrid IMRT plans requiring less
MUs can achieve dosimetric equivalence to 4-field IP-IMRT. These
hybrid IMRTs are also more flexible in terms of positioning
repeatability. In the case of hybrid IMRT, the dosimetric
equivalence is achieved by combining the same amount of IMRT beams
to the standard tangential conformal beams. In this manner, hybrid
IMRT plans not only reduce hot spots outside the target volume but
also improve dose homogeneity within the target volume.
Heart and lung are the primary organs of concern. In
our study, for the IP-IMRT technique, the relative volume of
ipsilateral lung or heart receiving high-dose (40 Gy) was
significantly reduced. However, the relative volume of tissue
receiving low-dose (13 Gy to total lung or 10 Gy to heart)
significantly increased, as compared to the other 2 techniques.
With the multi-beam IMRT technique, the relative volume of
bilateral lungs and heart receiving even lower dose (5 Gy)
comparatively increased while employing IP-IMRT and hybrid
techniques. Moreover, the relative V5 of contralateral lung was
significantly larger for IP-IMRT than for the hybrid plan. Thus,
compared with IP-IMRT, the hybrid technique cannot achieve
equivalent reduction in exposure of lung or heart volumes to
high-doses (>40 Gy). However, it does contribute to compromising
low-dose volumes. Among all these techniques, the V20 and V30 of
the total lung and heart were comparable with each other. Moreover,
the max doses to heart were also comparable for all 3 techniques.
While devising a planning approach, the physician’s clinical
judgment plays an important role in deciding how to balance the
risks of low-dose levels against high-dose levels.
In clinical practice, we have come across cases of
radiation-induced heart disease, wherein the patients’ heart
partially received therapeutic doses of approximately ≥35 Gy
(37). Recent research studies were
performed on atom bomb survivors. These studies also suggested a
relationship between low-radiation doses in the range of ≤4 Gy and
cardiac mortality (38–41). It is reported that 1 Gy added to the
mean heart dose could increase the cardiotoxic risk by 4% (42). The complex process of
radiation-induced heart disease involved different heart structures
with different radiosensitivities. Under these circumstances, we
have not been able to successfully decipher the associated
pathomechanisms in patients (43,44).
Furthermore, pre-existing cardiovascular risk factors, such as
smoking, hypertension, obesity, and the use of cardiotoxic agents
generally instigate the development of radiation-induced heart
disease. In view of the potential risks, it has been recommended
that all measures should be used to reduce radiation exposure to
cardiac tissues (43).
Radiation pneumonitis is a rare complication of
breast RT, affecting ~1% of patients receiving breast irradiation
(45). In a research study
exploring the efficacy of chemoradiation therapy in the treatment
of esophageal cancer, Lee et al (46) indicated the importance of volume of
lung tissue receiving at least 10 Gy. They illustrated that a
similar incidence of complications was reported in cases where
>40% of lung tissues received at least 10 Gy and >20% of lung
received at least 20 Gy. It has been confirmed that total lung V20
is an independent predictor of pneumonitis in lung cancer patients
(47). Schallenkamp et al
(48) also noted that intrathoracic
radiotherapy should be planned cautiously, especially while using
radiotherapy techniques delivering doses of 10-5 Gy to large lung
volumes. In our study, total lung V20 was <8% and comparable for
all techniques; total lung V13 significantly increased in the case
of the IP-IMRT plans: this increase was ~3% as compared to the
3D-CRT and hybrid IMRT plans.
While treating breast cancer patients, the dose to
the contralateral breast is of paramount significance. In the cases
of women diagnosed with breast cancer, the risk of contralateral
breast cancer is estimated to be within 2–11% (49). However, we have not yet been able to
comprehend the association with low-dose irradiation. In our plans,
for the hybrid technique, the mean dose of the contralateral breast
was comparable with the 3D-CRT technique, but it was significantly
lower than that for IP-IMRT. The contralateral breast V5 for the
hybrid technique was comparable with IP-IMRT, but it was
significantly greater than that for the 3D-CRT technique. In
summary, hybrid IMRT plans compromised the mean dose and low-dose
volume of contralateral breast as compared with the 3D-CRT and
IP-IMRT plans.
Whole body dose may be an area of concern; however,
we have limited data to estimate the associated risk. Hall and Wuu
(50) reported that vulnerability
to developing secondary cancer after 10 years may increase in 1% of
patients subjected to conventional radiation therapy and 1.75%
patients subjected to IMRT. In this study, comparing the 3
techniques, the relative volume of soft tissue surrounding the
breast receiving at least 5 Gy was the largest for IP-IMRT (20.25%)
and the smallest for 3D-CRT (11.8%) (P<0.017). Similarly, hybrid
IMRT plans compromised the VOB receiving low-dose irradiation
between 3D-CRT and IP-IMRT only plans.
As compared to 3D-CRT plans, the hybrid IMRT and
IP-IMRT plans were comparable as they improved dose homogeneity and
conformity. Owing to these techniques, we could also witness a
significant reduction in the magnitude of hot regions outside the
target. However, the first trade-off is that these techniques
increased low-dose radiation to surrounding areas, including the
lungs, heart, contralateral breast and the soft tissue surrounding
breast. However, we do not yet know if these increases in low-dose
exposure translate into long-term complications or induction of
secondary cancer. However, as long as the primary aim of treatment
is not affected, these low-dose regions should be minimized.
Another trade-off, of course, is the additional time required to
perform quality assurance on the IMRT beams. Hence, we need to
carefully select patients who would appreciably benefit from IMRT
beams; it will also help us in mitigating the impact on clinical
resources.
As compared to the IP-IMRT plans, the hybrid IMRT
plans compromised the increase of low-dose volume; however, they
promoted the increase of high-dose (40 Gy) volume in important
normal tissues. We need to strike a balance between the risks of
low-dose levels against high-dose levels. This plays a pivotal role
in influencing the clinical selection of a proper planning
technique. In general, multi-beam IMRT plans may benefit elder
patients or those patients with cardiopulmonary insufficiency by
preventing the heart and lungs from being exposed to high-dose
irradiation. Hybrid IMRT may benefit patients in good
cardiopulmonary health as the hybrid technique not only improves
the cosmetic outcome but also reduces the volume of healthy tissue
receiving low-dose irradiation.
In conclusion, compared to the conventional 3D-CRT
technique, both the IP-IMRT and hybrid IMRT techniques improved the
PTV dose uniformity and conformity. Compared to the hybrid IMRT and
the 3D-CRT plans, the IP-IMRT plans achieved significant reduction
in the volume of heart and ipsilateral lung exposed to high-dose
(≥40 Gy). In general, the multi-beam inverse planned IMRT technique
probably benefits patients whose cardiopulmonary condition is poor.
Hybrid IMRT plans allowed a PTV coverage and dose homogeneity as
good as IP-IMRT only plans. Moreover, it also compromised the
low-dose volumes of normal tissues and the demanding clinic
resource for IP-IMRT only plans. Therefore, hybrid IMRT plans were
preferred as the standard practice for left-whole-breast
irradiation. However, no evidence was found for differences in
disease control. Furthermore, an additional time was required for
executing IMRT-involved plan quality assurance. In conclusion, the
3D-CRT technique should not be excluded as a good selection
technique, especially for patients with relatively smaller
curvature of thorax, smaller breast volume, and good-ordered
cardiopulmonary health.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81301942).
References
|
1
|
Fisher B, Anderson S, Bryant J, Margolese
RG, Deutsch M, Fisher ER, Jeong JH and Wolmark N: Twenty-year
follow-up of a randomized trial comparing total mastectomy,
lumpectomy, and lumpectomy plus irradiation for the treatment of
invasive breast cancer. N Engl J Med. 347:1233–1241.
2002.PubMed/NCBI
|
|
2
|
Veronesi U, Cascinelli N, Mariani L, Greco
M, Saccozzi R, Luini A, Aguilar M and Marubini E: Twenty-year
follow-up of a randomized study comparing breast-conserving surgery
with radical mastectomy for early breast cancer. N Engl J Med.
347:1227–1232. 2002.PubMed/NCBI
|
|
3
|
Das IJ, Cheng CW, Fein DA and Fowble B:
Patterns of dose variability in radiation prescription of breast
cancer. Radiother Oncol. 44:83–89. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Back M, Guerrieri M, Wratten C and
Steigler A: Impact of radiation therapy on acute toxicity in breast
conservation therapy for early breast cancer. Clin Oncol. 16:12–16.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schubert LK, Gondi V, Sengbusch E,
Westerly DC, Soisson ET, Paliwal BR, Mackie TR, Mehta MP, Patel RR,
Tomé WA and Cannon GM: Dosimetric comparison of left-sided whole
breast irradiation with 3DCRT, forward-planned IMRT,
inverse-planned IMRT, helical tomotherapy, and topotherapy.
Radiother Oncol. 100:241–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kelly CA, Wang XY, Chu JC and Hartsell WF:
Dose to contralateral breast: a comparison of four primary breast
irradiation techniques. Int J Radiat Oncol Biol Phys. 34:727–732.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Unnithan J and Macklis RM: Contralateral
breast cancer risk. Radiother Oncol. 60:239–246. 2001. View Article : Google Scholar
|
|
8
|
Gyenes G, Rutqvist LE, Liedberg A and
Fornander T: Long-term cardiac morbidity and mortality in a
randomized trial of pre- and postoperative radiation therapy versus
surgery alone in primary breast cancer. Radiother Oncol.
48:185–190. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bird BR and Swain SM: Cardiac toxicity in
breast cancer survivors: review of potential cardiac problems. Clin
Cancer Res. 14:14–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Donovan E, Bleakley N, Denholm E, Evans P,
Gothard L, Hanson J, Peckitt C, Reise S, Ross G, Sharp G,
Symonds-Tayler R, Tait D and Yarnold J; Breast Technology Group:
Randomized trial of standard 2D radiotherapy (RT) versus intensity
modulated radiotherapy (IMRT) in patients prescribed breast
radiotherapy. Radiother Oncol. 82:254–264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pignol JP, Olivotto I, Rakovitch E,
Gardner S, Sixel K, Beckham W, Vu TT, Truong P, Ackerman I and
Paszat L: A multicenter randomized trial of breast
intensity-modulated radiation therapy to reduce acute radiation
dermatitis. J Clin Oncol. 26:2085–2092. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beckham WA, Popescu CC, Patenaude VV, Wai
ES and Olivotto IA: Is multibeam IMRT better than standard
treatment for patients with left-sided breast cancer? Int J Radiat
Oncol Biol Phys. 69:918–924. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Selvaraj RN, Beriwal S, Pourarian RJ,
Lalonde RJ, Chen A, Mehta K, Brunner G, Wagner KA, Yue NJ, Huq SM
and Heron DE: Clinical implementation of tangential field intensity
modulated radiation therapy (IMRT) using sliding window technique
and dosimetric comparison with 3D conformal therapy (3DCRT) in
breast cancer. Med Dosim. 32:299–304. 2007. View Article : Google Scholar
|
|
14
|
Kestin LL, Sharpe MB, Frazier RC, Vicini
FA, Yan D, Matter RC, Martinez AA and Wong JW: Intensity modulation
to improve dose uniformity with tangential breast radiotherapy:
initial clinical experience. Int J Radiat Oncol Biol Phys.
48:1559–1568. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mundt AJ, Mell LK and Roeske JC:
Preliminary analysis of chronic gastrointestinal toxicity in
gynecology patients treated with intensity-modulated whole pelvic
radiation therapy. Int J Radiat Oncol Biol Phys. 56:1354–1360.
2003. View Article : Google Scholar
|
|
16
|
Mundt AJ, Lujan AE, Rotmensch J, Waggoner
SE, Yamada SD, Fleming G and Roeske JC: Intensity-modulated whole
pelvic radiotherapy in women with gynecologic malignancies. Int J
Radiat Oncol Biol Phys. 52:1330–1337. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roeske JC, Lujan A, Rotmensch J, Waggoner
SE, Yamada D and Mundt AJ: Intensity-modulated whole pelvic
radiation therapy in patients with gynecologic malignancies. Int J
Radiat Oncol Biol Phys. 48:1613–1621. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roeske JC, Bonta D, Mell LK, Lujan AE and
Mundt AJ: A dosimetric analysis of acute gastrointestinal toxicity
in women receiving intensity-modulated whole-pelvic radiation
therapy. Radiother Oncol. 69:201–207. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kachnic LA and Powell SN: IMRT for breast
cancer - balancing outcomes, patient selection, and resource
utilization. J Natl Cancer Inst. 103:777–779. 2011.PubMed/NCBI
|
|
20
|
Mayo CS, Urie MM and Fitzgerald TJ: Hybrid
IMRT plans - concurrently treating conventional and IMRT beams for
improved breast irradiation and reduced planning time. Int J Radiat
Oncol Biol Phys. 61:922–932. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Farace P, Zucca S, Solla I, Fadda G, Durzu
S, Porru S, Meleddu G, Deidda MA, Possanzini M, Orrù S and Lay G:
Planning hybrid intensity modulated radiation therapy for
whole-breast irradiation. Int J Radiat Oncol Biol Phys.
84:e115–e122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thilmann C, Zabel A, Milker-Zabel S,
Schlegel W, Wannenmacher M and Debus J: Number and orientation of
beams in inversely planned intensity-modulated radiotherapy of the
female breast and the parasternal lymph nodes. Am J Clin Oncol.
26:e136–e143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Van Dyk J: The Modern Technology of
Radiation Oncology. 2. Medical Physics Publishing; Madison, WI:
2005
|
|
24
|
Caudell JJ, De Los Santos JF, Keene KS,
Fiveash JB, Wang W, Carlisle JD and Popple R: A dosimetric
comparison of electronic compensation, conventional intensity
modulated radiotherapy, and tomotherapy in patients with
early-stage carcinoma of the left breast. Int J Radiat Oncol Biol
Phys. 68:1505–1511. 2007. View Article : Google Scholar
|
|
25
|
Ragaz J, Jackson SM, Le N, Plenderleith
IH, Spinelli JJ, Basco VE, Wilson KS, Knowling MA, Coppin CM,
Paradis M, Coldman AJ and Olivotto IA: Adjuvant radiotherapy and
chemotherapy in node-positive premenopausal women with breast
cancer. N Engl J Med. 337:956–962. 1997. View Article : Google Scholar
|
|
26
|
Overgaard M, Hansen PS, Overgaard J, Rose
C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen
MB and Zedeler K: Postoperative radiotherapy in high-risk
premenopausal women with breast cancer who receive adjuvant
chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N
Engl J Med. 337:949–955. 1997. View Article : Google Scholar
|
|
27
|
Lo YC, Yasuda G, Fitzgerald TJ and Urie
MM: Intensity modulation for breast treatment using static
multi-leaf collimators. Int J Radiat Oncol Biol Phys. 46:187–194.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Evans PM, Donovan EM, Partridge M, Childs
PJ, Convery DJ, Eagle S, Hansen VN, Suter BL and Yarnold JR: The
delivery of intensity modulated radiotherapy to the breast using
multiple static fields. Radiother Oncol. 57:79–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Donovan EM, Johnson U, Shentall G, Evans
PM, Neal AJ and Yarnold JR: Evaluation of compensation in breast
radiotherapy: a planning study using multiple static fields. Int J
Radiat Oncol Biol Phys. 46:671–679. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zackrisson B, Arevärn M and Karlsson M:
Optimized MLC-beam arrangements for tangential breast irradiation.
Radiother Oncol. 54:209–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McDonald MW, Godette KD, Butker EK, Davis
LW and Johnstone PA: Long-term outcomes of IMRT for breast cancer:
a single-institution cohort analysis. Int J Radiat Oncol Biol Phys.
72:1031–1040. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morganti AG, Cilla S, Valentini V, Digesu’
C, Macchia G, Deodato F, Ferrandina G, Cece MG, Cirocco M,
Garganese G, Di Lullo L, Traficante D, Scarabeo F, Panunzi S, De
Gaetano A, Sallustio G, Cellini N, Sofo L, Piermattei A and Scambia
G: Phase I–II studies on accelerated IMRT in breast carcinoma:
technical comparison and acute toxicity in 332 patients. Radiother
Oncol. 90:86–92. 2009.
|
|
33
|
Chang SX, Deschesne KM, Cullip TJ, Parker
SA and Earnhart J: A comparison of different intensity modulation
treatment techniques for tangential breast irradiation. Int J
Radiat Oncol Biol Phys. 45:1305–1314. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hong L, Hunt M, Chui C, Spirou S, Forster
K, Lee H, Yahalom J, Kutcher GJ and McCormick B:
Intensity-modulated tangential beam irradiation of the intact
breast. Int J Radiat Oncol Biol Phys. 44:1155–1164. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hurkmans CW, Cho BC, Damen E, Zijp L and
Mijnheer BJ: Reduction of cardiac and lung complication
probabilities after breast irradiation using conformal radiotherapy
with or without intensity modulation. Radiother Oncol. 62:163–171.
2002. View Article : Google Scholar
|
|
36
|
Fogliata A, Bolsi A and Cozzi L: Critical
appraisal of treatment techniques based on conventional photon
beams, intensity modulated photon beams and proton beams for
therapy of intact breast. Radiother Oncol. 62:137–145. 2002.
View Article : Google Scholar
|
|
37
|
Brosius FC 3rd, Waller BF and Roberts WC:
Radiation heart disease. Analysis of 16 young (aged 15 to 33 years)
necropsy patients who received over 3,500 rads to the heart. Am J
Med. 70:519–530. 1981.PubMed/NCBI
|
|
38
|
McGale P and Darby SC: Low doses of
ionizing radiation and circulatory diseases: a systematic review of
the published epidemiological evidence. Radiat Res. 163:247–257.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Preston DL, Shimizu Y, Pierce DA, Suyama A
and Mabuchi K: Studies of mortality of atomic bomb survivors.
Report 13: solid cancer and noncancer disease mortality: 1950–1997.
2003. Radiat Res. 178:AV146–AV172. 2012.
|
|
40
|
Ozasa K, Shimizu Y, Suyama A, Kasagi F,
Soda M, Grant EJ, Sakata R, Sugiyama H and Kodama K: Studies of the
mortality of atomic bomb survivors, Report 14, 1950–2003: an
overview of cancer and noncancer diseases. Radiat Res. 177:229–243.
2012.
|
|
41
|
Taylor CW, McGale P and Darby SC: Cardiac
risks of breast-cancer radiotherapy: a contemporary view. Clin
Oncol. 18:236–246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mège A, Ziouèche A, Pourel N and Chauvet
B: Radiation-related heart toxicity. Cancer Radiother. 15:495–503.
2011.(In French).
|
|
43
|
Senkus-Konefka E and Jassem J:
Cardiovascular effects of breast cancer radiotherapy. Cancer Treat
Rev. 33:578–593. 2007. View Article : Google Scholar
|
|
44
|
Darby SC, Cutter DJ, Boerma M, Constine
LS, Fajardo LF, Kodama K, Mabuchi K, Marks LB, Mettler FA, Pierce
LJ, Trott KR, Yeh ET and Shore RE: Radiation-related heart disease:
current knowledge and future prospects. Int J Radiat Oncol Biol
Phys. 76:656–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lingos TI, Recht A, Vicini F, Abner A,
Silver B and Harris JR: Radiation pneumonitis in breast cancer
patients treated with conservative surgery and radiation therapy.
Int J Radiat Oncol Biol Phys. 21:355–360. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee HK, Vaporciyan AA, Cox JD, Tucker SL,
Putnam JB Jr, Ajani JA, Liao Z, Swisher SG, Roth JA, Smythe WR,
Walsh GL, Mohan R, Liu HH, Mooring D and Komaki R: Postoperative
pulmonary complications after preoperative chemoradiation for
esophageal carcinoma: correlation with pulmonary dose-volume
histogram parameters. Int J Radiat Oncol Biol Phys. 57:1317–1322.
2003.
|
|
47
|
Graham MV, Purdy JA, Emami B, Harms W,
Bosch W, Lockett MA and Perez CA: Clinical dose-volume histogram
analysis for pneumonitis after 3D treatment for non-small cell lung
cancer (NSCLC). Int J Radiat Oncol Biol Phys. 45:323–329.
1999.PubMed/NCBI
|
|
48
|
Schallenkamp JM, Miller RC, Brinkmann DH,
Foote T and Garces YI: Incidence of radiation pneumonitis after
thoracic irradiation: dose-volume correlates. Int J Radiat Oncol
Biol Phys. 67:410–416. 2007.PubMed/NCBI
|
|
49
|
Chen Y, Thompson W, Semenciw R and Mao Y:
Epidemiology of contralateral breast cancer. Cancer Epidemiol
Biomarkers Prev. 8:855–861. 1999.PubMed/NCBI
|
|
50
|
Hall EJ and Wuu CS: Radiation-induced
second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol
Biol Phys. 56:83–88. 2003. View Article : Google Scholar : PubMed/NCBI
|