Introduction
Melanoma is a common form of aggressive cancer that
originates from melanocytes. The prognosis for patients with early
stage melanoma is 90% survival by surgical treatment. In contrast,
the prognosis for advanced melanoma is restricted largely due to
chemoresistance of the cancer cells (1). In recent years, numerous chemical
agents have been developed and used in the treatment of melanoma.
Although these agents have immensely contributed to improve
treatment outcomes for patients, they harbor some limitations, in
that they are tumor resistance-prone and induce
chemotherapy-related systemic toxicity (2). Consequently, the search for novel and
non-toxic anti-melanoma agents remains urgent.
Curcumin is the primary bioactive component of
turmeric, a dietary spice derived from the rhizome of Curcuma
longa (3). It has long been
used in Southeastern Asian medicine, and also in cooking to give
food a natural yellow color. Cucumin possesses potent
anti-inflammatory, antioxidant and above all, anticancer properties
(4). Curcumin induces
antiproliferative and apoptotic effects on several human melanoma
cell lines (4–9). In addition, preclinical animal
experiments and phase I clinical trials have certified curcumin to
be of minimal toxicity, even at relatively high doses (12 g/day)
(10). Although most
chemotherapeutic drugs exert their cytotoxic effect by promoting
apoptosis, recent studies indicate that autophagy could hold a
promise in cancer therapy (11–14).
However, there is paucity of information on the effect of curcumin
on autophagy in melanoma. In the present study, we investigated the
effects of curcumin on human melanoma A375 and C8161 cells through
in vitro assessment of cell proliferation, cell cycle, cell
invasion, autophagy and the activation of AKT, mTOR and P70S6K
proteins. A murine explanted melanoma model was further used to
evaluate the anticancer property of curcumin in vivo. We
present evidence that curcumin induces autophagy, and inhibits
proliferation and invasion of human melanoma cells by blocking the
AKT/mTOR signaling pathway.
Materials and methods
Chemicals and reagents
Curcumin (no. A0086, CAS: 458-37-7, purity >98%)
was purchased from Must Biotechnology Inc. (Chengdu, China).
Dulbecco's modified Eagle's medium (DMEM) was purchased from
Gibco-BRL (Grand Island, NY, USA). Dimethylsulfoxide (DMSO),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
Triton X-100, rapamycin and anti-β-actin antibody were purchased
from Sigma Chemical (St. Louis, MO, USA). Matrigel was purchased
from BD Company (Franklin Lakes, NJ, USA). Propidium iodide (PI)
and RNase were purchased from Takara Biotechnology Inc. (Otsu,
Shiga, Japan). Lipofectamine 2000 was purchased from Life
Technologies (Carlsbad, CA, USA). The antibodies for LC3-I and
LC3-II were purchased from Sigma-Aldrich (Shanghai, China). The
antibodies for AKT, phosphorylated-AKT (Ser473), mTOR,
phosphorylated-mTOR (Ser2448), P70S6K, phosphorylated-P70S6K
(Thr389) were all purchased from Cell Signaling Technology
(Beverly, MA, USA).
Cell culture and treatment
Human malignant melanoma A375 and C8161 cell lines
were obtained from Union Cell Resource Center (Beijing, China) and
were cultured and maintained in DMEM supplemented with 1%
penicillin-streptomycin and 10% fetal bovine serum (FBS) at 37°C
and 5% CO2 in a humid incubator. Curcumin (dissolved in
DMSO) was used to treat A375 and C8161 cells. Control cells were
treated with equivalent volume of DMSO.
Cell viability
The effect of curcumin on the viability of the cells
was determined by MTT assay. Briefly A375 and C8161 cells were
plated at 1×104 cells/well in 200 µl culture
medium into 96-well plates. Different concentrations of curcumin
(15–75 µM) were added to different wells and incubated at
37°C for 24, 48, 72 and 96 h. Following incubation for the
specified time, 20 µl MTT solution [5 mg/ml in
phosphate-buffered saline (PBS)] was added to each well and
incubated for additional 4 h. The culture medium in each well was
replaced with 200 µl of DMSO to dissolve the formazan
crystals that were formed from the MTT assay. Absorbance of the
solution was read at a wavelength of 490 nm on a microplate reader
(T17108U; Perkin-Elmer, USA).
Cell invasion assay
To measure the three-dimensional movement of the
cells, cell invasion assay was performed using Transwell chambers
with 8-µm pore polycarbonate filter inserts (Corning, New
York, NY, USA). The upper side of each insert was coated with 10
µl of Matrigel (3 mg/ml; Becton-Dickinson, Mountain View,
CA, USA). A375 and C8161 cells (1×106/ml) were
separately cultured on Matrigel-coated Transwell inserts in DMEM
supplemented with curcumin (25 and 15 µM, respectively), or
DMSO. The lower chamber contained DMEM culture medium appended with
10% FBS as a chemoattractant. After 72 h of incubation, the invaded
cells on the lower surface of the membranes were fixed with chilled
3.7% methanol for 15 min and stained with 0.5% crystal violet for
15 min. Cells were visualized using inverted light microscope, and
invasiveness was determined by counting the number of cells
appearing on the undersurface of the polycarbonate membranes in
five random, non-overlapping fields at a magnification of ×200. The
average number of invaded cells in the five fields was taken to
represent the mean cell invasion. The experiments were performed in
triplicate.
DNA cell cycle analysis
A375 and C8161 cells were treated with curcumin (25
and 15 µM, respectively) at 37°C and 5% CO2
atmosphere for 24 h. Cells were harvested and fixed in chilled 75%
alcohol for 4 h. Prior to analysis, a staining solution containing
480 µl PBS, 5 µl PI (5 mg/ml), 5 µl RNase (10
mg/ml) and 10 µl Triton X-100 (10%) was added to resuspend
the cell pellet and incubated in the dark for 30 min at room
temperature. Cells were analyzed by flow cytometer (Accuri C6; BD,
USA) for cell cycle phase distribution.
Transfection and fluorescence
microscopy
Cells were transfected with a plasmid expressing
GFP-LC3 using Lipofectamine 2000 according to the manufacturer's
instructions. After 16 h post-transfection with GFP-LC3, A375 and
C8161 cells were incubated with curcumin (25 and 15 µM,
respectively) or rapamycin (100 nM, used as a positive control) or
DMSO at 37°C for 24 h. Dot formation by GFP-LC3 was detected under
a fluorescence microscope (DP73; Olympus, Japan) following drug
treatment. Transfected cells were considered to have accumulated
autophagosomes when five or more puncta were observed. A total of
100 transfected cells were examined/well, and the percentage of
cells showing autophagy were counted in triplicate. The experiment
was independently repeated three times.
Transmission electron microscopy
Cells were harvested by scraping from the plates.
They were then washed twice with PBS and fixed with 2.5%
glutaraldehyde and 1% (v/v) osmic acid, followed by an increasing
gradient dehydration step using ethanol and acetone. Cells were
then embedded in epoxy resin and ultrathin sections were cut, and
stained with 0.2% lead citrate and 1% uranyl acetate. Images were
captured with a transmission electron microscope (JEM 1011CX; JEOL,
Japan).
Western blotting
After treatment of A375 and C8161 cells with
curcumin (25 and 15 µM, respectively) for 24 h, the cells
were harvested and incubated in lysis buffer on ice for 30 min.
Then the lysate was clarified by centrifugation at 12,000 x g for
10 min at 4°C to obtain the supernatant (total cell lysate). The
total protein concentration was determined using the Coomassie
brilliant blue (CBB) method. For western blotting, 25 µg of
total protein was separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto nitrocellulose membranes. After blocking of
non-specific binding sites with 5% non-fat dry milk for 2 h at room
temperature, membranes were incubated with respective primary
antibodies at appropriate concentrations overnight at 4°C. After
washing the membranes to remove unbound primary antibodies, they
were incubated with either horseradish peroxidase-conjugated
anti-rabbit or anti-mouse secondary antibody (1:5,000) for 1 h at
room temperature. Finally the membranes were washed with PBS and
chemiluminescence developed using ECL kit (APG BIO, Shanghai,
China) for 1 min. Protein bands were visualized by image scanning
and the optical density for each band was measured using Image Lab
software (version 4.0; Bio-Rad, USA) after data were normalized to
β-actin as an internal control.
In vivo antitumor activity assay
All the animal experiments were carried out in
strict accordance with the institutional guidelines for the ethical
treatment of animals. The protocol was approved by the Ethics
Committee of the Laboratory Animal of Dalian Medical University
(permit no. L2015012). Six-week-old female BALB/c nude mice
(Institute of Animal Center, Chinese Academy of Sciences, Shanghai,
China) were housed in laminar flow rooms with stable temperature
and humidity conditions. Human melanoma A375 cells
(1×107/ml) resuspended in PBS were injected
subcutaneously into the right flank of each mouse. The mice were
randomly assigned to two groups (n=5/group). Therapy was initiated
on the eighth day post-inoculation with A375 melanoma cells. Mice
from the control and therapeutic groups received intraperitoneal
injections of DMSO or curcumin (25 mg/kg), respectively, every day
for 3 weeks. Tumor size was monitored before every injection using
calipers at 3 days interval, and tumor volume was calculated using
the formula: Volume (mm3) = (maximal length) ×
(perpendicular width)2/2. All the mice were sacrificed
21 days after treatment. In addition, the tumors were resected for
analyses.
Statistical analysis
All the experiments were carried out in triplicate
and values are expressed as mean ± standard deviation (SD). SPSS
v17.0 software (SPSS, Inc., Chicago, IL, USA) was used for
statistical analysis. The Student's t-test was used for the
assessment of differences between groups. A probability of ≤0.05
was deemed statistically significant. In the figures:
*P<0.05, **P<0.01 and
***P<0.001, relative to controls.
Results
Curcumin exhibits antiproliferative
effect on A375 and C8161 cells
We first studied the inhibitory effect of curcumin
on the growth of A375 and C8161 cells by employing MTT assay. Cells
were treated with different doses of curcumin (15–75 µM) for
different periods of time (24–96 h). MTT assay showed that curcumin
was effective in inhibiting the proliferation of A375 and C8161
cells in a dose-dependent, as well as time-dependent manner
(ranging from 15–35 and 15–25 µM, respectively, within 48 h
(Fig. 1A and B). The
IC50 value at 24 h was estimated to be 25 and 15
µM, respectively (Fig. 1A and
B).
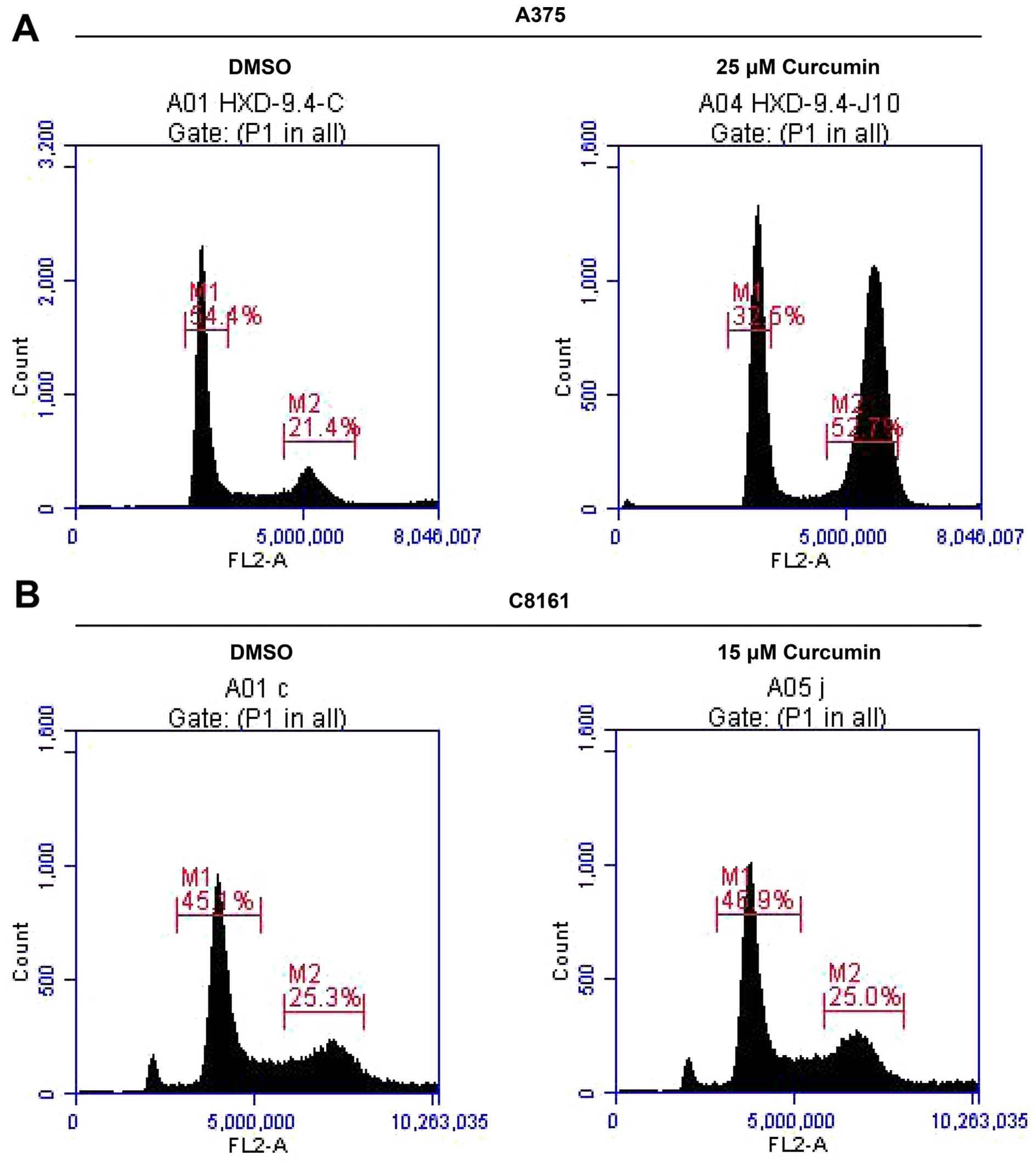
Curcumin arrests A375 and C8161 cells at
G2/M phase in the cell cycle
Since curcumin treatment inhibited A375 and C8161
cell growth, we next examined whether growth inhibitory effect of
curcumin was mediated through cell cycle arrest. For this purpose,
the effect of curcumin on cell cycle progression in A375 and C8161
cells was determined by flow cytometry. Treatment of A375 and C8161
cells with curcumin (25 and 15 µM, respectively) resulted in
a remarkable accumulation of cells in G2/M phase and a
reduction in G0/G1 cell population during the
cell cycle (Fig. 2, Table I). This profound decrease in
G0/G1 phase cell population suggests a
G2/M phase cell cycle arrest of melanoma cells upon
exposure to curcumin and that blockage of cell cycle progression
may be one of the mechanisms by which curcumin inhibits A375 and
C8161 cells growth.
 | Table IEffect of curcumin on cell cycle
progression in A375 and C8161 cells. |
Table I
Effect of curcumin on cell cycle
progression in A375 and C8161 cells.
| Curcumin
concentrations | A375
| C8161
|
|---|
|
G0/G1 (%) | G2/M
(%) |
G0/G1 (%) | G2/M
(%) |
|---|
| 0 (µM) | 51.80±3.29 | 21.30±0.75 | 44.57±1.00 | 25.23±2.31 |
| 15 (µM) | – | – | 43.53±2.33 | 33.80±1.25a |
| 25 (µM) | 33.40±6.84 | 48.07±7.09a | – | – |
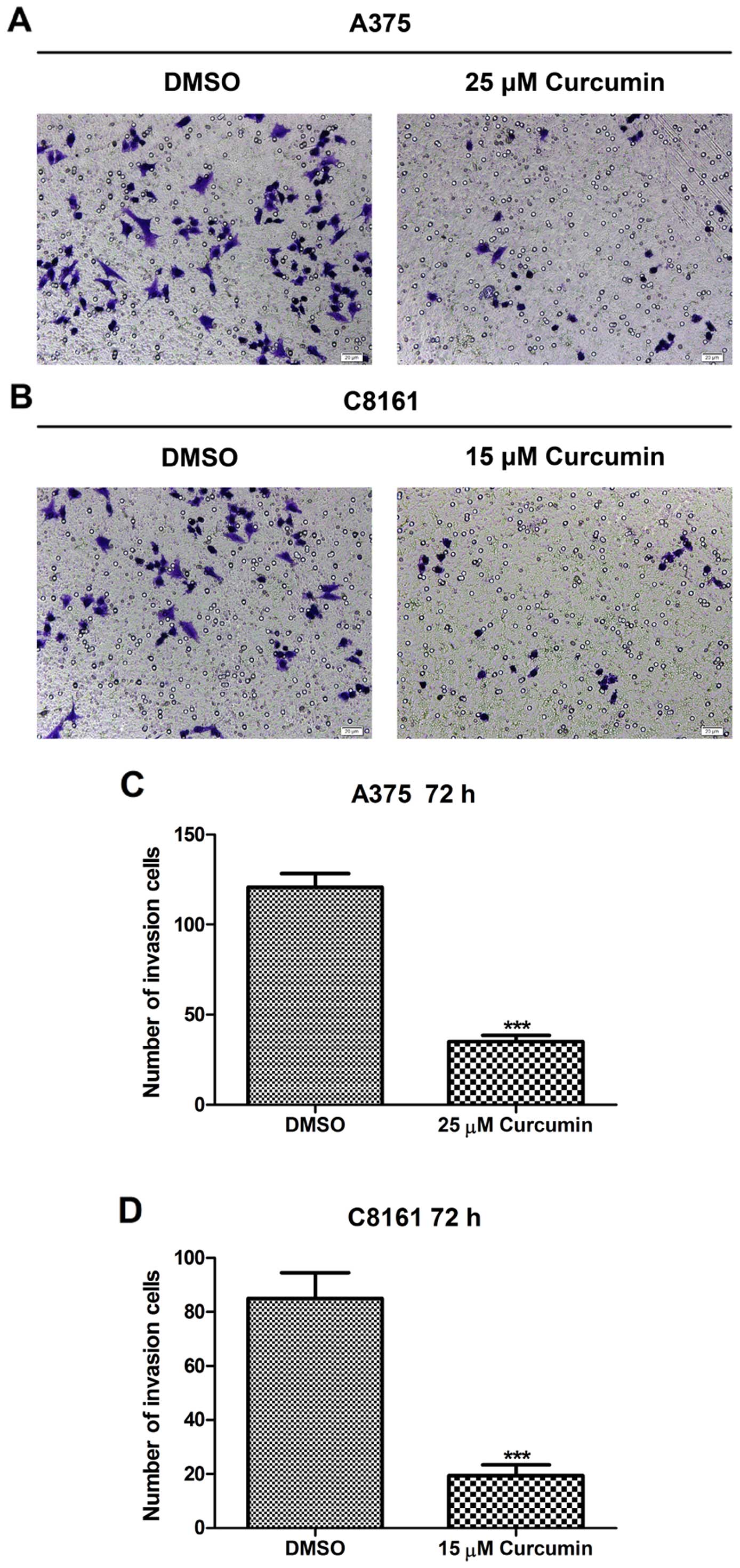
Curcumin inhibits A375 and C8161 cell
invasion potential
The Matrigel model of the basement membrane was
employed as a cell invasion barrier to quantify the invasive
potential of A375 and C8161 cells. The density of invaded cells on
the membrane and the number of invaded cells/microscopic field are
shown in Fig. 3. Treatment with
curcumin (25 and 15 µM, respectively) for 72 h significantly
reduced the invasive potential of A375 and C8161 melanoma cells as
compared to their untreated control cells (P<0.001). When A375
cells were treated with ≥35 µM curcumin and C8161 cells
treated with ≥25 µM curcumin for 72 h, no invading cells
could be seen (data not shown), suggesting that curcumin may
antagonize the invasive potential of melanoma cells, as evidenced
on A375 and C8161 cells.
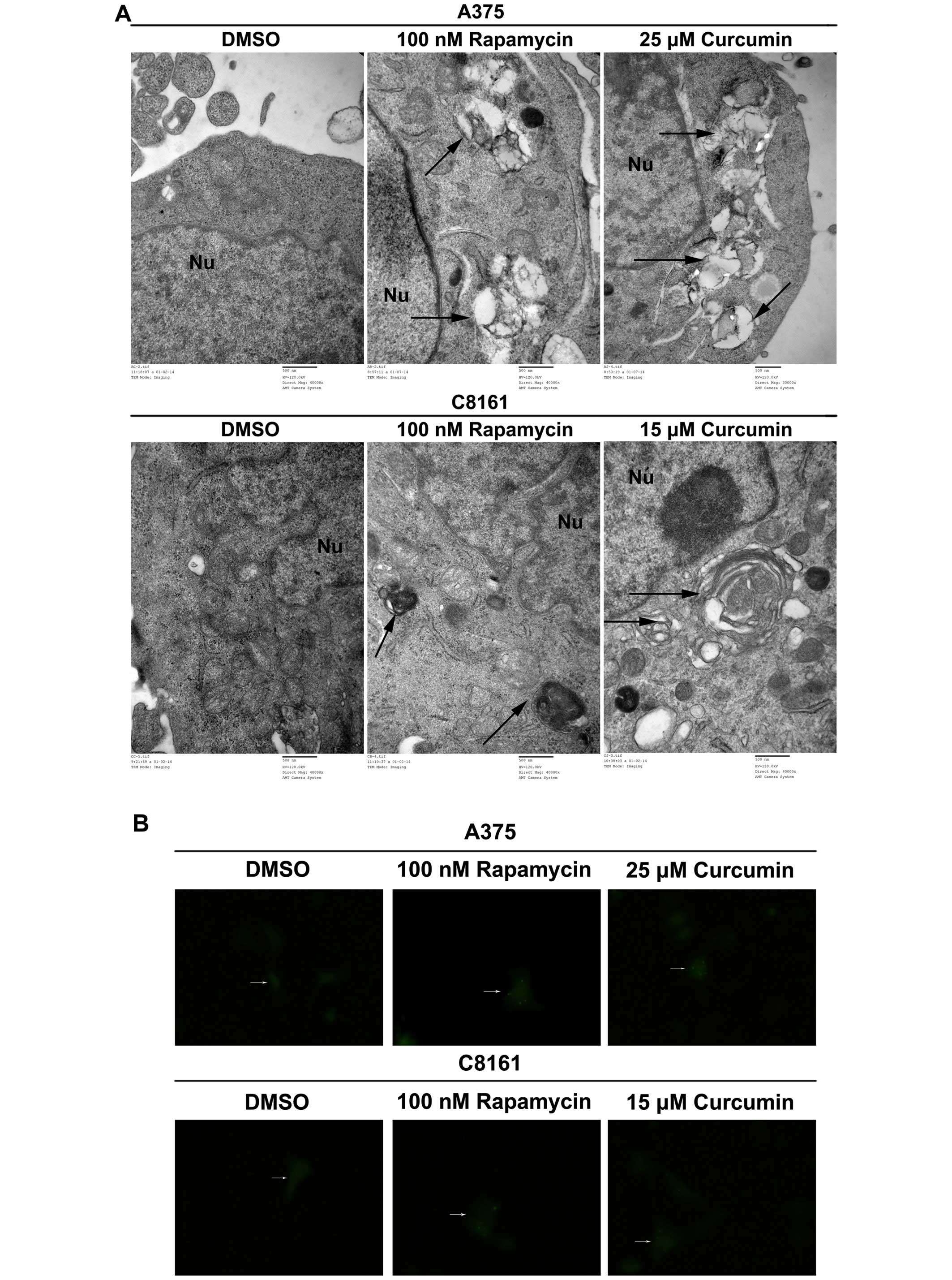
Curcumin promotes autophagy in A375 and
C8161 cells
LC3 is now believed to be a reliable marker for
autophagy. A375 and C8161 cells were treated with DMSO, rapamycin
(100 nM) or curcumin (25 and 15 µM, respectively). As shown
in Fig. 4A, autophagic vacuoles and
autolysosomes accumulated in rapamycin- and curcumin-treated
groups, while none was observed in DMSO-treated group (Fig. 4A). In the absence of any treatment,
diffuse LC3 fluorescence was observed. Following treatment with
curcumin, punctate fluorescence, which was similar to that produced
by the canonical autophagy inducer rapamycin, was observed
(Fig. 4B). Curcumin caused an
obvious increase in the number of cells with GFP-LC3 punctate dots
in both A375 and C8161 cells (Fig.
4C, P<0.001). During autophagy, LC3-II is recruited to the
autophagosome. We found that LC3-II level was significantly
increased with 25 and 15 µM curcumin treatment of A375 and
C8161 cells as compared to DMSO treatment (Fig. 4D, P<0.001, P<0.05). These
results revealed that curcumin could potently induce autophagy in
both A375 and C8161 cells.
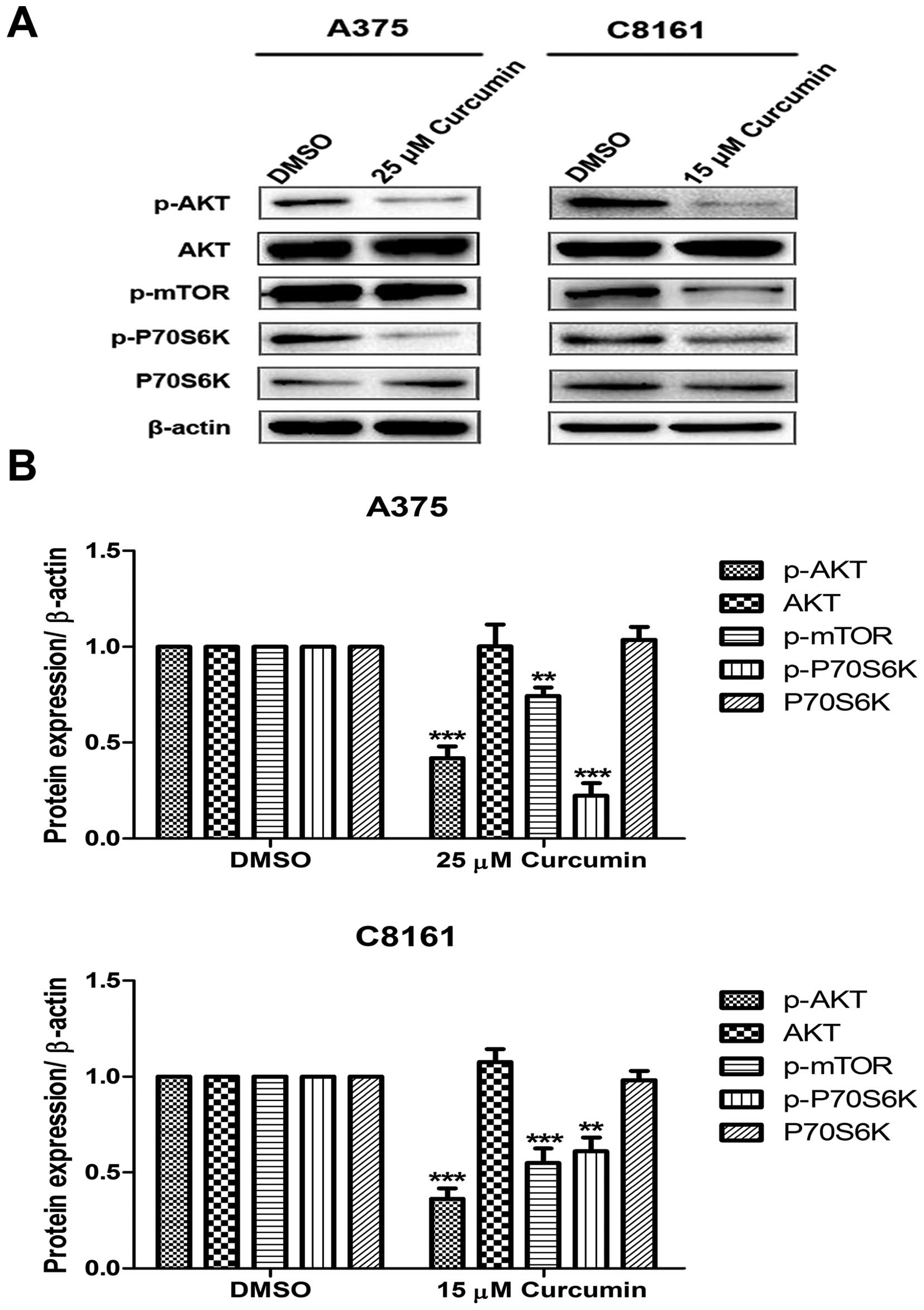
Curcumin inhibits the activation of
PI3K/AKT/mTOR signaling pathway
Since growth factor signaling can directly regulate
autophagy through mTOR pathway, western blotting against
phospho-AKT, AKT, phospho-mTOR, phospho-P70S6K and P70S6K were
performed. Under the indicated concentration of curcumin treatment
of A375 and C8161 cells, the expression of PI3K downstream
activated proteins, p-AKT, p-mTOR and p-P70S6K, significantly
decreased as compared to DMSO treated cells (Fig. 5).
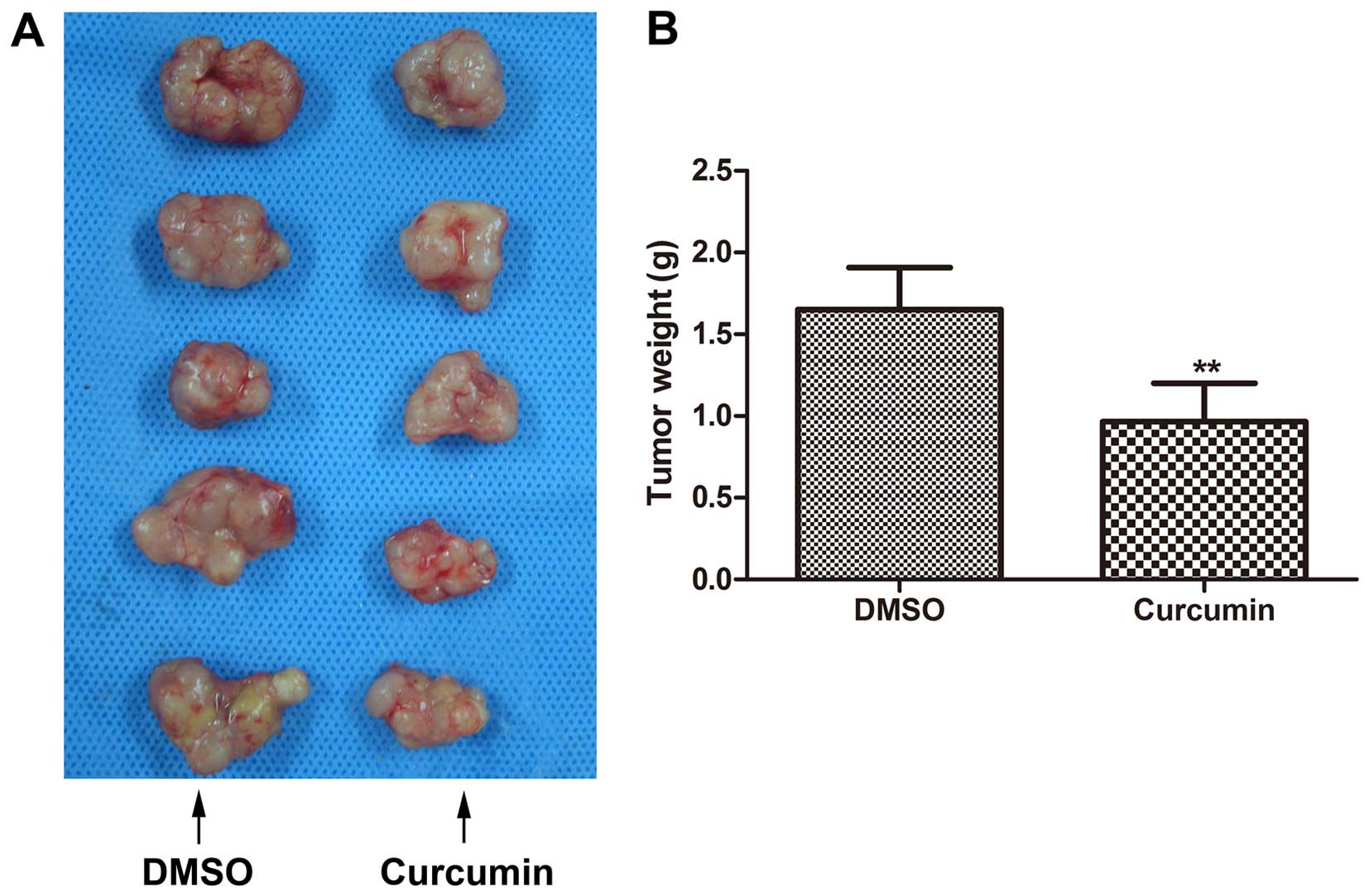
Inhibition of melanoma growth in vivo by
curcumin treatment
BALB/c nude mice were inoculated with A375 melanoma
cells to establish an explanted in vivo melanoma model.
After 21 days of treatments with either curcumin or DMSO, tumors in
the curcumin-treated group were generally smaller than tumors in
corresponding DMSO-treated animals (Fig. 6A). The average tumor weight in
curcumin-treated mice was also markedly less as compared to tumors
from control mice (Fig. 6B).
However, the difference between the average tumor volumes of the
two groups was not statistically significant (data not shown).
Curcumin, thus may suppress melanoma growth in vivo.
Discussion
Melanoma is regarded as one of the most malignant
cancers. In 2014, ~76,100 new cases of melanoma was diagnosed in
the US alone, and an estimated 9,710 deaths occurred from the
disease (15). Currently, the major
challenges to conventional antitumor agents include
chemoresistance, and severe-systemic adverse effects (16–18).
Therefore, growing interests have focused on the search for novel
alternative medicines both safe and effective against melanoma.
Phytochemical compounds have shown fairly promising potential as
anti-carcinogen (19). In the
present study, we explored the chemotherapeutic capacity of
curcumin against human melanoma.
We used human melanoma A375 and C8161 cell lines
with different BRAF mutation status. A375 cells harbor the
BRAFV600E mutation, unlike C8161 cells which
harbor BRAF wild-type gene. Curcumin, as evidenced in the present
study, observably inhibited the growth of both A375 and C8161 cells
(Fig. 1). It could also
significantly decrease the tumor weight in a murine melanoma model
(Fig. 6). These data, in
vitro and in vivo, strongly suggest curcumin have
chemotherapeutic potential against melanoma.
G2/M checkpoint is one of the well-known
cell cycle checkpoints. Defective checkpoint function fosters
genetic modifications that contribute to tumorigenesis. The
regulation of checkpoint signaling offers important clinical
influences because the abrogation of checkpoint function can alter
the sensitivity of tumor cells to chemotherapeutics (20). Moreover, cell cycle arrest is
considered to be an effective strategy for eliminating cancer cells
(21). We found that curcumin
inhibited the growth of A375 and C8161 cells by inducing cell
arrest at G2/M phase of the cell cycle (Fig. 2). Our data support an earlier
assertion by Zheng et al that curcumin may arrest cells at
the G2/M transition in human melanoma cells (6).
It has been reported that the antiproliferative
property of D6, a curcumin analogue, in melanoma could be partially
due to the downregulation of the PI3K/AKT pathway (22). Proverbially PI3K/AKT/mTOR/P70S6K
pathway is a critical intracellular signaling pathway with respect
to cell survival and death (23).
The present study revealed that curcumin inhibited the activation
of AKT, mTOR and P70S6K proteins (Fig.
5). AKT is active during mitosis and suppression of PI3K/AKT
pathway facilitates cell cycle to arrest at G2/M
transition through the regulation of CDK1 expression rather than
cyclin B1 expression (24).
The pivotal signaling pathway that modulates
invasion of tumor cells is the PI3K/AKT pathway (25). This pathway promotes resistance to
chemotherapy-induced apoptosis in many types of cancer including
melanoma (26). Several studies
have reported that P70S6K has the potential to regulate cell
motility (27). The effects of mTOR
and P70S6K on migration may be correlated to synthesis of proteins
required for cytoskeleton reorganization (28). Our results suggested that curcumin
exhibited anti-invasion effect on A375 and C8161 cells (Fig. 3) and this could be via the
downregulation of phospho-AKT, phospho-mTOR and phospho-P70S6K
(Fig. 5).
Apoptosis has been generally recognized to be
associated with oncogenic transformation and tumor development.
However, an increasingly growing body of evidence indicates that
autophagy may be of equivalent importance in tumorigenesis and as
such a momentous target for cancer therapy (29). Recent studies suggest autophagy may
play a role in resistance to chemotherapy (30–32).
Autophagy is hence being explored as a therapeutic option for
advanced cancers, such as in melanoma treatment (33). The present study revealed that
curcumin significantly elevated the expression of LC3 which is a
prominent marker of autophagy (Fig.
4). Chatterjee and Pandey (4)
have also reported that curcumin treatment could induce autophagy
in A375 cells. We demonstrated here that curcumin may not only
induce autophagy in A375 cells, but also in BRAF mutation negative
melanoma cells such as C8161. This is essential in that curcumin
may be effective against melanoma subtypes which are resistant to
BRAF inhibitors. However, further studies are needed for validation
of this phenomenon. AKT negatively regulates autophagy in response
to mitogens via activation of target of rapamycin (mTOR), which
suppresses multiple autophagy-promoting proteins via
phosphorylation (34). In the
present study, we found for the first time that treatment of
curcumin-induced autophagy via decreasing the phosphorylation of
AKT, mTOR and P70S6K. Chatterjee and Pandey (4) have shown that curcumin in combination
with tamoxifen, both at low doses, induce a synergistic increase in
cell death of chemoresistant human melanomas as compared to the use
of either drug alone. This observation was associated with
increased induction of autophagy. Given this evidence, nontoxic
curcumin is worthy of future clinical trials against chemoresistant
human malignant melanoma.
In conclusion, curcumin is a potent suppressor of
cell viability and invasion, and simultaneously an inducer of
autophagy in melanoma cells. These activities are associated with
inhibition of the PI3K/AKT/mTOR/P70S6K pathway. Consequently,
curcumin may possess an effective antitumoral potency and provides
a promising treatment paradigm for melanoma.
Acknowledgments
We would like to thank Quentin Liu for guidance in
our research. The present study was supported by grants from the
National Natural Science foundation of China (81472865, 81171491
and 30670650), and the Natural Science foundation of Liaoning
Province (201102056 and 2014023005).
References
|
1
|
Russo A, Ficili B, Candido S, Pezzino FM,
Guarneri C, Biondi A, Travali S, McCubrey JA, Spandidos DA and
Libra M: Emerging targeted therapies for melanoma treatment
(Review). Int J Oncol. 45:516–524. 2014.PubMed/NCBI
|
|
2
|
Voskoboynik M and Arkenau HT: Combination
therapies for the treatment of advanced melanoma: A review of
current evidence. Biochem Res Int. 2014:3070592014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anand P, Sundaram C, Jhurani S,
Kunnumakkara AB and Aggarwal BB: Curcumin and cancer: An 'old-age'
disease with an 'age-old' solution. Cancer Lett. 267:133–164. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chatterjee SJ and Pandey S:
Chemo-resistant melanoma sensitized by tamoxifen to low dose
curcumin treatment through induction of apoptosis and autophagy.
Cancer Biol Ther. 11:216–228. 2011. View Article : Google Scholar
|
|
5
|
Siwak DR, Shishodia S, Aggarwal BB and
Kurzrock R: Curcumin-induced antiproliferative and proapoptotic
effects in melanoma cells are associated with suppression of
IkappaB kinase and nuclear factor kappaB activity and are
independent of the B-Raf/mitogen-activated/extracellular
signal-regulated protein kinase pathway and the Akt pathway.
Cancer. 104:879–890. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng M, Ekmekcioglu S, Walch ET, Tang CH
and Grimm EA: Inhibition of nuclear factor-kappaB and nitric oxide
by curcumin induces G2/M cell cycle arrest and apoptosis in human
melanoma cells. Melanoma Res. 14:165–171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Odot J, Albert P, Carlier A, Tarpin M,
Devy J and Madoulet C: In vitro and in vivo anti-tumoral effect of
curcumin against melanoma cells. Int J Cancer. 111:381–387. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marín YE, Wall BA, Wang S, Namkoong J,
Martino JJ, Suh J, Lee HJ, Rabson AB, Yang CS, Chen S, et al:
Curcumin downregulates the constitutive activity of NF-kappaB and
induces apoptosis in novel mouse melanoma cells. Melanoma Res.
17:274–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oelkrug C, Lange CM, Wenzel E, Fricke S,
Hartke M, Simasi J and Schubert A: Analysis of the tumoricidal and
anti-cachectic potential of curcumin. Anticancer Res. 34:4781–4788.
2014.PubMed/NCBI
|
|
10
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu H, He Z and Simon HU: Targeting
autophagy as a potential therapeutic approach for melanoma therapy.
Semin Cancer Biol. 23:352–360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hönscheid P, Datta K and Muders MH:
Autophagy: Detection, regulation and its role in cancer and therapy
response. Int J Radiat Biol. 90:628–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chu YL, Raghu R, Lu KH, Liu CT, Lin SH,
Lai YS, Cheng WC, Lin SH and Sheen LY: Autophagy therapeutic
potential of garlic in human cancer therapy. J Tradit Complement
Med. 3:159–162. 2013. View Article : Google Scholar
|
|
14
|
Thorburn A, Thamm DH and Gustafson DL:
Autophagy and cancer therapy. Mol Pharmacol. 85:830–838. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alifrangis C, Koizia L, Rozario A, Rodney
S, Harrington M, Somerville C, Peplow T and Waxman J: The
experiences of cancer patients. QJM. 104:1075–1081. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Slevin ML, Stubbs L, Plant HJ, Wilson P,
Gregory WM, Armes PJ and Downer SM: Attitudes to chemotherapy:
Comparing views of patients with cancer with those of doctors,
nurses, and general public. BMJ. 300:1458–1460. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thornton M, Parry M, Gill P, Mead D and
Macbeth F: Hard choices: A qualitative study of influences on the
treatment decisions made by advanced lung cancer patients. Int J
Palliat Nurs. 17:68–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chinembiri TN, du Plessis LH, Gerber M,
Hamman JH and du Plessis J: Review of natural compounds for
potential skin cancer treatment. Molecules. 19:11679–11721. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stewart ZA, Westfall MD and Pietenpol JA:
Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol
Sci. 24:139–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho JH, Lee JG, Yang YI, Kim JH, Ahn JH,
Baek NI, Lee KT and Choi JH: Eupatilin, a dietary flavonoid,
induces G2/M cell cycle arrest in human endometrial cancer cells.
Food Chem Toxicol. 49:1737–1744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rozzo C, Fanciulli M, Fraumene C, Corrias
A, Cubeddu T, Sassu I, Cossu S, Nieddu V, Galleri G, Azara E, et
al: Molecular changes induced by the curcumin analogue D6 in human
melanoma cells. Mol Cancer. 12:372013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Wang H, Xu J, Zhu J and Ding K:
Inhibition of cathepsin S induces autophagy and apoptosis in human
glioblastoma cell lines through ROS-mediated PI3K/AKT/mTOR/p70S6K
and JNK signaling pathways. Toxicol Lett. 228:248–259. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ornelas IM, Silva TM, Fragel-Madeira L and
Ventura AL: Inhibition of PI3K/Akt pathway impairs G2/M transition
of cell cycle in late developing progenitors of the avian embryo
retina. PLoS One. 8:e535172013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin HP, Jiang SS and Chuu CP: Caffeic acid
phenethyl ester causes p21 induction, Akt signaling reduction, and
growth inhibition in PC-3 human prostate cancer cells. PLoS One.
7:e312862012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou HY and Wong AS: Activation of p70S6K
induces expression of matrix metalloproteinase 9 associated with
hepatocyte growth factor-mediated invasion in human ovarian cancer
cells. Endocrinology. 147:2557–2566. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berven LA, Willard FS and Crouch MF: Role
of the p70S6K pathway in regulating the actin
cytoskeleton and cell migration. Exp Cell Res. 296:183–195. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Corazzari M, Fimia GM, Lovat P and
Piacentini M: Why is autophagy important for melanoma? Molecular
mechanisms and therapeutic implications. Semin Cancer Biol.
23:337–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu S, Wang X, Chen J and Chen Y: Autophagy
of cancer stem cells is involved with chemoresistance of colon
cancer cells. Biochem Biophys Res Commun. 434:898–903. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han Z, Jing Y, Xia Y, Zhang S, Hou J, Meng
Y, Yu F, Liu X, Wu M, Zhang P, et al: Mesenchymal stem cells
contribute to the chemoresistance of hepatocellular carcinoma cells
in inflammatory environment by inducing autophagy. Cell Biosci.
4:222014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ge J and Chen Z, Huang J, Chen J, Yuan W,
Deng Z and Chen Z: Upregulation of autophagy-related gene-5 (ATG-5)
is associated with chemoresistance in human gastric cancer. PLoS
One. 9:e1102932014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Armstrong JL, Corazzari M, Martin S,
Pagliarini V, Falasca L, Hill DS, Ellis N, Al Sabah S, Redfern CP,
Fimia GM, et al: Oncogenic B-RAF signaling in melanoma impairs the
therapeutic advantage of autophagy inhibition. Clin Cancer Res.
17:2216–2226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Janku F, McConkey DJ, Hong DS and Kurzrock
R: Autophagy as a target for anticancer therapy. Nat Rev Clin
Oncol. 8:528–539. 2011. View Article : Google Scholar : PubMed/NCBI
|