Introduction
Pancreatic cancer is a highly malignant tumor with
an extremely poor prognosis, partially due to the lack of early
diagnosis and treatment options (1). Although surgery remains the only way
to cure this severe disease, the majority of patients present at an
advanced inoperable stage and only 20% of patients are with
localized disease amenable for surgery (2). Even those seemingly resectable
pancreatic tumors often fail to be cured due to the microscopic
systemic spread of the cancer that occurs before the surgical
intervention (3). Understanding the
molecular basis of the disease is highly desirable for developing
new strategies to prevent and treat pancreatic cancer.
Low oxygen tension is most commonly presented in the
microenvironment of solid tumors (4). Tumor hypoxia is associated with
enhanced tumor invasiveness, angiogenesis and distant metastasis
(5,6). Hypoxia-inducible factor-1 (HIF-1),
which belongs to the basic helix-loop-helix-periodic acid-Schiff
domain transcription factor family, is the most important
transcription factor as a result of intratumoral hypoxia (7). HIF-1 consists of two subunits, HIF-1α
and HIF-1β. Only the expression and activation of HIF-1α is tightly
regulated by the cellular oxygen concentration (8). In pancreatic cancer, the level of
HIF-1α expression is overexpressed and is associated with tumor
progression, angiogenesis, cell migration and hepatic metastasis
(9,10). Our previous study identified that
Hedgehog (Hh) signaling modulated hypoxia-induced pancreatic cancer
epithelial to mesenchymal transition (EMT) and invasion (11).
The Hh signaling pathway, which is considered to
play an important role in vertebrate development, the homeostatic
process and tumorigenesis (12), is
normally quiescent in the adult pancreas and has been shown to be
very active in pancreatic cancer (11). The Hh signaling pathway, initiated
through the binding of secreted Hh ligands to the membrane receptor
patched 1 (PTCH1), results in smoothened (SMO) dissociation,
nuclear translocation and activation of the transcription factors
of the GLI family (11,13). The expression of SMO and GLI1 is
presumed to be markers of Hh pathway activation (11). Our previous study confirmed that
hypoxia-induced invasion and the EMT process is intimately related
with the Hh signaling pathway (11). In addition, inhibition of Hh
signaling also enhanced vascular density and delivery of
gemcitabine in a mouse model of pancreatic cancer (14).
Reactive oxygen species (ROS) generated by the
mitochondrial respiratory chain, consist of a number of chemically
reactive molecules derived from oxygen, including hydrogen peroxide
(H2O2). Malignant tumor cells commonly have
increased levels of ROS, which plays a significant role in cancer
progression (15,16). Our recent study showed that
hypoxia-induced ROS production is intimately related with
pancreatic stellate cell (PSC) activation and pancreatic cancer
cell invasion (17).
Resveratrol
(trans-3,4′,5-trihydroxystilbene), a natural polyphenolic
phytoalexin, is widely found in plants (such as grape skin, red
wine, berries and peanuts) and in traditional Chinese medicines
(such as Rheum officinale Baill. and Polygonum
cuspidatum) (18). Recent
studies have shown that resveratrol has many biological and
pharmaceutical properties, including anti-inflammatory,
antioxidant, anti-aging, neuroprotective and antitumorigenic
capabilities (19–21). Our previous study demonstrated that
resveratrol plays an important role in suppressing the
proliferation and EMT of pancreatic cancer cells via the
PI-3K/Akt/NF-κB signaling pathway (22). In addition, we also confirmed that
resveratrol inhibited the growth of human pancreatic cancer cells
in vitro by inhibiting cell proliferation and promoting cell
apoptosis via inhibition of the Hh signaling pathway (23).
In the present study, we tested the hypothesis that
resveratrol is able to inhibit hypoxia-induced ROS production and
the invasive and migratory ability of pancreatic cancer cells. We
also investigated the effect of resveratrol on hypoxia-induced
activation of the Hh pathway. Results from the present study
suggest that resveratrol treatment may be a novel option for the
therapy of pancreatic cancer via inhibition of the Hh signaling
pathway.
Materials and methods
Preparation of chemicals
Dulbecco's modified eagle's medium (DMEM) and fetal
bovine serum (FBS) were purchased from Gibco (Grand Island, NY,
USA). Resveratrol (>99% pure) was acquired from Xi'an Chongxin
Natural Additive Company (Xi'an, China). N-acetylcysteine (NAC) was
purchased from Sigma. Millicell Transwells for the invasion assays
were obtained from Millipore (Billerica, MA, USA). Matrigel was
from BD (Biosciences, Bedford, MA, USA). Primary antibodies against
HIF-1α, MMP-2, uPA, SHH, SMO as well as GLI1 were from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Nitrocellulose membranes were
from Millipore. The BCA assay kit and the chemiluminescence kit
were from Pierce (Rockford, IL, USA). Other reagents were purchased
from common commercial sources. All drug solutions were freshly
prepared on the day of testing.
Cell cultures and treatments
The human pancreatic cancer cell lines, BxPC-3 and
Panc-1, were obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA). The cells were cultured in DMEM
containing 10% dialyzed heat-inactivated FBS, 100 U/ml penicillin
and 100 μg/ml streptomycin in a humidified atmosphere of 5%
CO2 at 37°C. In experiments designed to assess the role
of hypoxia, cells were first cultured in normoxic conditions to
obtain the desired subconfluence level (65–70%), and were then
incubated in strictly controlled hypoxic conditions (1%
O2). Exponentially growing cells in complete medium were
pretreated for 1 h with different concentrations of resveratrol,
followed by continual incubation in normal culturing conditions or
hypoxic conditions for the indicated time intervals according to
the aim of the experiment.
Measurement of intracellular ROS
The level of intracellular ROS was measured using
the ROS assay kit. In brief, cells were incubated with
2,7-dichlorodihydrofluorecein diacetate (DCFDA) for 30 min, washed
in phosphate-buffered saline (PBS) 3 times, and fluorescence
intensity was measured using a fluorometer (Becton-Dickinson, USA)
with excitation at 488 nm and emission at 525 nm.
Wound healing assay
Cell migratory ability was detected by a
wound-healing assay. Pancreatic cancer cells were seeded into
24-well plates (1.0×105 cells/500 μl). After the
cells grew to 90–100% confluency, a sterile pipette tip was used to
produce a wound line between the cells. Cellular debris was removed
by washing with PBS and then allowed to migrate for 24 h. Images
were captured at time 0 and 24 h post-wounding under a Nikon
Diaphot TMD inverted microscope (magnification, ×10). The relative
distance traveled by the leading edge from 0 to 24 h was assessed
using Photoshop software (n=5).
Transwell Matrigel invasion assays
The invasion of pancreatic cancer cells was
performed in Transwell chambers. The 8.0-μm pore inserts
were coated with 25 μl Matrigel. The cell suspensions
(5×104) were added to the upper chambers in DMEM
containing 1% FBS. Simultaneously, 500 ml of DMEM containing 20%
FBS was placed in the lower chambers. The cells were allowed to
migrate for 48 h at 37°C. The non-invading cells were removed from
the upper surface by scraping with a wet cotton swab. After rinsing
with PBS, the filter was fixed and stained with crystal violet.
Invasion ability was determined by counting the stained cells. The
invasion ability was determined by counting the stained cells on
the bottom surface. Three random fields were captured at a
magnification of ×20 (n=3).
Real-time quantitative PCR (qRT-PCR)
Total RNA was extracted from the pancreatic cancer
cells using the Fastgen200 RNA isolation system (Fastgen, Shanghai,
China) according to the manufacturer's protocol. Total RNA was
reverse-transcribed into cDNA using a PrimeScript RT reagent kit
(Takara, Dalian, China). The primer sequences were as follows:
HIF-1α-F, 5′-AAG TCT AGG GAT GCA GCA-3′ and HIF-1α-R, 5′-CAA GAT
CAC CAG CAT CAT G-3′; MMP-2-F, 5′-GAT GAT GCC TTT GCT CGT GC-3′ and
MMP-2-R, 5′-CAA AGG GGT ATC CAT CGC CA-3′; uPA-F, 5′-TAA GAG CTG
GTG TCT GAT TG-3′ and uPA-R, 5′-TTG GAT GAA CTA GGC TAA AA-3′;
SHH-F, 5′-TCC AGA AAC TCC GAG CGA TTT AAG-3′ and SHH-R, 5′-CAC TTC
CTG GCC ACT GGT TCA-3′; SMO-F, 5′-ACG AGG ACG TGG AGG GCT G-3′ and
SMO-R, 5′-CGC ACG GTA TCG GTA GTT CT-3′; GLI1-F, 5′-GGG ATG ATC CCA
CAT CCT CAG TC-3′ and GLI1-R, 5′-CTG GAG CAG CCC CCC CAG T-3′;
β-actin-F, 5′-GAC TTA GTT GCG TTA CAC CCT TTC T-3′ and β-actin-R,
5′-GAA CGG TGA AGG TGA CAG CAG T-3′. The PCR reactions consisted of
30 sec at 95°C followed by 40 cycles at 95°C for 5 sec, at 60°C for
30 sec and at 72°C for 30 sec. After each qRT-PCR, a dissociation
curve analysis was conducted. Relative gene expression was
calculated using the 2−ΔΔCt method as previously
reported (24).
Western blotting
Proteins were electrophoretically resolved on a
denaturing SDS-polyacrylamide gel and electrotransferred onto
nitrocellulose membranes. The membranes were initially blocked with
5% non-fat dry milk in Tris-buffered saline (TBS) for 2 h and then
probed with antibodies against HIF-1α, MMP-2, uPA, SHH, SMO, GLI1
or β-actin (loading control). After co-incubation with the primary
antibodies at 4°C overnight, the membranes were blotted with the
secondary antibody for 2 h at 37°C. The results were visualized
using the ECL western blotting substrate and photographed by
GeneBox (SynGene).
Immunofluorescence microscopy
After the designated treatment, pancreatic cancer
cells were fixed with 4% paraformaldehyde for 10 min at room
temperature, permeabilized in 0.5% Triton X-100 for 10 min and
blocked in 1% BSA for 1 h. Fixed cells were then incubated with
primary antibody against GLI1 (1:100) at 4°C overnight. Cells were
washed and incubated with fluorescein isothiocyanate
(FITC)-conjugated goat anti-rabbit IgG for 1 h in a darkroom.
Nuclei were stained with DAPI for 5 min. The cells were visualized
by a fluorescence microscope (Nikon, Japan) using appropriate
excitation and emission spectra at a magnification of ×400.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). Data are
presented as the means ± SEM of three replicate assays. Differences
between groups were analyzed by analysis of variance (ANOVA).
Statistical significance was set at P<0.05. All experiments were
independently repeated at least three times.
Results
Resveratrol decreases hypoxia-induced
production of ROS in pancreatic cancer cells
The intracellular ROS levels in BxPC-3 and Panc-1
cells treated with different concentrations of resveratrol under
hypoxia conditions were determined using cell-permeable and
redox-sensitive compound DCFDA by flow cytometry. Our previous
study confirmed that the 50% inhibitory concentration
(IC50) for both BxPC-3 and Panc-1 cells is ~50 μM
of resveratrol, which exhibits no cytotoxic effects on BxPC-3 and
Panc-1 cells (22). Therefore,
treatment concentrations of 12.5, 25 and 50 μM of
resveratrol on the cells were used for the present experiments. As
shown in Fig. 1, a hypoxia
condition significantly increased intracellular levels of ROS, and
resveratrol suppressed these effects in a concentration-dependent
manner after incubation for 24 h.
Resveratrol suppresses hypoxia-induced
invasive ability of pancreatic cancer cells
A vital step of cancer metastasis is invasion of
cancer cells through the basement membrane. In order to confirm
whether resveratrol influences hypoxia-induced cancer cell invasive
ability, we used a Transwell invasion assay. As shown in Fig. 2, hypoxia exposure significantly
increased pancreatic cancer invasive ability, while the average
cell number that invaded into the lower chamber decreased as the
resveratrol concentration increased from 12.5 to 50 μM.
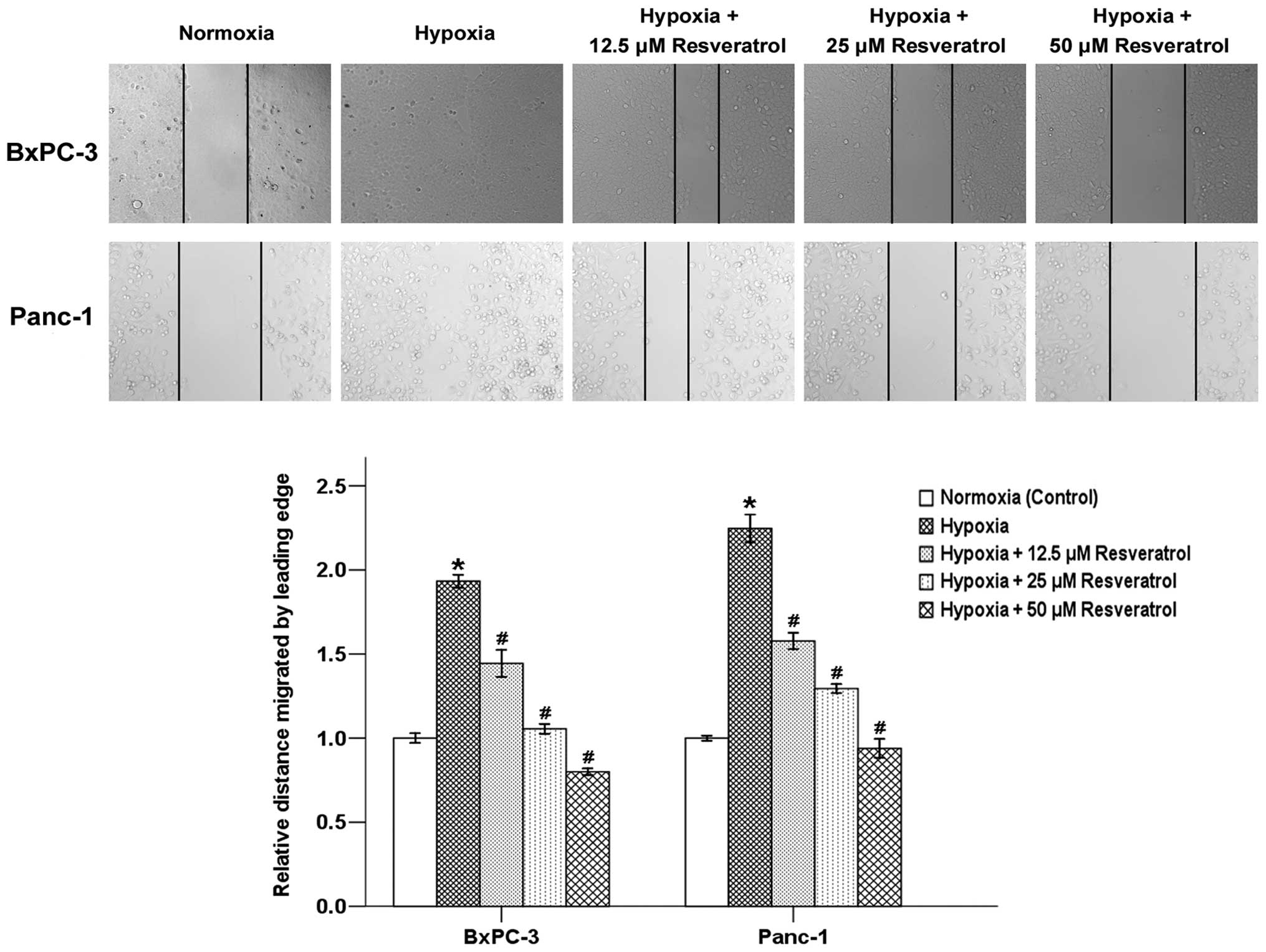
Resveratrol inhibits hypoxia-induced
wound closure of pancreatic cancer cells
Migration and invasion are two important aspects
that lead to the ability of cancer cells to form metastases. The
effect of resveratrol on hypoxia-induced pancreatic cancer cell
motility was determined using a wound-healing assay. Results showed
that a hypoxic condition caused a significant increase in the
migration of both BxPC-3 and Panc-1 cells after incubation for 24
h. Resveratrol suppressed these effects in a dose-dependent manner
(Fig. 3). This finding revealed
that resveratrol may be an effective inhibitor of hypoxia-induced
migration and invasion of pancreatic cancer cells.
Resveratrol inhibits the expression of
hypoxia-induced HIF-1α and metastatic-related factors
Previous studies have demonstrated that the effect
induced by hypoxia is mainly mediated by HIF-1α (25). In order to investigate the effect of
hypoxia on pancreatic cancer cells, both BxPC-3 and Panc-1 cells
were exposed to hypoxic conditions (1% O2) for up to 48
h. As shown in Fig. 4, the
expression levels of HIF-1α, uPA and MMP-2 were markedly increased
in both cell lines, compared with normoxic conditions. Resveratrol
decreased hypoxia-induced HIF-1α protein expression (Fig. 4A), while no apparent changes in
HIF-1α mRNA were observed in both the BxPC-3 and Panc-1 cells
(Fig. 4B), which indicated that
resveratrol inhibits hypoxia-induced HIF-1α protein expression
through a post-transcriptional mechanism as metastatic-related
factors, uPA and MMP-2, have been implicated in cancer invasion and
metastasis. Our results showed that resveratrol suppressed
hypoxia-induced uPA and MMP-2 expression at both the mRNA and
protein levels (Fig. 4).
Resveratrol downregulates the
hypoxia-activated Hh signaling pathway
Hh signaling plays an important role in the
initiation and progression of pancreatic cancer (26). As shown in Fig. 4, the mRNA and protein expression
levels of SHH, SMO and GLI1 were significantly increased in both
the BxPC-3 and Panc-1 cancer cells, compared with the normal
controls, which indicated that Hh signaling was activated in both
cell lines under hypoxic condition. Resveratrol markedly decreased
hypoxia-induced expression levels of SHH, SMO and GLI1. In
addition, immunofluorescence staining of these treated cells also
confirmed that a hypoxic condition could induce GLI1 expression in
the nucleus of BxPC-3 and Panc-1 cells, while resveratrol obviously
decreased the nuclear translocation of GLI1 (Fig. 5).
NAC suppresses hypoxia-induced ROS
generation as well as the invasive and migratory ability of
pancreatic cancer cells
NAC, a precursor of L-cysteine, is thought to be a
scavenger of free radicals such as hydroxyl radical,
H2O2 and superoxide. To explore whether
hypoxia-induced invasive and migratory ability of pancreatic cancer
cells is related with ROS production, we cultured the cells under a
hypoxic condition in the presence or absence of 20 mM NAC. The
results showed that NAC efficiently reduced ROS levels under a
hypoxic condition in both the BxPC-3 and Panc-1 cells (Fig. 6A). The average cell number that
invaded into the lower chamber decreased with NAC treatment
(Fig. 6B). The cell migration
ability (Fig. 6C) was also
significantly inhibited 24 h after the addition of NAC.
Additionally, the mRNA expression of MMP-2, uPA and GLI1 was
downregulated by NAC under a hypoxic condition (Fig. 6D). Taken together, our results
demonstrated that resveratrol inhibits hypoxia-driven ROS-induced
cancer progression via suppression of the Hh signaling pathway in
both the BxPC-3 and Panc-1 cells.
Discussion
Pancreatic cancer is a malignant carcinoma of the
digestive system with an extremely high mortality rate, due to both
the inherently aggressive biology of the disease and its late
diagnosis in most cases (2). A
hypoxic microenvironment is commonly found in the central region of
solid tumors, including pancreatic cancer (11). Tumor hypoxia not only increases the
metastatic capacity of cancer cells, but also leads to resistance
to chemotherapy and radiotherapy. Hypoxia can also induce altered
transcription and translation of a number of DNA damage response
and repair genes, which further leads to inhibition of
recombination-mediated repair of DNA double-strand breaks. In
addition, hypoxia can increase the rate of mutation (27). Overexpression of HIF-1α has been
shown in many human cancers and their metastases and is closely
associated with a more aggressive tumor progression (28). Our previous study demonstrated that
resveratrol inhibits the growth of human pancreatic cancer cells
in vitro by inhibiting cell proliferation and promoting cell
apoptosis via inhibition of the Hh signaling pathway (23). In the present study, we focused on
whether resveratrol is able to suppress hypoxia-induced cancer
invasive and migratory ability and its underlying mechanism.
Our data showed that a hypoxic condition could
significantly increase the production of ROS and the expression of
HIF-1α as well as cancer metastatic-related factors, uPA and MMP-2,
in BxPC-3 and Panc-1 cells, which further enhanced the capacity of
the pancreatic cancer cells to migrate and invade the extracellular
matrix. Resveratrol was able to abrogate these effects of a hypoxic
condition. A previous study confirmed that hypoxia activates
canonical Hh signaling through accumulation of HIF-1α (29). In the present study, we tested the
effects of a hypoxic condition and resveratrol on the activation of
SHH, SMO and GLI1. The data showed that a hypoxic condition
significantly increased the expression levels of SHH, SMO and GLI1
in both the BxPC-3 and Panc-1 cancer cells, whereas the addition of
resveratrol to the cell culture resulted in a decrease in these Hh
pathway-related factors. In addition, the hypoxia-enhanced nuclear
translocation of GLI1 was decreased by resveratrol.
ROS generated by the mitochondrial respiratory
chain, consist of a number of chemically reactive molecules derived
from oxygen. As a double-edged sword, excess ROS production can
kill cancer cells, whereas sublethal concentrations of ROS can
stimulate tumor progression by promoting cell proliferation,
survival, invasion and metastasis (30). Our previous studies confirmed that
both a hyperglycemic condition and SOD-induced ROS production were
able to promote the invasive and migratory activity of pancreatic
cancer (15,16). Our recent study also showed that
hypoxia-induced ROS production is intimately related with
pancreatic stellate cell (PSC) activation and pancreatic cancer
cell invasion (17). In the present
study, we found that hypoxia-induced ROS production was suppressed
by resveratrol in a concentration-dependent manner.
Resveratrol and its analogues have been proven to
inhibit the invasion of many tumor types, including pancreatic
cancer (31). Recent studies have
focused on the relationship between resveratrol and hypoxia-induced
cancer progression. Wu et al (8) demonstrated that the anti-metastatic
effect of resveratrol was associated with the restriction of
invasion, mobility, adhesion and MMP expression under both normoxic
and hypoxic conditions in colon carcinoma. Mitani et al
(32) showed that dietary
resveratrol inhibited β-catenin-mediated androgen receptor function
by decreasing the expression of HIF-1α protein in hypoxic LNCaP
cells and consequently suppressed prostate cancer cell growth in
vivo. They also confirmed that resveratrol suppressed
hypoxia-induced resistance to cytotoxicity of doxorubicin and
repressed the expression of CBR1 in breast cancer cells (33). In the present study, we also found
that resveratrol was able to decrease hypoxia-induced pancreatic
cancer invasion and migration, which may be attributed to the
reduction of ROS.
Resveratrol can inhibit tumor biological behavior
through multiple signaling pathways. Our previous study indicated
that resveratrol plays an important role in suppressing the
proliferation, migration and invasion of pancreatic cancer cells
in vitro by modulating EMT-related factors via the
PI-3K/Akt/NF-κB signaling pathway. We also showed that resveratrol
was able to suppress the migration and invasion of pancreatic
cancer cells by inhibiting TGF-β-mediated EMT (22). Ji et al (34) confirmed that resveratrol
downregulated MALAT1 and decreased nuclear localization of
β-catenin, which in turn attenuated the Wnt/β-catenin signaling
pathway leading to the inhibition of invasion and metastasis of
colorectal cancer cells. Sun et al (35) showed that resveratrol activated
SIRT1, which further hampered lung cancer cell metastasis in
vivo. In addition, resveratrol also inhibited hypoxia-induced
HIF-1α accumulation and vascular endothelial growth factor (VEGF)
expression in both human tongue squamous cell carcinomas and
hepatoma cells via the suppression of ERK1/2 and Akt signaling
pathway (36).
Hh signaling activation is a common event in
pancreatic cancer. The Hh signaling pathway is composed of patched
(PTCH), a 12-transmembrane receptor, smoothened (SMO), a
7-transmembrane receptor and the GLI transcription factor family. A
previous study demonstrated that hypoxia could activate canonical
Hh signaling through accumulation of HIF-1α in vitro and
in vivo (26). Our recent
study also confirmed that accumulated HIF-1α could also trigger
non-canonical Hh signaling to facilitate hypoxia-induced EMT and
invasion processes in pancreatic cancer (11). Qin et al (23) recently indicated that resveratrol
inhibited pancreatic cancer cell proliferation and promoted cell
apoptosis via inhibition of the Hh signaling pathway. Gao et
al (37) also demonstrated that
resveratrol was able to inhibit gastric cancer cell invasion and
metastasis in vitro by inhibiting the Hh signaling pathway
and EMT. In addition, resveratrol could not only effectively
downregulate interleukin-6-stimulated SHH signaling in human acute
myeloid leukemia (38), but also
inhibited both SHH signaling and Bcr-Abl expression in human
chronic myeloid leukemia cells (39), indicating that resveratrol
may have potential as a treatment for myeloid leukemia. In the
present study, we showed that resveratrol was able to suppress
hypoxia-induced activation of the Hh pathway and thus inhibited
pancreatic cancer cell invasive and migratory ability.
In conclusion, the present study demonstrated that
resveratrol plays an important role in suppressing hypoxia-induced
migration and invasion of pancreatic cancer cells in vitro
by inhibiting the Hh signaling pathway. These results suggest that
resveratrol may be a potential anticancer agent for the treatment
of pancreatic cancer.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (grant serial no.
81301846).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Castellanos EH, Cardin DB and Berlin JD:
Treatment of early-stage pancreatic cancer. Oncology. 25:182–189.
2011.PubMed/NCBI
|
|
4
|
Semenza GL: Hypoxia-inducible factors:
Mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoffmann AC, Mori R, Vallbohmer D,
Brabender J, Klein E, Drebber U, Baldus SE, Cooc J, Azuma M,
Metzger R, et al: High expression of HIF1α is a predictor of
clinical outcome in patients with pancreatic ductal adenocarcinomas
and correlated to PDGFA, VEGF, and bFGF. Neoplasia. 10:674–679.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao T, Gao S, Wang X, Liu J, Duan Y, Yuan
Z, Sheng J, Li S, Wang F, Yu M, et al: Hypoxia-inducible factor-1α
regulates chemotactic migration of pancreatic ductal adenocarcinoma
cells through directly transactivating the CX3CR1 gene. PLoS One.
7:e433992012. View Article : Google Scholar
|
|
7
|
Pouysségur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu H, Liang X, Fang Y, Qin X, Zhang Y and
Liu J: Resveratrol inhibits hypoxia-induced metastasis potential
enhancement by restricting hypoxia-induced factor-1 alpha
expression in colon carcinoma cells. Biomed Pharmacother.
62:613–621. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuo Y, Ding Q, Desaki R, Maemura K,
Mataki Y, Shinchi H, Natsugoe S and Takao S: Hypoxia inducible
factor-1 alpha plays a pivotal role in hepatic metastasis of
pancreatic cancer: An immunohistochemical study. J Hepatobiliary
Pancreat Sci. 21:105–112. 2014. View
Article : Google Scholar
|
|
10
|
Zhao X, Gao S, Ren H, Sun W, Zhang H, Sun
J, Yang S and Hao J: Hypoxia-inducible factor-1 promotes pancreatic
ductal adenocarcinoma invasion and metastasis by activating
transcription of the actin-bundling protein fascin. Cancer Res.
74:2455–2464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lei J, Ma J, Ma Q, Li X, Liu H, Xu Q, Duan
W, Sun Q, Xu J, Wu Z, et al: Hedgehog signaling regulates hypoxia
induced epithelial to mesenchymal transition and invasion in
pancreatic cancer cells via a ligand-independent manner. Mol
Cancer. 12:662013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McMahon AP, Ingham PW and Tabin CJ:
Developmental roles and clinical significance of hedgehog
signaling. Curr Top Dev Biol. 53:1–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nguyen NT, Lin DP, Yen SY, Tseng JK,
Chuang JF, Chen BY, Lin TA, Chang HH and Ju JC: Sonic hedgehog
promotes porcine oocyte maturation and early embryo development.
Reprod Fertil Dev. 21:805–815. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olive KP, Jacobetz MA, Davidson CJ,
Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA,
Caldwell ME, Allard D, et al: Inhibition of Hedgehog signaling
enhances delivery of chemotherapy in a mouse model of pancreatic
cancer. Science. 324:1457–1461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li W, Cao L, Han L, Xu Q and Ma Q:
Superoxide dismutase promotes the epithelial-mesenchymal transition
of pancreatic cancer cells via activation of the
H2O2/ERK/NF-κB axis. Int J Oncol.
46:2613–2620. 2015.
|
|
16
|
Li W, Ma Q, Li J, Guo K, Liu H, Han L and
Ma G: Hyperglycemia enhances the invasive and migratory activity of
pancreatic cancer cells via hydrogen peroxide. Oncol Rep.
25:1279–1287. 2011.PubMed/NCBI
|
|
17
|
Lei J, Huo X, Duan W, Xu Q, Li R, Ma J, Li
X, Han L, Li W, Sun H, et al: α-Mangostin inhibits hypoxia-driven
ROS-induced PSC activation and pancreatic cancer cell invasion.
Cancer Lett. 347:129–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen BY, Kuo CH, Liu YC, Ye LY, Chen JH
and Shieh CJ: Ultrasonic-assisted extraction of the botanical
dietary supplement resveratrol and other constituents of Polygonum
cuspidatum. J Nat Prod. 75:1810–1813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albani D, Polito L, Signorini A and
Forloni G: Neuroprotective properties of resveratrol in different
neurodegenerative disorders. Biofactors. 36:370–376. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fulda S: Resveratrol and derivatives for
the prevention and treatment of cancer. Drug Discov Today.
15:757–765. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jha RK, Ma Q, Sha H and Palikhe M:
Emerging role of resveratrol in the treatment of severe acute
pancreatitis. Front Biosci. 2:168–175. 2010. View
Article : Google Scholar
|
|
22
|
Li W, Ma J, Ma Q, Li B, Han L, Liu J, Xu
Q, Duan W, Yu S, Wang F, et al: Resveratrol inhibits the
epithelial-mesenchymal transition of pancreatic cancer cells via
suppression of the PI-3K/Akt/NF-κB pathway. Curr Med Chem.
20:4185–4194. 2013. View Article : Google Scholar
|
|
23
|
Qin Y, Ma Z, Dang X, Li W and Ma Q: Effect
of resveratrol on proliferation and apoptosis of human pancreatic
cancer MIA PaCa-2 cells may involve inhibition of the Hedgehog
signaling pathway. Mol Med Rep. 10:2563–2567. 2014.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408.
2001. View Article : Google Scholar
|
|
25
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spivak-Kroizman TR, Hostetter G, Posner R,
Aziz M, Hu C, Demeure MJ, Von Hoff D, Hingorani SR, Palculict TB,
Izzo J, et al: Hypoxia triggers hedgehog-mediated tumor-stromal
interactions in pancreatic cancer. Cancer Res. 73:3235–3247. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luoto KR, Kumareswaran R and Bristow RG:
Tumor hypoxia as a driving force in genetic instability. Genome
Integr. 4:52013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ye LY, Zhang Q, Bai XL, Pankaj P, Hu QD
and Liang TB: Hypoxia-inducible factor 1α expression and its
clinical significance in pancreatic cancer: A meta-analysis.
Pancreatology. 14:391–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bijlsma MF, Groot AP, Oduro JP, Franken
RJ, Schoenmakers SH, Peppelenbosch MP and Spek CA: Hypoxia induces
a hedgehog response mediated by HIF-1alpha. J Cell Mol Med.
13:2053–2060. 2009. View Article : Google Scholar
|
|
30
|
Nishikawa M, Hashida M and Takakura Y:
Catalase delivery for inhibiting ROS-mediated tissue injury and
tumor metastasis. Adv Drug Deliv Rev. 61:319–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu Q, Zong L, Chen X, Jiang Z, Nan L, Li
J, Duan W, Lei J, Zhang L, Ma J, et al: Resveratrol in the
treatment of pancreatic cancer. Ann NY Acad Sci. 1348:10–19. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mitani T, Harada N, Tanimori S, Nakano Y,
Inui H and Yamaji R: Resveratrol inhibits hypoxia-inducible
factor-1α-mediated androgen receptor signaling and represses tumor
progression in castration-resistant prostate cancer. J Nutr Sci
Vitaminol. 60:276–282. 2014. View Article : Google Scholar
|
|
33
|
Mitani T, Ito Y, Harada N, Nakano Y, Inui
H, Ashida H and Yamaji R: Resveratrol reduces the hypoxia-induced
resistance to doxorubicin in breast cancer cells. J Nutr Sci
Vitaminol. 60:122–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L,
Sun J, Cai J, Qin J, Ren J, et al: Resveratrol inhibits invasion
and metastasis of colorectal cancer cells via MALAT1 mediated
Wnt/β-catenin signal pathway. PLoS One. 8:e787002013. View Article : Google Scholar
|
|
35
|
Sun L, Li H, Chen J, Dehennaut V, Zhao Y,
Yang Y, Iwasaki Y, Kahn-Perles B, Leprince D, Chen Q, et al: A
SUMOylation-dependent pathway regulates SIRT1 transcription and
lung cancer metastasis. J Natl Cancer Inst. 105:887–898. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Q, Tang X, Lu QY, Zhang ZF, Brown J
and Le AD: Resveratrol inhibits hypoxia-induced accumulation of
hypoxia-inducible factor-1alpha and VEGF expression in human tongue
squamous cell carcinoma and hepatoma cells. Mol Cancer Ther.
4:1465–1474. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao Q, Yuan Y, Gan HZ and Peng Q:
Resveratrol inhibits the hedgehog signaling pathway and
epithelial-mesenchymal transition and suppresses gastric cancer
invasion and metastasis. Oncol Lett. 9:2381–2387. 2015.PubMed/NCBI
|
|
38
|
Su YC, Li SC, Wu YC, Wang LM, Chao KS and
Liao HF: Resveratrol downregulates interleukin-6-stimulated sonic
hedgehog signaling in human acute myeloid leukemia. Evid Based
Complement Alternat Med. 2013:5474302013. View Article : Google Scholar : PubMed/NCBI
|