Introduction
Hepatocellular carcinoma (HCC) is the sixth most
prevalent cancer worldwide and the third most frequent cause of
cancer-related mortality (1).
Surgical resection and liver trans-plantation are currently the
main options for the treatment of HCC, however, the high frequency
of tumor recurrence is almost inevitable due to therapy resistance
(2). Accumulating scientific
evidence indicates that tumor formation is driven by a
subpopulation of self-renewing cells known as cancer stem cells
(CSCs) or tumor-initiating cells (TICs) (3). CSCs have been shown to be responsible
for tumor initiation, metastasis, recurrence and chemoresistance
(4). CSCs in liver cancer cells can
be identified and enriched by several specific surface markers,
such as CD90, CD44 and most recently, CD133 (5). CD133 (also known as AC133 or
prominin-1), a 5 transmembrane cell surface glycoprotein, has been
used to extract a subset of putative stem cells in HCC. Moreover,
CD133+ liver cancer cells possess many stem cell
properties, including self-renewal, high proliferation,
differentiation and have greater tumorigenicity and chemoresistance
(6–8). As a liver CSC marker, CD133 also
serves as an important indicator for tumor malignant progression,
patient survival and recurrence rates (9,10).
The epithelial-mesenchymal transition (EMT) is a
transdifferentiation process that converts adherent epithelial
cells into migratory mesenchymal cells. Activation of the EMT
program is considered an important step in the embryonic
development, tumorigenic progression and cancer metastasis
(11). Previous studies have also
linked EMT with the properties of CSCs in HCC (12). Several associated signalling
pathways are able to induce EMT, such as nuclear factor-κB (NF-κB),
Notch, TGF-β and Wnt/β-catenin, which are also known to regulate
CSCs (13). As a transcription
factor, NF-κB has been reported to play critical roles in the
processes of EMT, tumor invasion and metastasis and the maintenance
of CSCs in various types of cancer, including liver cancer
(14). Activation of NF-κB
typically involves the phosphorylation of the inhibitor of κB-α
(IκBα) by the IκB kinase (IKK) complex. Moreover, NF-κB activation
through IKK activity modulation leads to EMT marker changes and the
development of CSCs (15).
With the emergence of new biotechnologies in gene
delivery, ultrasound-targeted microbubble destruction (UTMD) has
evolved into a new, safe, non-viral gene transfection tool for
site-specific drug and gene delivery (16,17).
It has been shown that ultrasound microbubble-mediated delivery
enhances the efficacy of gene transfection and reduces the
side-effects of other bioactive transfection agents, such as
liposomes and viral vectors. Recombinant expression plasmid of the
shRNA targeting gene mediated by the UTMD technique specifically
and effectively regulated the expression of target genes in several
studies (18,19). Based on those studies, we
hypothesized that combining UTMD and shRNA is a promising strategy
for gene delivery in liver cancer stem cells (LCSCs).
Mounting evidence suggests that the stem
cell-related CD133 gene and the EMT process have a linear
relationship. However, few studies have shown this association in
LCSCs. In the present study, using the UTMD technique, we
demonstrated that CD133 plays a vital functional role in the
regulation of EMT, tumor-initiating properties and migratory
ability of LCSCs in vitro and in vivo. Additionally,
we found that the reversal of EMT and the impaired invasion and
migration of LCSCs by CD133 downregulation may be in part,
associated with the repression of the NF-κB pathway. Findings from
the present study provide insight into the regulatory effects of
CD133 on EMT transformation in LCSCs and lead to the development of
new and more effective therapeutics for HCC.
Materials and methods
Cell lines and culture
The human SMMC-7721 liver cancer cell line was
obtained from the Institute of Life Sciences of Chongqing Medical
University (Chongqing, China). SMMC-7721 cells were maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) (both from Gibco, Life Technologies,
Carlsbad, CA, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin (Invitrogen Life Technologies, Carlsbad, CA, USA).
CD133+ cells sorted from the SMMC-7721 cells were
performed in serum-free culture medium (SFM) composed of DMEM/F12
(Gibco) plus 20 ng/ml basic fibroblast growth factor (bFGF), 20
ng/ml epidermal growth factor (EGF) (both from PeproTech, Rocky
Hill, NJ, USA) and 2% B27 supplement (Life Technologies). The cells
were cultured in a humidified incubator with 5% CO2 at
37°C.
Flow cytometric analysis and
fluorescence-activated cell sorting (FACS)
SMMC-7721 cells were subjected to FACS. The cells
were dissociated, washed and resuspended in phosphate-buffered
saline (PBS). Human-specific anti-CD133/1 (AC133) conjugated to
R-phycoerythrin (PE; Milyteni Biotec, Bergisch Gladbach, Germany)
and anti-CD133-florescein isothiocyanate mouse monoclonal antibody
were used for FACS analysis. All the procedures were performed
according to the manufacturer's instructions. The labeled cells
were analyzed and sorted by the FACS-LSR II flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA). The fresh isolated
CD133+ SMMC-7721 cells were collected and cultured in
the SFM.
Experimental grouping
To analyze the effect of UTMD and liposomes on CD133
transfection and the biological characteristics of LCSCs, the fresh
sorted CD133+ SMMC-7721 cells were divided into the
following three groups: the control (control); shCD133 plasmid +
Lipofectamine 2000 (P+L); and the shCD133 plasmid + ultrasound
exposure + microbubbles group (P+UTMD).
Lipofectamine-mediated gene transfection
and ultrasound-targeted microbubble destruction (UTMD) exposure
protocols
The gene-specific short hairpin RNA (shRNA) (HuSH
29-mer shRNA constructs against PROM1 in pGFP-V-RS vector) were
obtained from OriGene (Rockville, MD, USA). The cells in the P+L
group were transfected with shCD133 and Lipofectamine 2000 protocol
(Invitrogen Life Technologies) in Opti-MEM medium without serum
with a ratio of 1:2 according to the manufacturer's instructions.
The mixture was incubated for 5 min at room temperature prior to
being added to the cells. A therapeutic ultrasound machine
(Institute of Ultrasound Imaging, Chongqing Medical University) was
used and the area of the probe (1 MHz) was ~5 cm2.
Microbubbles (SonoVue; Bracco, Milan, Italy) were lipid-shelled
ultrasound contrast agents containing sulfur hexafluoride gas
(diameter, 1.0–10.0 µm) and used at a concentration of
~2×108 bubbles/ml. SonoVue was reconstituted in saline
solution according to the manufacturer's instructions prior to
transfection. Plasmid DNA and SonoVue complexes were gradually
added to cell suspensions in the P+UTMD group. After protocol
optimization with various settings, the UTMD parameters were set at
the radiation frequency of 1 MHz, 20% duty cycle and sound
intensity of 1 W/cm2 for 60 sec. The plates were
supplemented with 2 ml serum-free culture medium and incubated in
an incubator at 37°C and 5% CO2 until gene expression
analysis was performed. The shRNA expression vectors expressed
green fluorescent protein (GFP) that was used to track transfection
efficiency. At 12, 24 and 48 h after transfection, transfection
efficiency was evaluated by observing the expression of GFP in the
living cells with fluorescent microscopy (Olympus, Tokyo, Japan).
After 48 h, RT-qPCR and western blotting were used to determine the
levels of related gene expression.
Reverse transcriptase-quantitative PCR
analysis
Total RNA from cells was isolated using the TRIzol
reagent (Takara, Dalian, China) according to the manufacturer's
instructions. The concentrations and purity of the total RNA were
evaluated using a UV spectrophotometer (Ultrospec 2100 Pro;
Amersham, USA). Total RNA was reverse transcribed into cDNA using
the PrimeScript RT reagent kit (Takara). Quantitative PCR (RT-qPCR)
was performed using SYBR Premix Ex Taq™ II (Takara) with the
CFX96™ Real-Time System (Bio-Rad, Hercules, CA, USA). GAPDH was
used as an internal control. The relative expression levels of
mRNAs were calculated using the 2−ΔΔCt method. RT-qPCR
reactions were run in triplicate. Primer sequences for the genes
analyzed were: CD133 forward, 5′-AGG ACA AGG CGT TCA CAG AT-3′ and
reverse, 5′-ACC AAG CAC AGA GGG TCA TT-3′; CD44 forward, 5′-AAG GAG
CAG CAC TTC AGG AG-3′ and reverse, 5′-ATC CCA GGT TTC TTG CCT
CT-3′; CD90 forward, 5′-GAC CCG TGA GAC AAA GAA GC-3′ and reverse,
5′-GCC CTC ACA CTT GAC CAG TT-3′; Oct4 forward, 5′-ACA TGT GTA AGC
TGC GGC C-3′ and reverse, 5′-GTT GTG CAT AGT CGC TGC TTG-3′; Sox2
forward, 5′-TCC CGT ATG AAA GCA TCG TGG-3′ and reverse, 5′-CCC ATT
TGG GTA GAT CAG GTA AC-3′; E-cadherin forward, 5′-GGA TGT GCT GGA
TGT GAA TG-3′ and reverse, 5′-TG GGC AGT GTA GGA TGT GAT-3′;
N-cadherin forward, 5′-CGT GAA GGT TTG CCA GTG T-3′ and reverse,
5′-CAG CAC AAG GAT AAG CAG GA-3′; vimentin forward, 5′-AGA GAA CTT
TGC CGT TGA AGC-3′ and reverse, 5′-ACG AAG GTG ACG AGC CAT T-3′;
GAPDH forward, 5′-AGA AGG CTG GGG CTC ATT TG-3′ and reverse, 5′-AGG
GGC CAT CCA CAG TCT TC-3′.
Cell sphere formation and colony
formation assays
For sphere formation assays, single
CD133+ SMMC-7721 cells of the three groups were plated
in a 6-well ultra-low attachment plate at a density of
1×103 cells/ml (Corning, Steuben, NY, USA) in the SFM.
Sphere formation (diameter, >50 µm) in each well was
calculated under an inverted microscope (Olympus) on the 14th day
after seeding.
Colony formation assays were carried out two days
after transfection. Cells (500/group) were resuspended in DMEM with
10% FBS and were plated in the 6-well plates. The culture medium
was changed twice/week. After 14 days of incubation, the cells were
fixed in 4% formaldehyde and stained with Giemsa staining (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Colonies with
>50 cells were counted.
Cell proliferation assay and apoptosis
detection
Cell prolife-ration was evaluated by the Cell
Counting Kit-8 (CCK-8) (Dojindo, Kumamoto, Japan) assays. The cells
were plated at a density of 2×103 cells/well in 96-well
plates containing serum-free culture medium. Cell proliferation was
examined after 1, 2, 3, 4 and 5 days, respectively. CCK-8 reagent
(10 µl) was added to each well and incubated for 4 h at
37°C. The spectrophotometric absorbance at 450-nm wavelength was
recorded using a microplate reader (Bio-Rad).
Flow cytometry was used to investigate cell
apoptosis. The target cells were collected and resuspended in PBS
buffer at a concentration of 1×106 cells/tube. The
apoptotic rate of cells was detected with an Annexin V-FITC
apoptosis detection kit (eBioscience, Inc., San Diego, CA, USA) and
propidium iodide (PI; Sigma-Aldrich) double staining according to
the manufacturer's guidelines. Flow cytometric analysis was
performed by a FACS-LSR II flow cytometer.
Cell migration and invasion assays
For the Transwell migration assays, 1×105
cells of the three groups were, respectively, resuspended in 200
µl of serum-free culture medium and plated in the upper
chamber of a 24-well 8-µm pore size Transwell plate
(Corning, New York, NY, USA). The lower chamber of Transwell was
loaded with 600 µl culture medium supplemented with 10% FBS
as a chemoattractant. The plates were incubated at 37°C in 5%
CO2 for 24 h. Non-migrating cells on the upper chamber
were removed from the surface of the membrane with cotton-tipped
swabs. The cells migrating through the lower membrane were fixed
with 4% paraformaldehyde for 30 min and stained with 0.1% crystal
violet for 15 min. The cells were counted under a light microscope
(Olympus) at a magnification of ×200 in five randomized fields.
Transwell invasion assay was similarly performed through the Boyden
chamber with polycarbonate membrane inserts that were coated with
BD Matrigel Basement Membrane Matrix (BD Biosciences, San Jose, CA,
USA).
Western blot analysis
Protein was extracted from the cells using RIPA
lysis buffer (Beyotime, Shanghai, China) with protease and
phosphatase inhibitors (Thermo Scientific, Waltham, MA, USA). The
protein concentration was deter-mined using a BCA kit (Beyotime,
Nanjing, China). Equal amounts of protein cell lysates were
separated on SDS-PAGE gels and transferred to PVDF membranes
(Millipore, Billerica, MA, USA). The membranes were blocked in 5%
non-fat dry milk in Tris-buffered saline with 0.1% Tween-20 (TBST)
for 1 h at room temperature and incubated with primary antibodies
overnight at 4°C. The primary antibody dilutions used were:
anti-E-cadherin, anti-N-cadherin and anti-vimentin (Abcam,
Cambridge, UK) at a dilution of 1:1,000; anti-β-actin (Santa Cruz
Biotechnology, Inc.), 1:2,000; anti-IKKβ, anti-p-IKKβ, anti-IκBα,
anti-p-IκBα, anti-RelA and anti-p-RelA (Cell Signaling Technology,
Inc., Danvers, MA, USA) at a dilution of 1:1,000. The membranes
were washed with TBST and then incubated with the secondary
antibodies for 1 h. The protein bands were then visualized using an
enhanced chemiluminescence (ECL) reagent (KeyGen, Nanjing, China)
and quantified using the ChemiDoc™ XRS detection system
(Bio-Rad).
Xenograft tumorigenicity assay
Four-week-old male BALB/c nude mice were purchased
from the Laboratory Animal Center of Chongqing Medical University
(Chongqing, China) to examine tumorigenicity. The mice were raised
in a specific pathogen-free unit under isothermal conditions. To
evaluate the role of CD133 in tumor formation, three groups of
cells (5×105) were suspended in 100 µl of
serum-free medium and inoculated subcutaneously into the flanks of
nude mice. Tumor diameters were measured every week using vernier
calipers. After 5 weeks, the mice were euthanized, and tumors were
weighed. Tumor volumes were calculated according to the formula: v
(mm3) = length × width2/2. The tissue samples
were preserved for subsequent experiments. All the experimental
protocols were approved by the Animal Ethics Committee of Chongqing
Medical University. All the procedures involving animals were
conducted as indicated by the guidelines of the National Institutes
of Health Guide for the Care and Use of Laboratory Animals.
Immunohistochemistry
Briefly, tissue samples preserved in formalin were
embedded in paraffin blocks and sectioned into positively charged
microscope slides. The sections were deparaffinized in xylene and
rehydrated in graded alcohols, and antigens were retrieved by
heating at 95°C for 15 min. The slides were incubated in 3%
hydrogen peroxide for 20 min, and then incubated at 4°C overnight
with primary antibodies anti-E-cadherin, anti-N-cadherin and
anti-vimentin (1:100 dilution; Abcam), respectively. Following
incubation with appropriate secondary antibodies for 30 min at
37°C, the sections were exposed to streptavidin-HRP label
(Zhongshan Chemical, Beijing, China) for 30 min at 37°C and
incubated for 15 min with the chromogen 3,3′-diaminobenzidine and
counterstained with hematoxylin. The sections were then dehydrated
and mounted with neutral gum (Bioworld Technology, Inc., St. Louis
Park, MN, USA). The slides were then observed under a light
microscope.
Statistical analysis
Experiments were performed in triplicate.
Statistically significant values were determined using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The data are presented as
the means ± standard deviation (SD). Group comparisons were
evaluated with the Student's t-test or analysis of variance
(ANOVA). P<0.05 was considered to indicate statistically
significant differences.
Results
Isolation of CD133+ cells from
the SMMC-7721 HCC cell line
It has been reported that the liver CSC marker CD133
is represented only in a small subset of the tumor population in
liver cancer cells (5). In an
attempt to characterize the molecular mechanisms by which
CD133+ liver CSCs mediate tumor formation and growth, we
sorted out the CD133+ liver CSCs from the SMMC-7721 HCC
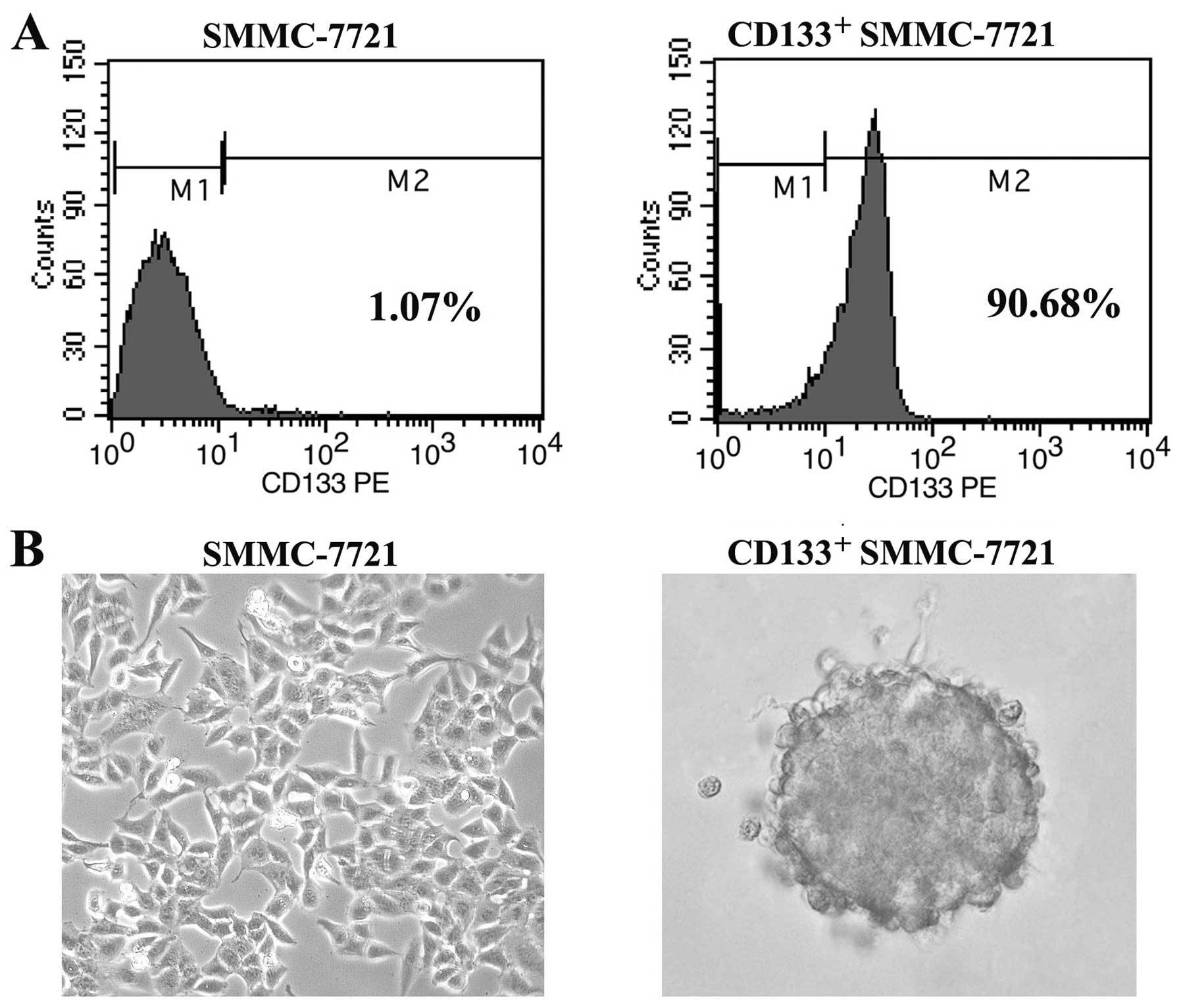
cell line by FACS. Following sorting, CD133+ SMMC-7721
cells were analyzed by flow cytometry, resulting in a considerable
enrichment in the CD133+ cell population (purity,
>90%) compared to the unsorted SMMC-7721 cells (purity, 0.2–2%)
(Fig. 1A). To establish a long-term
culture from CD133+ SMMC-7721 cells that possess cancer
stem cell-like properties, we performed the tumorsphere formation
assay by culturing the CD133+ cells in serum-free
culture medium. Within 14 days of culture, we obtained liver cancer
spheroids in CD133+ cells which grew in aggregate
clusters and increased in size and amount in a timely manner
(Fig. 1B).
Downregulation of CD133 reduces stemness
properties in LCSCs
Since CD133 is a known liver CSC marker, we examined
whether the downregulation of CD133 had any effect on the
inhibition of cancer and stem cell-like properties,
CD133+ SMMC-7721 cells were transfected with shCD133
mediated by UTMD and Lipofectamine 2000, respectively. The effects
of the transfection were assessed with fluorescence, RT-qPCR and
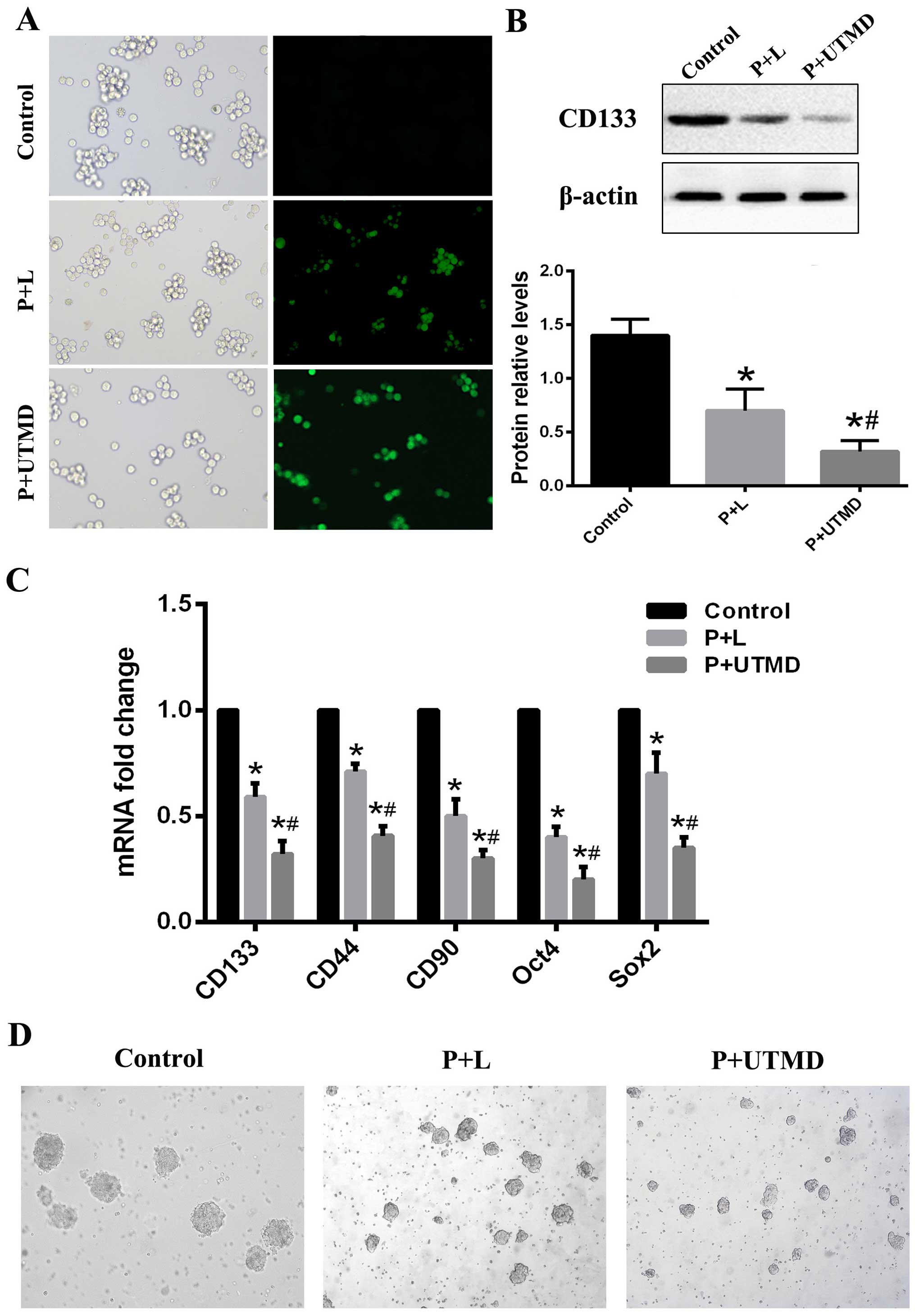
western blot analysis. We observed the expression of GFP under an
inverse fluorescence microscope 12 h after transfection (Fig. 2A). Forty-eight hours after the
transfection, the expression of GFP was the strongest. The
expression of GFP was attenuated gradually 72 h after transfection.
The CD133 expression levels of the groups were determined by
RT-qPCR and western blot analysis. We observed that the relative
mRNA and protein levels of CD133 in the UTMD group of cells were
significantly decreased compared to the Lipofectamine or control
group cells (Fig. 2B). Furthermore,
the mRNAs of several stem cell associated genes, including
CD44, CD90, Oct4 and SOX2, were all
downregulated in the CD133 knockdown LCSCs, which was confirmed by
RT-qPCR (Fig. 2C). We also examined
the effect of the downregulation of CD133 on self-renewal and
sphere-forming ability. The UTMD-transfected CD133+
SMMC-7721 cells formed much smaller and fewer spheroids than cells
transduced with Lipofectamine or the control group (Fig. 2D). Of note, CD133-transduced
spheroids were not passaged from one generation to another, whereas
the CD133+ SMMC-7721 cells spheres were passaged,
demonstrating their reduced self-renewal ability in vitro.
These data suggested that the downregulation of CD133 inhibited
self-renewal and the sphere-forming ability of LCSCs and UTMD
exerted a more significant knockdown effect on gene transfection in
CD133+ SMMC-7721 cells than the
Lipofectamine-transfected group.
Downregulation of CD133 suppresses
proliferation, colony formation ability and promotes apoptosis in
CD133+ SMMC-7721 cells
To detect the biological characteristics of
CD133-downregulated LCSCs, colony-forming and proliferation assays
were performed. CCK-8 assays showed that CD133+
SMMC-7721 cells transfected with shCD133 mediated by UTMD (P+UTMD
group) had a significantly reduced proliferation rate, compared to
the cells transfected with shCD133 mediated by Lipofectamine (P+L)
and the control groups (P<0.05; Fig.
3A). In the colony-forming assays, the P+UTMD group presented a
significant decrease in the number and size of colonies (P<0.05;
Fig. 3B). We detected cell
apoptosis using Annexin V/PI staining. LCSCs transfected with
shCD133 mediated by UTMD significantly increased the percentage of
total apoptotic cells (early + late apoptotic) (Fig. 3C). These results suggested that the
downregulation of CD133 mediated by UTMD resulted in a reduction of
proliferation and cell viability in CD133+ LCSCs.
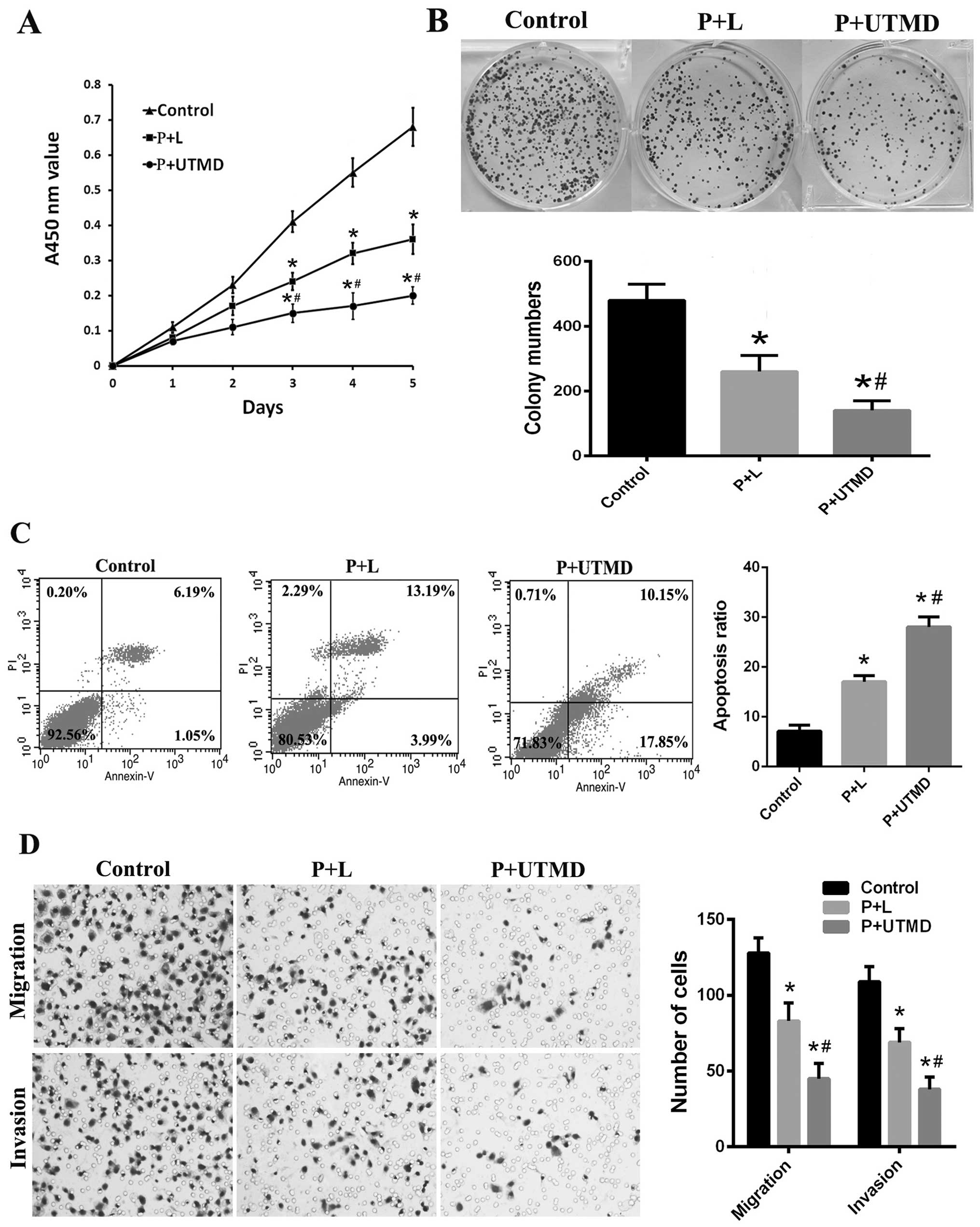 | Figure 3Downregulation of CD133 suppresses
proliferation, clonogenicity, migration and invasion and promotes
apoptosis in CD133+ SMMC-7721 cells. (A) The
proliferation rate was decreased in the P+UTMD group, compared to
P+L or the control group, based on the CCK-8 assays. (B) In
colony-forming assays, the P+UTMD group presented a marked decrease
in the number and size of colonies. Upper, representative images;
lower, colony numbers in three independent experiments. (C) The
percentage of total apoptotic cells (early + late apoptotic) in the
P+UTMD group was significantly increased compared with the P+L or
the control groups based on flow cytometric analysis. (D)
Representative images (left, magnification, ×200) and the
quantifications (right) of cell migration and invasion assays
showing that the downregulation of CD133 mediated by UTMD
attenuated the migration and invasion of LCSCs.
*P<0.05 vs. control groups; #P<0.05 vs.
P+L groups. UTMD, ultrasound-targeted microbubble destruction;
CCK-8, Cell Counting Kit-8; LCSCs, liver cancer stem cells. |
Downregulation of CD133 inhibits invasion
and migration in CD133+ SMMC-7721 cells
To assess whether the downregulation of CD133
affected the malignant properties of CD133+ LCSCs, we
performed the Transwell migration and Boyden chamber assays. As
shown in Fig. 3D, the number of
LCSCs in the CD133 transfected by UTMD group, migrating or invading
through the Boyden chamber pores decreased significantly compared
with the P+L and control groups (P<0.05). These results
suggested that the downregulation of CD133 inhibited invasion and
migration in CD133+ LCSCs and that inhibition of CD133
by UTMD may be an attractive method to regulate malignant
properties of LCSCs.
Downregulation of CD133 attenuates
tumorigenicity in vivo
Based on the observed decreases in proliferative,
invasive and migratory behaviors in LCSCs transfected with shCD133
mediated by UTMD, we then determined the effect of CD133
downregulation on the tumorigenic ability of LCSCs using a nude
mouse xenograft model. CD133+ SMMC-7721 cells were
collected by FACS, transfected with shCD133 mediated by UTMD or
Lipofectamine for 48 h and injected into the flank regions of nude
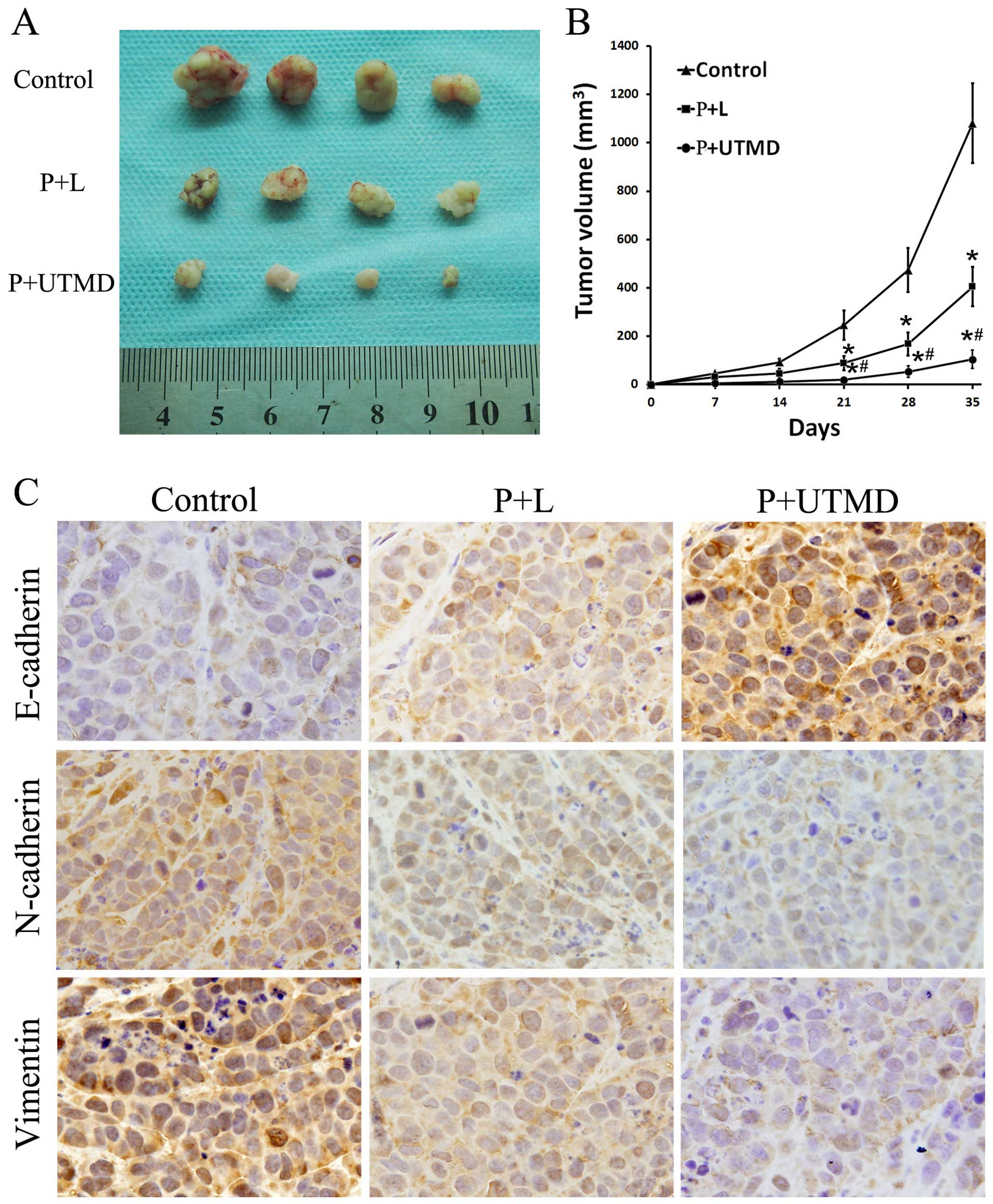
mice. During a 5-week follow-up period, it was observed that the
tumor volumes varied between groups. CD133-downregulated groups
generated small tumors in nude mice in contrast to the large tumors
generated in the CD133+ LCSCs groups. The weights and
volumes of tumors in the P+UTMD group were significantly reduced
compared to those of the P+L and control groups (P<0.05;
Fig. 4A and B). The results
suggested the downregulation of CD133 attenuated the ability
CD133+ LCSCs to initiate tumors in vivo. Thus,
CD133 served as a target for modulating liver cancer. CD133
downregulated by the UTMD technology exerted a significant effect
on the inhibition of tumor growth.
Downregulation of CD133 reverses EMT in
the CD133+ SMMC-7721 cells
EMT has been recognized as a de-differentiation
program attributed to the generation of CSCs, which is also
important in the maintenance of CSCs properties. Based on the
earlier results, we examined whether CD133 influenced the
expression of several EMT-associated genes. The expression levels
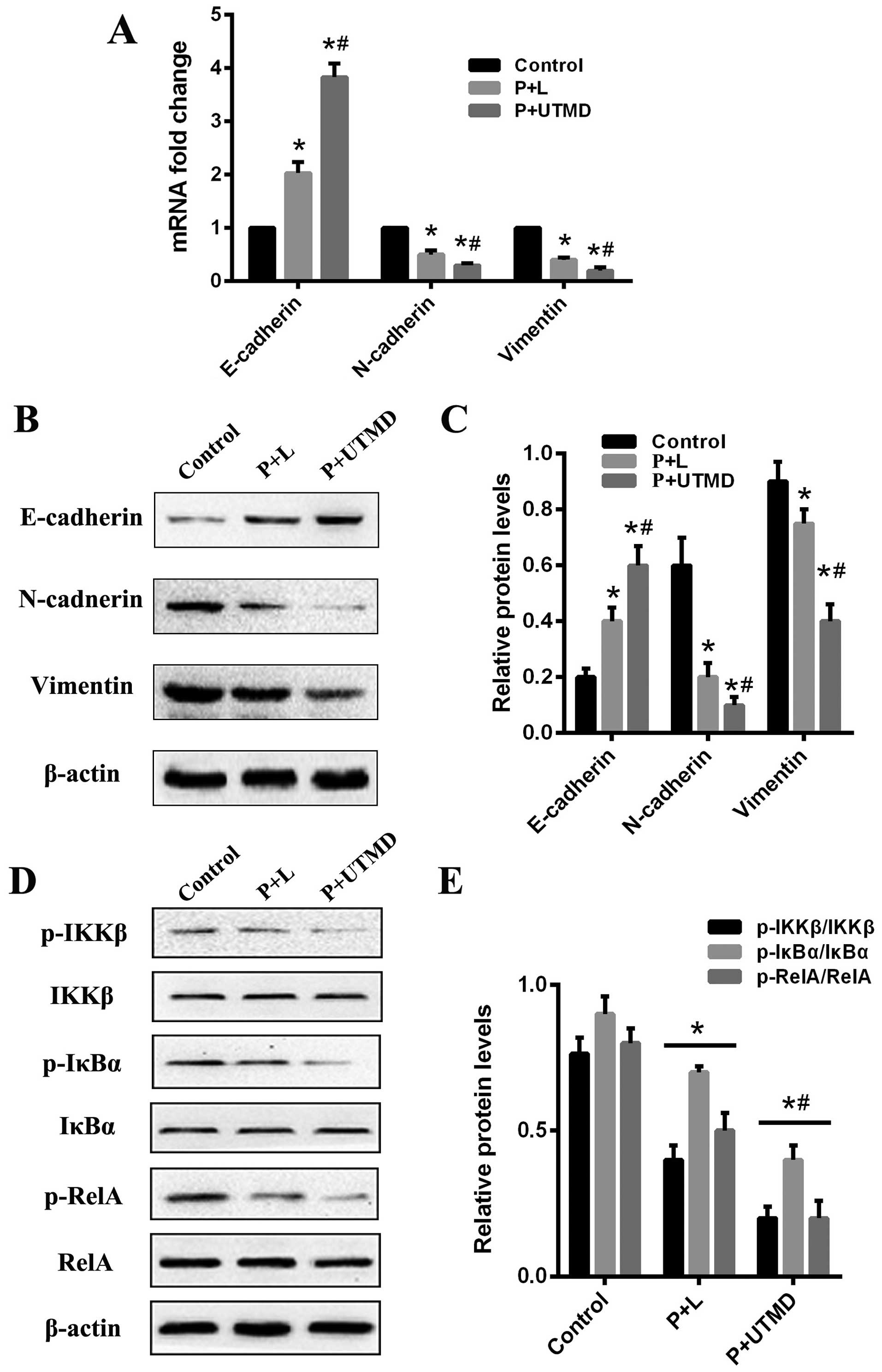
of EMT markers were detected by RT-qPCR and western blotting. The
results revealed that E-cadherin was enhanced, whereas N-cadherin
and vimentin were decreased in CD133-downregulated LCSCs when
compared with the CD133+ SMMC-7721 cells. The expression
of the N-cadherin and vimentin protein in the UTMD-transfected
group was lower than that of the Lipofectamine-transfected and
control groups, while the expression level of E-cadherin in the
UTMD-transfected group was significantly increased (P<0.05;
Fig. 5A–C). To investigate the
potential mechanisms of CD133 and EMT progress, xenograft tissue
samples were analyzed by immunohistochemical staining for the
EMT-related proteins E-cadherin, N-cadherin and vimentin. As is
evident in Fig. 4C, the expression
levels of E-cadherin were increased in the UTMD group compared to
the P+L and control groups (P<0.05). By contrast, N-cadherin and
vimentin exhibited reduced expression levels in the P+UTMD group
with respect to the P+L and control groups, which was consistent
with our earlier in vitro studies. These results
demonstrated that the EMT process may be reversed by the
downregulation of CD133 which contributed to the decreased
invasiveness of LCSCs.
Reversal of EMT mediated by CD133
downregulation may be achieved by suppressing the NF-κB
pathway
To elucidate the underlying mechanism of CD133
regulation of EMT traits, we focused on the NF-κB pathway since it
has been reported to regulate EMT and the development of CSCs. We
identified that the expression of CD133 regulated tumor-initiating
properties and the EMT traits. To investigate whether CD133-induced
EMT is associated with the NF-κB signaling pathway in
CD133+ LCSCs, we examined the expression of IκB kinase β
(IKKβ), inhibitor nuclear factor-κBα (IκBα), and nuclear factor κB
(NF-κB) RelA using western blotting. By shRNA-mediated knockdown of
CD133, a decreased expression of the classical NF-κB signaling
pathway (IKKβ-IκBα-RelA) was observed in the UTMD- and
Lipofectamine-transfected groups, as compared with the control
group (Fig. 5D and E). This
downregulation of the NF-κB signaling pathway further correlated
with our previous migration analysis and reversal of the EMT
phenotype in the LCSCs. Taken together, the results indicated that
the NF-κB pathway mediates the role of CD133 in regulating EMT
phenotype.
Discussion
Cancer stem cells (CSCs) are defined as a small
minority of heterogeneous tumorigenic cells that have the ability
for self-renewal, differentiation and the potential to proliferate
extensively (3). CSCs are
considered to be integral to the initiation, progression and
metastasis of human types of cancer (4). CD133 has recently been identified as
one of the most important CSCs marker for many types of tumor,
including liver cancer (5–7). CD133+ LCSCs bear features
that include the ability to self-renew, differentiate, initiate
tumors in vivo and resist standard chemotherapy (8). The underlying biological functions of
CD133 remain to be elucidated. Although various studies have shown
that CD133 is involved in metastasis, the expression of CD133 has
also been identified as an important risk factor of advanced
disease stage and worst overall survival in HCC (9,10). In
order to determine the molecular mechanisms of CD133 and identify
new effective therapeutic approaches for liver cancer, we sorted
CD133+ cells from the SMMC-7721 cell line and
subsequently inhibited CD133 expression in these cells using a UTMD
technique. We demonstrated that the downregulation of CD133
mediated by UTMD significantly attenuated self-renewal, cell
proliferation, repressed cell invasion and migration, and inhibited
the tumorigenicity in vivo, which was consistent with
previous findings for LCSCs (8,20).
CD133 expression was also found to be associated with several stem
cell-associated genes, including CD44, CD90,
Oct4 and Sox2. Thus, we confirm that CD133 plays a
function rule in regulating proliferative, migratory behaviors and
tumorigenesis in LCSCs.
EMT is a key developmental program that generates
cells with properties of stem cells and contributes to tumor
initiation, invasion and metastatic spread (11,12).
Previous studies have attempted to determine the functional
relevance of CD133 and the EMT process in several types of cancer
(21–24). The results identified indicated that
CD133 may be a critical mediator facilitating migration and
invasion through the EMT progress. In the present study, we showed
that CD133 regulated the invasive ability and properties of LCSCs.
We also examined whether CD133 modulated the EMT pathway in LCSCs.
Western blotting and immunohistochemical stainings showed that an
epithelial-like protein expression pattern (E-cadherin) was
enhanced but a mesenchymal-like protein expression pattern
(N-cadherin and vimentin) was decreased in CD133-downregulated
LCSCs. These results indicate that the downregulation of CD133
reversed the EMT process and that EMT mediated by CD133 may be a
mechanism for the regulation LCSC initiation, invasion and
migration.
NF-κB is thought to initiate and accelerate
tumorigenesis (25,26). It has been shown that NF-κB
activation, through regulation of the expression of several
transcription factors, promotes the EMT program in cancer cells
(27). It has also been suggested
that EMT and the process of invasion are regulated by
NF-κB-mediated signaling in CD133+ cancer cells. Nomura
et al reported that NF-κB activation by CD133 surface
expression increased the metastatic potential and induced EMT in
pancreatic cancer (28). Since
NF-κB path-ways have been associated with EMT and the development
of CSCs, we hypothesized that the reversal of LCSC EMT by
downregulated CD133 may be mediated through the NF-κB signaling
pathway. Notably, in the CD133 downregulated LCSCs, E-cadherin
levels were increased, whereas N-cadherin and vimentin levels were
decreased. Furthermore, a decreased expression of IKKβ-IκBα-RelA
phosphorylation was observed, indicating that the downregulation of
CD133 reversed the progress of EMT and inhibited the classical
NF-κB signaling pathway in the malignant transformation of LCSCs.
These results suggest that NF-κB pathway may partially, mediate the
role of CD133 in regulating EMT phenotype.
UTMD, as a promising method for gene and drug
delivery, may be combined with RNAi technique successfully
(16). The UTMD system is the
combination of ultrasound and microbubbles, which is safer and more
effective compared with other methods. SonoVue is an aqueous
suspension of stabilized sulfur hexafluoride microbubbles, which is
widely used in the clinic. Ultrasound microbubble-mediated
destruction may increase cell membrane permeability and
synergistically promote gene delivery. UTMD has previously been
used to efficiently deliver plasmid DNA to a variety of cancer
cells, including glioma (17), yolk
sac carcinoma (18), human cervical
cancer (19), bone marrow stromal
(29) and HCC cells (30). The aim of the present study was to
evaluate the possibility of shRNA vector transfection mediated by
UTMD in LCSCs. We also addressed the issue of whether the
UTMD-based shRNA delivery system facilitated gene delivery in
LCSCs. Under the selected condition, it was found that the
transfection mediated by UTMD was higher than that of the
Lipofectamine group and showed a significantly decreased expression
of CD133, which was in agreement with the results of RT-qPCR and
western blot analysis. In addition, the invasiveness and
tumorigenicity of LCSCs were significantly decreased by the UTMD
transfection of CD133. The present study demonstrates that UTMD is
a safe and efficient technique for gene delivery to LCSCs.
In conclusion, the present findings have shown that
CD133 expression regulated EMT and stem cell properties in LCSCs
in vitro and in vivo. Downregulation of CD133 reduced
tumor-initiating activities and inhibited invasion and migratory
ability of LCSCs. Notably, the downregulation of CD133 led to a
reduced mesenchymal marker (N-cadherin and vimentin) but induced
epithelial markers (E-cadherin) in LCSCs, and supported a potential
connection between CD133 and EMT transformation. Furthermore,
downregulation of the classical NF-κB pathway (IKKβ-IκBα-RelA
phosphorylation) was observed in the CD133-downregulated LCSCs,
indicating the reversal of EMT and impaired migratory potential of
CD133+ LCSCs may be in part mediated by suppressing the
NF-κB signaling pathway. UTMD effectively transfered shCD133 into
CD133+ LCSCs and led to the inhibition of CD133
expression and the properties of LCSCs. Thus, UTMD may be a
powerful and effective tool for the transfection of specific genes
and functional analysis of genes, which may be explored as a useful
therapeutic option for liver cancer therapy.
Acknowledgments
This study was supported by the Research Projects of
the Chongqing Municipal Health Bureau (grant nos. 2013-2-080 and
2013-1-022).
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tanaka S and Arii S: Molecularly targeted
therapy for hepatocellular carcinoma. Cancer Sci. 100:1–8. 2009.
View Article : Google Scholar
|
|
3
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: Models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar
|
|
4
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suetsugu A, Nagaki M, Aoki H, Motohashi T,
Kunisada T and Moriwaki H: Characterization of CD133+
hepatocellular carcinoma cells as cancer stem/progenitor cells.
Biochem Biophys Res Commun. 351:820–824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin S, Li J, Hu C, Chen X, Yao M, Yan M,
Jiang G, Ge C, Xie H, Wan D, et al: CD133 positive hepatocellular
carcinoma cells possess high capacity for tumorigenicity. Int J
Cancer. 120:1444–1450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO,
Zheng BJ and Guan XY: Identification and characterization of
tumorigenic liver cancer stem/progenitor cells. Gastroenterology.
132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang KH, Ma S, Lee TK, Chan YP, Kwan PS,
Tong CM, Ng IO, Man K, To KF, Lai PB, et al: CD133+
liver tumor-initiating cells promote tumor angiogenesis, growth,
and self-renewal through neurotensin/interleukin-8/CXCL1 signaling.
Hepatology. 55:807–820. 2012. View Article : Google Scholar
|
|
9
|
Yang XR, Xu Y, Yu B, Zhou J, Qiu SJ, Shi
GM, Zhang BH, Wu WZ, Shi YH, Wu B, et al: High expression levels of
putative hepatic stem/progenitor cell biomarkers related to tumour
angiogenesis and poor prognosis of hepatocellular carcinoma. Gut.
59:953–962. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song W, Li H, Tao K, Li R, Song Z, Zhao Q,
Zhang F and Dou K: Expression and clinical significance of the stem
cell marker CD133 in hepatocellular carcinoma. Int J Clin Pract.
62:1212–1218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song R, Song H, Liang Y, Yin D, Zhang H,
Zheng T, Wang J, Lu Z, Song X, Pei T, et al: Reciprocal activation
between ATPase inhibitory factor 1 and NF-κB drives hepatocellular
carcinoma angiogenesis and metastasis. Hepatology. 60:1659–1673.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang R, Li Y, Xu Y, Zhou Y, Pang Y, Shen
L, Zhao Y, Zhang J, Zhou J, Wang X, et al: EMT and CSC-like
properties mediated by the IKKβ/IκBα/RelA signal pathway via the
transcriptional regulator, Snail, are involved in the
arsenite-induced neoplastic transformation of human keratinocytes.
Arch Toxicol. 87:991–1000. 2013. View Article : Google Scholar
|
|
16
|
Li HL, Zheng XZ, Wang HP, Li F, Wu Y and
Du LF: Ultrasound-targeted microbubble destruction enhances
AAV-mediated gene transfection in human RPE cells in vitro and rat
retina in vivo. Gene Ther. 16:1146–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang JF, Wu CJ, Zhang CM, Qiu QY and Zheng
M: Ultrasound-mediated microbubble destruction facilitates gene
transfection in rat C6 glioma cells. Mol Biol Rep. 36:1263–1267.
2009. View Article : Google Scholar
|
|
18
|
He Y, Bi Y, Hua Y, Liu D, Wen S, Wang Q,
Li M, Zhu J, Lin T, He D, et al: Ultrasound microbubble-mediated
delivery of the siRNAs targeting MDR1 reduces drug resistance of
yolk sac carcinoma L2 cells. J Exp Clin Cancer Res. 30:1042011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hao Y, Guo L, Abudula A, Saidoula W and
Guo X: Proliferation inhibition and apoptosis enhancement of human
cervical cancer cells by ultrasound-targeted microbubble
destruction delivered double suicide genes. Int J Clin Exp Med.
7:5330–5335. 2014.
|
|
20
|
Lan X, Wu YZ, Wang Y, Wu FR, Zang CB, Tang
C, Cao S and Li SL: CD133 silencing inhibits stemness properties
and enhances chemoradiosensitivity in CD133-positive liver cancer
stem cells. Int J Mol Med. 31:315–324. 2013.
|
|
21
|
Na DC, Lee JE, Yoo JE, Oh B-K, Choi GH and
Park YN: Invasion and EMT-associated genes are up-regulated in B
viral hepatocellular carcinoma with high expression of CD133-human
and cell culture study. Exp Mol Pathol. 90:66–73. 2011. View Article : Google Scholar
|
|
22
|
Ding Q, Miyazaki Y, Tsukasa K, Matsubara
S, Yoshimitsu M and Takao S: CD133 facilitates
epithelial-mesenchymal transition through interaction with the ERK
pathway in pancreatic cancer metastasis. Mol Cancer. 13:152014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zarkoob H, Taube JH, Singh SK, Mani SA and
Kohandel M: Investigating the link between molecular subtypes of
glioblastoma, epithelial-mesenchymal transition, and CD133 cell
surface protein. PLoS One. 8:e641692013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen YS, Wu MJ, Huang CY, Lin SC, Chuang
TH, Yu CC and Lo JF: CD133/Src axis mediates tumor initiating
property and epithelial-mesenchymal transition of head and neck
cancer. PLoS One. 6:e280532011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Y, Li Y, Wang K, Wang Y, Yin W and Li
L: P38/NF-κB/snail pathway is involved in caffeic acid-induced
inhibition of cancer stem cells-like properties and migratory
capacity in malignant human keratinocyte. PLoS One. 8:e589152013.
View Article : Google Scholar
|
|
26
|
Kumar M, Allison DF, Baranova NN, Wamsley
JJ, Katz AJ, Bekiranov S, Jones DR and Mayo MW: NF-κB regulates
mesenchymal transition for the induction of non-small cell lung
cancer initiating cells. PLoS One. 8:e685972013. View Article : Google Scholar
|
|
27
|
Wamsley JJ, Kumar M, Allison DF, Clift SH,
Holzknecht CM, Szymura SJ, Hoang SA, Xu X, Moskaluk CA, Jones DR,
et al: Activin upregulation by NF-κB is required to maintain
mesenchymal features of cancer stem-like cells in non-small cell
lung cancer. Cancer Res. 75:426–435. 2015. View Article : Google Scholar
|
|
28
|
Nomura A, Banerjee S, Chugh R, Dudeja V,
Yamamoto M, Vickers SM and Saluja AK: CD133 initiates tumors,
induces epithelial-mesenchymal transition and increases metastasis
in pancreatic cancer. Oncotarget. 6:8313–8322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang G, Zhuo Z, Zhang Q, Xu Y, Wu S, Li L,
Xia H and Gao Y: Transfection of CXCR-4 using microbubble-mediated
ultrasound irradiation and liposomes improves the migratory ability
of bone marrow stromal cells. Curr Gene Ther. 15:21–31. 2015.
View Article : Google Scholar
|
|
30
|
Yu BF, Wu J, Zhang Y, Sung HW, Xie J and
Li RK: Ultrasound-targeted HSVtk and Timp3 gene delivery for
synergistically enhanced antitumor effects in hepatoma. Cancer Gene
Ther. 20:290–297. 2013. View Article : Google Scholar : PubMed/NCBI
|