Introduction
Lung cancer accounts for 12% of all newly diagnosed
cancer cases worldwide (1). Small
cell lung cancer (SCLC) is the most aggressive subtype of lung
cancer and it represents more than 15% of all lung cancer cases
(2). Cisplatin
[cis-diamminedichloroplatinum (CDDP)]-based combination
chemotherapy has markedly improved the survival rate of SCLC
patients (3), However, the median
survival time of SCLC patients with limited and advanced stage are
still less than 2 years, and the overall 5-year survival rate of
SCLC patients remains 5–10% owing to acquired multidrug resistance
(MDR) and intolerable toxicity (4).
It has been found that apoptosis blockade is one of the most
important mechanisms by which cancer cells escape from the
cytotoxicity of cisplatin, leading to MDR (5). Therefore, it is of great significance
for cancer treatments to explore the mechanisms of apoptotic
resistance after cisplatin stimulation and to accelerate apoptosis
in tumor cells in efficient ways.
Mitochondria are essential for regulation of the
intrinsic apoptotic pathway and they are the main generation spots
for reactive oxygen species (ROS) in most living cells (6). A low level of ROS generation exhibits
critical physiological effects in normal cells, whereas excessive
generation of ROS induces oxidative stress, which may further lead
to loss of cell function and cell apoptosis (7). It has been shown that DNA damage
caused by ROS can evoke p53 and result in mitochondrial-mediated
cell apoptosis through downregulation of anti-apoptotic Bcl-2
proteins and upregulation of various pro-apoptotic proteins, such
as Bax and apoptotic protease-activating factor-1 (Apaf-1)
(8). In contrast, the mitochondrial
membrane permeability is sensitive to redox stress and excessive
ROS can upgrade mitochondrial membrane permeability (9), which ultimately leads to mitochondrial
swelling, depolarization of mitochondrial membrane potential (MMP)
and release of apoptosis-inducing proteins (10). In addition, many types of
phytochemicals, including cisplatin, have been reported to kill
cancer cells via the ROS-mediated mitochondrial apoptotic pathway
(11).
Gallic acid [3,4,5-trihydroxybenzoic acid (GA)], a
natural botanic phenolic compound, is abundantly found in green
tea, grapes and red wine (12). GA
possesses a wide range of pharmacological properties including
anti-obesity, anti-inflammation and anticancer activities (13–15).
More and more attention has been given to the anticancer effects of
GA in recent years since it may induce cell apoptosis in various
types of cancers, such as lung and cervical cancer, and oral
squamous carcinoma (16–18). Evidence has also revealed that the
accumulation of intracellular ROS caused by oxidative stress
imbalance is an important predisposing factor for GA to exhibit its
anticancer effects. For example, it was demonstrated that GA
induced apoptosis in prostate cells and mouse lung fibroblasts via
the ROS-dependent intrinsic apoptotic pathway and the ROS-dependent
p53 activation pathway, respectively (15,19).
However, the mechanisms by which GA induces apoptosis in cancer
cells are not well illustrated and there is little evidence
revealing its anticancer effects on human SCLC cells.
In the present study, based on these clues, we
investigated the hypothesis that GA can exhibit an anticancer
effect in the human SCLC H446 cell line by interfering with the
generation of ROS and disrupting the function of the mitochondria.
Further exploration also revealed that GA enhanced the anticancer
effects of cisplatin via the ROS-dependent mitochondrial apoptotic
pathway in H446 cells.
Materials and methods
Materials
The human SCLC H446 cell line was obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA). GA
(Fig. 1) was purchased from Xi'an
Grass Plant Technology Co. (Xi'an, China) with a purity above 98%.
Fetal bovine serum (FBS) and RPMI-1640 medium were purchased from
Gibco-BRL (Grand Island, NY, USA). N-acetyl-l-cysteine (NAC)
was purchased from Sigma-Aldrich (St. Louis, MO, USA). The cell
apoptosis detection kit with Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI), MMP assay and intracellular ROS
detection kits were purchased from Beyotime Institute of
Biotechnology (Jiangsu, China). Primary antibodies against Bax,
Apaf-1, DIABLO, XIAP, p53 and β-actin were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-rabbit IgG
peroxidase conjugated secondary antibody, sodium salt
(SDS)-polyacrylamide gel electrophoresis (SDS-PAGE) gels and
polyvinylidene difluoride (PVDF) membrane were purchased from
Amersham Biosciences (Piscataway, NJ, USA).
Cell culture
The human SCLC H446 cell line was cultivated in
RPMI-1640 medium containing 10% FBS, 100 U/ml penicillin and 0.1
mg/ml streptomycin in a humidified atmosphere containing 5%
CO2 at 37°C.
Cell viability assay
The effects of cisplatin, GA and co-administration
of cisplatin and GA on cell viability were detected by MTT
colorimetric assay. H446 cells in logarithmic growth phase were
collected and seeded into 96-well plates at a density of
5×103 cells/well. After 12 h of incubation, the cells
were treated with different concentrations of cisplatin and GA or
their combination for 6–32 h. Then, 20 µl MTT was added to
each well and cultured for another 4 h. Finally, the medium was
removed and 150 µl dimethyl sulfoxide (DMSO) was added to
each well. The absorbance was recorded at 492 nm on an automated
Bio-Rad 550 microplate reader.
Morphological examination of the H446
cells
The individual or/and combined effects of cisplatin
and GA on morphological changes in the H446 cells were observed
under an inverted microscope (10×10 magnification). Briefly, SCLC
H446 cells were cultured in 6-well plates and treated with 5
µg/ml cisplatin and 3 µg/ml GA alone or in
combination for 24 h. After that, images of the morphological
features of the cells from the different groups were captured.
Cytotoxicity of the different treatments on the H446 cells was
evaluated according to the variations in cellular morphological
changes and the amount of adherent cells.
Analysis of cell apoptosis by flow
cytometry
The apoptosis of H446 cells was assessed by flow
cytometry using Annexin V-FITC/PI staining according to the
manufacturer's instructions. Firstly, H446 cells were treated with
5 µg/ml cisplatin and/or 3 µg/ml GA, in the presence
or absence of 6 mM NAC for 2 h. After 24 h, both suspension and
adherent cells were collected, washed twice with cold
phosphate-buffered saline (PBS), and then stained with Annexin V
and PI at room temperature for 15 min in the dark. Finally, the
cells were analyzed by a flow cytometer (Becton-Dickinson
Immunocytometry System, San Jose, CA, USA). As to the analysis,
Annexin V-positive and PI-negative cells represent early apoptotic
populations, Annexin V-positive and PI-positive cells represent
late apoptotic or dead proportions.
Measurement of ROS
2′7′-Dichlorofluorescein diacetate (DCFH-DA) is a
highly sensitive probe which is usually used for detection of
intracellular ROS. This non-fluorescent dye diffuses readily into
cells and yields DCFH which cannot pass out of cells. In the
presence of peroxidase, DCFH can generate the fluorescent compound
dichlorofluorescein (DCF), which can be observed by fluorescence
microscopy with an excitation wavelength of 488 nm and a 525-nm
emission filter. In the present study, H446 cells were divided into
four groups: the normal group, 5 µg/ml cisplatin group, 3
µg/ml GA group and a combination of the two drugs group.
Cells from each group were incubated with 10 µM DCFH-DA for
30 min after 24 h of drug treatment and finally covered with 1 ml
serum-free DMEM before observion. Green fluorescence was detected
to evaluate the concentration of intracellular ROS.
Measurement of mitochondrial membrane
permeability (MMP)
MMP was measured using a JC-1 assay kit. The JC-1
dye can enter the mitochondrial matrix in normal cells, form JC-1
aggregates and emit red fluorescence (excitation by 540 nm), while
JC-1 exists as a monomeric form and exhibits green fluorescence
(excitation by 490 nm), when MMP is decreased. In the present
study, the cells were seeded into 6-well plates and stimulated with
5 µg/ml cisplatin, 3 µg/ml GA and 5 µg/ml
cisplatin combined with 3 µg/ml GA for 24 h. After that,
cells in each well were incubated in the dark at 37°C with 1 ml
culture medium and 1 ml 5 µM JC-1 for 30 min. At last,
fluorescence was detected by an inverse fluorescence microscope
(DMI6000B; Leica, Germany). The relative ratio of green to red
fluorescence indicated the depolarization of MMP.
Western blotting
Cells from the different groups were collected and
lysed in RIPA lysis buffer containing protease inhibitor on ice.
Then, cell lysates were centrifuged at 12,000 rpm for 5 min at 4°C,
and cell supernatants were collected. Protein concentration was
confirmed by a BCA protein assay kit (Beyotime, China). Proteins
(20 µg) from each group were separated on 12%
SDS-polyacrylamide gels and subsequently transferred onto a PVDF
membrane (Amersham, Braunschweig, Germany). The membranes were
blocked in 5% skimmed milk dissolved in Tris-buffered saline and
Tween-20 (TBST) (20 mM Tris, pH 7.5) for 2 h and then incubated
with appropriate primary antibodies at 4°C overnight. After three
times washing in TBST, the membranes were incubated with the
secondary antibody conjugated with anti-rabbit IgG peroxidase for 2
h at room temperature. Bands were monitored with chemiluminescence
using an ECL detection system (Amersham Pharmacia Biotech).
Statistical analysis
All experiments were repeated three times. Numeric
data are expressed as mean ± SD. Statistical differences between
the groups were analyzed by one-way analysis of variance (ANOVA)
followed by Dunnett's test after these data were confirmed to have
a normal distribution. A p-value of <0.05 was considered to
indicate a statistically significant result.
Results
Cell viability of the H446 cells
The effects of cisplatin, GA and cisplatin combined
with GA on cell viability were evaluated by MTT assay. The results
revealed that both cisplatin and GA effectively suppressed the cell
viability in a dose-dependent manner, and the survival rate of the
H446 cells was reduced to 65 and 75% when treated with 5
µg/ml cisplatin and 3 µg/ml GA for 24 h, respectively
(Fig. 2A and B). Moreover, the
viability of the cells decreased in a time-dependent manner and was
reduced to 40% at 24 h when stimulated with a combination of 5
µg/ml cisplatin and 3 µg/ml GA (p<0.05) (Fig. 2C and D). In addition, the growth
inhibitory effects of their combination became more and more
dramatic with decreasing P-values with increasing time compared
with that of the single cisplatin-treated group (Fig. 2D).
Cell morphological changes in the H446
cells
When apoptosis occurs, cells present characteristic
structural changes which can be observed through inverted
microscopy. As shown in Fig. 3,
almost all cells were regular in shape and confluent, rarely
sloughing off in the normal control group, while many H446 cells
became smaller, round and blunt in size and some floated on the
medium when treated with cisplatin and GA. More importantly, the
above changes became more extreme in cells exposed to cisplatin
combined with GA. Moreover, the number of H446 cells adhering onto
the plates decreased according to the following order: normal group
> GA group > cisplatin group > cisplatin combined with GA
group.
Apoptosis of the H446 cells
To further explore the mechanisms underlying the
inhibitory effects of the two agents on H446 cells, the percentage
of apoptotic cells in each group was detected by flow cytometry. As
shown in Fig. 4, the percentage of
apoptotic cells increased in both the cisplatin and GA groups
compared with that of the control (30.47±1.56 and 12.43±2.89 vs.
1.53±1.25%) (p<0.05). Of particular note, joint application of
cisplatin and GA induced more apoptosis compared with that in the
cisplatin treatment alone group (40.97±1.92 vs. 30.47±1.56%)
(p<0.05).
Intracellular ROS levels in the H446
cells
Based on the results above, we hypothesized that
apoptosis induced by cisplatin, GA and combined treatment of the
two agents was related to the abnormal intracellular ROS level. We
then examined the generation of ROS using the DCFH-DA probe. As
shown in Fig. 5, there was rare ROS
detected in the normal cells, while a 24-h treatment with both
cisplatin and GA obviously increased the accumulation of ROS.
Moreover, the amount of intracellular ROS was maximally elevated
when H446 cells were treated with CDDP plus GA.
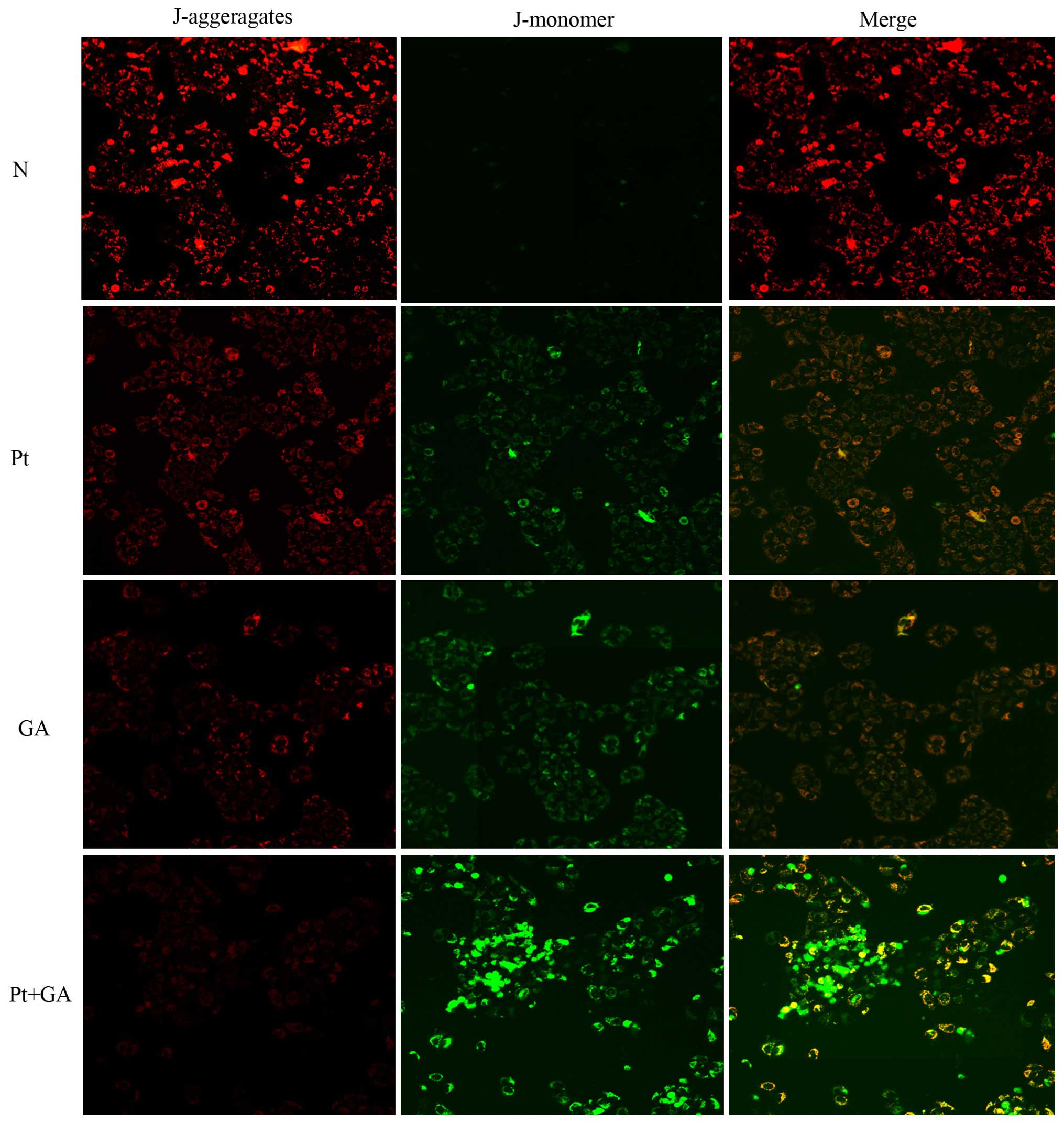
Loss of MMP in H446 cells
The depolarization of MMP reflects mitochondrial
dysfunction which is an early marker of mitochondrial-mediated cell
apoptosis. In the present study, we evaluated the changes in MMP by
a fluorescence microscope. As shown in Fig. 6, the red light was most obvious in
the normal group and it was markedly decreased 24 h after cells
were treated with cisplatin and GA. Strikingly, the red light
reached the lowest point when cells were exposed to a combination
of the two agents. In contrast, there was barely any green light
detected in the normal cells, while cisplatin and GA treatment
resulted in the generation of green light. Moreover, opposite to
that of red light, the brightness of green peaked when cells were
treated with cisplatin and GA in combination.
Expression of mitochondrial
apoptosis-related proteins in the H446 cells
In order to elucidate the molecular mechanisms
involved in the cell apoptosis induced by GA in H446 cells, we
measured the expression of several apoptosis-related proteins by
western blotting. As shown in Fig.
7, we found that the expression levels of Bax, Apaf-1, DIABLO
and p53 were markedly increased by GA accompanied with decreased
expression of XIAP in a time-dependent manner (p<0.05).
 | Figure 7Effect of gallic acid (GA) on the
expression of Bax, Apaf-1, DIABLO, p53 and XIAP in H446 cells. (A)
Cells were respectively challenged with 3 µg/ml GA for 0, 6,
12 and 24 h, and were then harvested. Western blotting was carried
out to investigate the contents of the proteins mentioned above.
The relative protein levels of the proteins were normalized to the
β-actin level. Representative bar diagrams show quantitative
results for the relative levels of (B) Bax, (C) Apaf-1, (D) DIABLO,
(E) p53 and (F) XIAP proteins. Data are mean ± SD, n=3.
*p<0.05 vs. the group preceding it. |
To further explore whether the changes in expression
of Bax, Apaf-1, DIABLO, p53 and XIAP were associated with enhanced
apoptosis-inducing effects by the combined treatment of cisplatin
and GA, we examined the expression levels of these proteins in the
H446 cells treated with cisplatin and/or GA for 24 h. As shown in
Fig. 8, combined treatment with
cisplatin and GA significantly increased the levels of Bax, Apaf-1,
DIABLO and p53, whereas it resulted in a decrease in XIAP levels
relative to the treatment with either drug alone (p<0.05).
Pretreatment with NAC prevents apoptosis
induced by cisplatin and GA
To confirm whether elevated intracellular ROS levels
are related to the apoptosis induced by cisplatin combined with GA.
The H446 cells were pretreated with 6 mM NAC for 2 h, followed by
treatment of cisplatin plus GA for 24 h. The proportion of
apoptotic cells was determined by flow cytometric analysis. As
shown in Fig. 9, the percentage of
apoptotic cells following the combination treatment was reduced
from 43.90±2.19% in the non-NAC treatment group to 32.57±0.69%
following NAC treatment; the difference was statistically
significant (p<0.05).
Discussion
Lung cancer is one of the leading causes of
cancer-related mortality worldwide (1). In addition, SCLC is the most deadliest
subtype with fast growth and early widespread dissemination as its
characteristic hallmark (20).
Although combined chemotherapy with cisplatin and etoposide has
been widely adopted to increase the remission rate of SCLC
(21), almost all patients suffer
from recurrent cancer with MDR and eventually succumb to this
disease. Recently, identification of novel effective
chemotherapeutic agents has become a research focus in the study of
SCLC treatments.
Polyphenols are abundant natural sources of
potential cancer chemotherapeutic agents, which can reduce the
toxicities of typical anticancer drugs and help to overcome drug
resistance of standard anticancer treatments (22). As one type of polyphenol acids,
gallic acid (GA) is attracting increased attention due to its high
bioavailability and non-toxicity (12). In the present study, we demonstrated
for the first time that GA inhibited the growth of SCLC H446 cells
and enhanced the apoptosis-inducing effects of cisplatin through an
increase in intracellular ROS, reduction in MMP, an increase in
BAX, Apaf-1, DIABLO and p53 expression and reduction in XIAP
expression. These results further indicate that GA may be an
effective supplementary drug in the clinical treatment of SCLC
which would help to reduce cisplatin-induced MDR and
toxicities.
Apoptosis, or programmed cell death, is a highly
regulated process and is characterized by a series of morphological
changes. Continuous studies have revealed that the activation of
apoptosis plays an important role in inhibiting the development and
progression of cancers (23,24).
It was firstly confirmed, in the present study, that GA altered
cell morphology, inhibited the growth and induced apoptosis in H446
cells. Moreover, combined treatment of GA and cisplatin exhibited a
better effect on cell morphological changes than that of single
treatment of cisplatin. These findings indicate that cell apoptosis
may be the mechanism by which cisplatin and GA exhibit their
anticancer effects in H446 cells.
As one of the by-products of normal cellular
oxidative processes, ROS is an important mediator of intracellular
signaling. Accumulating evidence suggests that a high level of ROS
induces oxidation of cellular macromolecules, disruption of MMP and
finally cell apoptosis (7,25). Ma et al believes there is a
positive feedback loop in ROS-mediated mitochondrial membrane
dysfunction which amplifies the death signals in liver cancer cells
(26). Accordingly, we investigated
the possibility that ROS may play a role in cisplatin and
GA-mediated apoptosis in H446 cells. We found that intracellular
ROS increased while MMP was reduced when H446 cells were exposed to
cisplatin, GA and the combination of the two agents for 24 h. In
addition, compared with individual treatment, the combined
treatment of cisplatin and GA obviously increased the accumulation
of ROS and the loss of MMP in H446 cells. Moreover, we found that
cisplatin and GA-induced apoptosis was greatly reduced by
pretreatment with the ROS scavenger NAC. These data indicated that
ROS acted as an upstream signaling molecule to initiate
mitochondrial-mediated apoptosis when H446 cells were treated with
cisplatin and GA.
Mitochondrial-mediated cell apoptosis mainly depends
on the abnormal activation and expression of the Bcl-2 family,
which consists of anti-apoptotic molecules, such as Bcl-2 and
Bcl-xL, and pro-apoptotic factors, such as Bak and Bax.
Overexpressed Bax may translocate and integrate into the
mitochondrial membrane resulting in altered protein interaction of
mitochondrial membranes, which may further lead to opening of the
permeability transition pore (PTP), disruption of MMP and finally
the release of secondary mitochondrial-derived activator of caspase
(SMAC, also called DIABLO) and cytochrome c (27,28).
DIABLO facilitates caspase processing and activation by
antagonizing an endogenous inhibitor of caspase called XIAP. Also,
cytochrome c initiates a caspase cascade by enhancing the
formation of apoptosomes through accelerating the expression of
Apaf-1 (29,30). Moreover, as a critical tumor
suppressor, p53 decreases cell proliferation and induces
caspase-mediated apoptosis via upregulation of its downstream
target Bax (31). Based on this
knowledge, we investigated the expression of the above
apoptosis-related proteins, and the results showed that both
cisplatin and GA markedly increased the expression of Bax, DIABLO,
Apaf-1 and p53 while decreased XIAP expression. More importantly,
GA markedly enhanced the effects of cisplatin on expression of
these proteins. These findings further confirmed that cisplatin and
GA-induced apoptosis of H446 cells occur through the
mitochondrial-mediated pathway.
In conclusion, we demonstrated for the first time
that GA suppressed the proliferation and induced the apoptosis of
H446 cells and enhanced the anticancer effects of cisplatin on
these cells through the ROS-mediated mitochondrial apoptotic
pathway. However, studies are still needed to fully evaluate the
anticancer effects of GA in combination with cisplatin in
vivo and to further assess whether GA could be adopted as a
novel auxiliary therapeutic in cancer treatment.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81071933).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sher T, Dy GK and Adjei AA: Small cell
lung cancer. Mayo Clin Proc. 83:355–367. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pelayo Alvarez M, Westeel V, Cortés-Jofré
M and Bonfill Cosp X: Chemotherapy versus best supportive care for
extensive small cell lung cancer. Cochrane Database Syst Rev.
11:CD0019902013.PubMed/NCBI
|
|
4
|
Lally BE, Urbanic JJ, Blackstock AW,
Miller AA and Perry MC: Small cell lung cancer: Have we made any
progress over the last 25 years? Oncologist. 12:1096–1104. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tyler A, Johansson A, Karlsson T, Gudey
SK, Brännström T, Grankvist K and Behnam-Motlagh P: Targeting
glucosylceramide synthase induction of cell surface
globotriaosylceramide (Gb3) in acquired cisplatin-resistance of
lung cancer and malignant pleural mesothelioma cells. Exp Cell Res.
336:23–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang J and Pervaiz S: Mitochondria: Redox
metabolism and dysfunction. Biochem Res Int. 2012:8967512012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slater AF, Stefan C, Nobel I, van den
Dobbelsteen DJ and Orrenius S: Signalling mechanisms and oxidative
stress in apoptosis. Toxicol Lett. 82–83:149–153. 1995. View Article : Google Scholar
|
|
8
|
Matés JM, Segura JA, Alonso FJ and Márquez
J: Oxidative stress in apoptosis and cancer: An update. Arch
Toxicol. 86:1649–1665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kroemer G and Blomgren K: Mitochondrial
cell death control in familial Parkinson disease. PLoS Biol.
5:e2062007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoboue ED and Devin A: Reactive oxygen
species-mediated control of mitochondrial biogenesis. Int J Cell
Biol. 2012:4038702012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fruehauf JP and Meyskens FL Jr: Reactive
oxygen species: A breath of life or death? Clin Cancer Res.
13:789–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shahrzad S, Aoyagi K, Winter A, Koyama A
and Bitsch I: Pharmacokinetics of gallic acid and its relative
bioavailability from tea in healthy humans. J Nutr. 131:1207–1210.
2001.PubMed/NCBI
|
|
13
|
Oi Y, Hou IC, Fujita H and Yazawa K:
Antiobesity effects of Chinese black tea (Pu-erh tea) extract and
gallic acid. Phytother Res. 26:475–481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsiang CY, Hseu YC, Chang YC, Kumar KJ, Ho
TY and Yang HL: Toona sinensis and its major bioactive compound
gallic acid inhibit LPS-induced inflammation in nuclear factor-κB
transgenic mice as evaluated by in vivo bioluminescence imaging.
Food Chem. 136:426–434. 2013. View Article : Google Scholar
|
|
15
|
Chuang CY, Liu HC, Wu LC, Chen CY, Chang
JT and Hsu SL: Gallic acid induces apoptosis of lung fibroblasts
via a reactive oxygen species-dependent ataxia telangiectasia
mutated-p53 activation pathway. J Agric Food Chem. 58:2943–2951.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
You BR and Park WH: The effects of
mitogen-activated protein kinase inhibitors or small interfering
RNAs on gallic acid-induced HeLa cell death in relation to reactive
oxygen species and glutathione. J Agric Food Chem. 59:763–771.
2011. View Article : Google Scholar
|
|
17
|
You BR, Kim SZ, Kim SH and Park WH: Gallic
acid-induced lung cancer cell death is accompanied by ROS increase
and glutathione depletion. Mol Cell Biochem. 357:295–303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chia YC, Rajbanshi R, Calhoun C and Chiu
RH: Anti-neoplastic effects of gallic acid, a major component of
Toona sinensis leaf extract, on oral squamous carcinoma cells.
Molecules. 15:8377–8389. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Russell LH Jr, Mazzio E, Badisa RB, Zhu
ZP, Agharahimi M, Oriaku ET and Goodman CB: Autoxidation of gallic
acid induces ROS-dependent death in human prostate cancer LNCaP
cells. Anticancer Res. 32:1595–1602. 2012.PubMed/NCBI
|
|
20
|
Govindan R, Page N, Morgensztern D, Read
W, Tierney R, Vlahiotis A, Spitznagel EL and Piccirillo J: Changing
epidemiology of small-cell lung cancer in the United States over
the last 30 years: Analysis of the surveillance, epidemiologic, and
end results database. J Clin Oncol. 24:4539–4544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schiller JH: Small cell lung cancer:
Defining a role for emerging platinum drugs. Oncology. 63:105–114.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bayet-Robert M, Kwiatkowski F, Leheurteur
M, Gachon F, Planchat E, Abrial C, Mouret-Reynier MA, Durando X,
Barthomeuf C and Chollet P: Phase I dose escalation trial of
docetaxel plus curcumin in patients with advanced and metastatic
breast cancer. Cancer Biol Ther. 9:8–14. 2010. View Article : Google Scholar
|
|
23
|
Kasibhatla S and Tseng B: Why target
apoptosis in cancer treatment? Mol Cancer Ther. 2:573–580.
2003.PubMed/NCBI
|
|
24
|
Singh BN, Shankar S and Srivastava RK:
Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms,
perspectives and clinical applications. Biochem Pharmacol.
82:1807–1821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fiers W, Beyaert R, Declercq W and
Vandenabeele P: More than one way to die: Apoptosis, necrosis and
reactive oxygen damage. Oncogene. 18:7719–7730. 1999. View Article : Google Scholar
|
|
26
|
Ma Y, Zhang J, Zhang Q, Chen P, Song J, Yu
S, Liu H, Liu F, Song C, Yang D, et al: Adenosine induces apoptosis
in human liver cancer cells through ROS production and
mitochondrial dysfunction. Biochem Biophys Res Commun. 448:8–14.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lotem J and Sachs L: Regulation of bcl-2,
bcl-XL and bax in the control of apoptosis by
hematopoietic cytokines and dexamethasone. Cell Growth Differ.
6:647–653. 1995.PubMed/NCBI
|
|
28
|
Chen Q, Liu XF and Zheng PS: Grape seed
proanthocyanidins (GSPs) inhibit the growth of cervical cancer by
inducing apoptosis mediated by the mitochondrial pathway. PLoS One.
9:e1070452014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Verhagen AM, Ekert PG, Pakusch M, Silke J,
Connolly LM, Reid GE, Moritz RL, Simpson RJ and Vaux DL:
Identification of DIABLO, a mammalian protein that promotes
apoptosis by binding to and antagonizing IAP proteins. Cell.
102:43–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang L, Wang P, Wang H, Li Q, Teng H, Liu
Z, Yang W, Hou L and Zou X: Fucoidan derived from Undaria
pinnatifida induces apoptosis in human hepatocellular carcinoma
SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar
Drugs. 11:1961–1976. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Qin CK, Lv W, Zhao Q and Qin CY:
OSU-03012, a non-Cox inhibiting celecoxib derivative, induces
apoptosis of human esophageal carcinoma cells through a
p53/Bax/cytochrome c/caspase-9-dependent pathway. Anticancer Drugs.
24:690–698. 2013. View Article : Google Scholar : PubMed/NCBI
|