Introduction
Colon cancer is one of the leading malignancies in
the digestive system, and in cancer-related mortality. The current
treatment for colon cancer includes surgery, radiotherapy,
chemotherapy and targeted therapy alone, or combination. The
treatment regimens for the late stage of colon cancer include
chemotherapy drugs, such as capecitabine, fluorouracil, irinotecan,
oxaliplatin and tegafur (1) or
targeted therapy drugs, such as bevacizumab, cetuximab and
panitumumab (2). Although the
treatment for colon cancer has been developed greatly, the
prognosis remains unsatisfactory (3,4). Thus,
there is still a great clinical need to develop drugs for colon
cancer treatment, particularly for the late stage of colon
cancer.
Traditional herb medicine or herbal derived
components have played an increasingly important role in the
prevention and treatment of cancers, such as camptothecin,
vincristine and taxol (5,6). In particular, the combination of these
medicines with traditional chemotherapy drugs has substantially
improved the prognosis of some cancers. Oridonin (ORI), a
diterpenoid extracted from Chinese herb Rabdosia rubescens
and/or related species, has attracted increasing attention in the
last decades for cancer biologists because of its notable
anticancer activity. It has been reported that ORI can induce
apoptosis in various cancer cell lines, such as colon cancer cells
(7), lymphoma cells (8), breast cancer cells (9) and leukemia cells (10), and this activity may be mediated by
inactivating PI3K/Akt and extracellular signal-regulated kinase
(ERK), activating p38 mitogen-activated protein kinases (MAPKs),
and increasing hydrogen peroxide (11–14).
Although substantial evidence supports that ORI can inhibit the
proliferation of colon cancer, the precise mechanism underlying
this process remains unclear.
In the present study, we investigated the
proliferation inhibitory effect of ORI in human colon cells, and
dissected the possible mechanism underlying this effect. Our
findings confirmed that ORI can inhibit the proliferation of colon
cancer cells and demonstrated that this activity may be mediated by
upregulating phosphatase and tensin homologue (PTEN) through at
least partly activating p38 MAPK.
Materials and methods
Chemicals and drug preparations
ORI was from Hao-Xuan Bio-Tech Co., Ltd. (Xi'an,
China). HCT116 cell line was purchased from the American Type
Culture Collection. (ATCC; Manassas, VA, USA). ORI was dissolved in
dimethyl sulfoxide (DMSO) or prepared with 0.4%
carboxymethylcellulose sodium (CMC-Na) as suspension for in
vitro or in vivo experiments, respectively. All
antibodies were ordered from Santa Cruz Biotechnology (Santa Cruz,
CA, USA). SB203580 (#S1076) was ordered from Selleckchem (Houston,
TX, USA). Cells were maintained in Dulbecco's modified Eagle's
medium (DMEM) at 37°C in 5% CO2, with 10% fetal bovine
serum (FBS), 100 U/ml of penicillin and 100 µg/ml of
streptomycin.
Cell viability assay
Cell viability was measured using crystal violet
staining. Briefly, HCT116 cells were seeded into a 24-well plate
and treated with different concentrations of ORI, the same volume
of DMSO was used as solvent control. Cells were washed carefully
with cold (4°C) phosphate-buffered saline (PBS) and stained with
0.5% crystal violet formalin solution at room temperature to
visualize the cell viability at the scheduled time points. For
quantification, the crystal violet was extracted with 1 ml 20%
acetic acid at room temperature for 20 min with shaking. The
absorbance at 570 nm was measured. Each assay was carried out in
triplicate.
Construction of recombinant
adenovirus
Recombinant adenoviruses expressing PTEN (AdPTEN)
and small interference RNA fragments targeting PTEN (AdsiPTEN) were
constructed with the AdEasy system (15), tagged with green fluorescence
protein (GFP) or red fluorescence protein (RFP), respectively. The
recombinant adenovirus expression GFP only was used as vector
control.
Flow cytometric analysis for apoptosis
and cell cycle
HCT116 cells were seeded in a 6-well plate. For
apoptosis assay, cells were treated with different concentrations
of ORI or DMSO for 48 h. Then, cells were harvested, washed with
cold PBS, and incubated with Annexin V-EGFP and propidium iodide
(PI) following the introduction of the kits (KeyGen Biotech,
Nanjing, China). The cells were subsequently analyzed by
fluorescence activated cell sorting (FACS). For cell cycle
analysis, cells were treated with different concentrations of ORI
for 24 h. Then, cells were collected, washed with PBS, fixed with
cold (4°C) 70% ethanol, washed with 50 and 30% ethanol, and PBS.
Finally, stained with 1 ml of PI (20 mg/ml) containing RNase (1
mg/ml) in PBS for 30 min, and followed by FACS for cycle analysis.
Each assay was carried out in triplicate.
Western blot assay
Sub-confluent HCT116 cells were seeded in 6-well
plate and treated with different concentrations of ORI or DMSO. At
the scheduled time points, cells were washed with cold PBS and
lysed with 300 µl lysis buffer. Cell lysates were boiled for
10 min, then subjected to SDS-PAGE separation and transfered to
polyvinylidene fluoride (PVDF) membranes. The membranes were
blotted with corresponding primary antibodies, followed by
incubating with HRP-conjugated second antibodies. The target
proteins were developed with the SuperSignal West Pico Substrate
(Pierce, Rockford, IL, USA). Each assay was carried out in
triplicate.
Reverse transcription and polymerase
chain reaction analysis (RT-PCR)
Cells were seeded in T25 flasks and treated with
different concentrations of ORI or DMSO. At the corresponding time
points, total RNA was extracted with TRIzol reagents (Invitrogen,
Carlsbad, CA, USA) and subjected to RT reaction for cDNA templates.
Then, the cDNAs were used for detecting the expression level of
target genes by PCR. The primer sequences are available upon
request. Each assay was carried out in triplicate.
Xenograft tumor model of human colon
cancer
The animal experiment was approved by the
Institutional Animal Care and Use Committee (IACUC) of Chongqing
Medical University. Athymic nude mice (female, 4–6 weeks old) were
from the animal centre of Chongqing Medical University (Chongqing,
China). Cells were harvested and resuspended in cold PBS to
2×107 cells/ml. Cells in 50 µl of cold PBS were
injected into the flank of athymic mice (16). One week after injection, animals
were treated with different dose of ORI (50 or 100 mg/kg) or
solvent by intragastric administration, once a day. Four weeks
after injection, all animals were sacrificed and the tumors were
harvested for histological evaluation.
Histological evaluation and
immunohistochemical staining
Retrieved tumor masses were fixed with 10% formalin
and embedded with paraffin. Serial sections were stained with
hematoxylin and eosin (H&E). For immunohistochemical staining,
slides were deparaffinized and rehydrated in a graduated fashion,
then subjected to antigen retrieval and probed with PTEN antibody
or isotype IgG as control. Finally, incubated with biotinylated
secondary antibodies and streptavidin conjugated horseradish
peroxidase. PTEN was visualized with DAB staining and imaged under
a microscope.
Statistical analysis
The results of all experiments are expressed as the
mean ± standard deviation (SD) of at least three independent tests.
A Student's t-test was used for single-variable comparisons, and a
p-value <0.05 was considered statistically significant.
Results
ORI inhibits the proliferation of HCT116
cells
We explored the antiproliferation effect of ORI on
HCT116 cells to validate whether ORI could be used as
chemotherapeutic agent for human colon cancer. The results of
crystal violet staining showed that ORI effectively inhibits the
proliferation of HCT116 cells time- and concentration-dependently
(Fig. 1A and B). Cell cycle
analysis results showed that ORI induces cell cycle arrest
apparently at S phase in HCT116 cells (Fig. 1C). Western blot assay showed that
ORI also significantly suppresses the expression of proliferating
cell nuclear antigen (PCNA) (Fig.
1D). These data suggested ORI is capable of inhibiting
proliferation in HCT116 cells.
ORI induces apoptosis in HCT116
cells
We next tested whether ORI could induce HCT116 cells
to undergo apoptosis. HCT116 cells were treated with different
concentrations of ORI or DMSO for 24 or 48 h. Then, cells were
subjected with flow cytometry or western blot analyses. The results
showed that ORI increases the apoptotic cell rate
concentration-dependently (Fig.
2A). Western blot analysis showed that ORI substantially
increases the level of Bad and reduces the level of Bcl-2 in HCT116
cells (Fig. 2B). These results
indicated that ORI is a potent apoptosis inducer for colon cancer
cells.
ORI inhibits tumor growth in an ectopic
human cancer model
We next investigated the in vivo anticancer
activity of ORI with a well-established xenograft colon cancer
model (17). We injected HCT116
cells subcutaneously into the flanks of athymic nude mice. One week
after injection, the mice were treated with different doses of ORI
(50 or 100 mg/kg) through intragastric administration, once a day
up to 4 weeks. Suspension of 0.4% CMC-Na were administrated as
control. The results showed that tumor masses of ORI treated group
are smaller than those of the control group, and ORI inhibits the
tumor growth dose dependently (Fig.
3A). H&E staining results showed that more necrotic cells
were found in ORI treated group, comparing with the solvent group
(Fig. 3B). These in vivo
results further demonstrated that ORI may be a potential anticancer
reagent for colon cancer treatment.
ORI upregulates PTEN in HCT116 cells
Cell proliferation is well-regulated by various
signalings or growth factors. Using western blot assay, we found
that ORI can reduce the phosphorylation of Akt1/2 (Fig. 4A), which suggested that the PI3K/Akt
signaling was inhibited by ORI in HCT116 cells. PTEN, as a tumor
suppressor, can negatively regulate PI3K/Akt signaling. For this
reason, we speculated that the inhibition of PI3K/Akt signaling may
be contributed to PTEN upregulation by ORI in HCT116 cells.
However, with PCR assay, we found ORI exerts no substantial effect
on the mRNA level of PTEN in HCT116 cells (Fig. 4B). Further results showed that ORI
can markedly increase the protein level of PTEN and decrease the
phosphorylation of PTEN, concentration-and time-dependently
(Fig. 4C). The immunohistochemical
staining results showed that PTEN is upregulated also in colon
cancer dose-dependently (Fig. 4D).
These results suggested that the antiproliferation effect of ORI in
colon cancer cells may be associated with PTEN upregulation.
PTEN partly mediates the
antiproliferation effect of ORI in HCT116 cells
We next investigated the role of PTEN in the
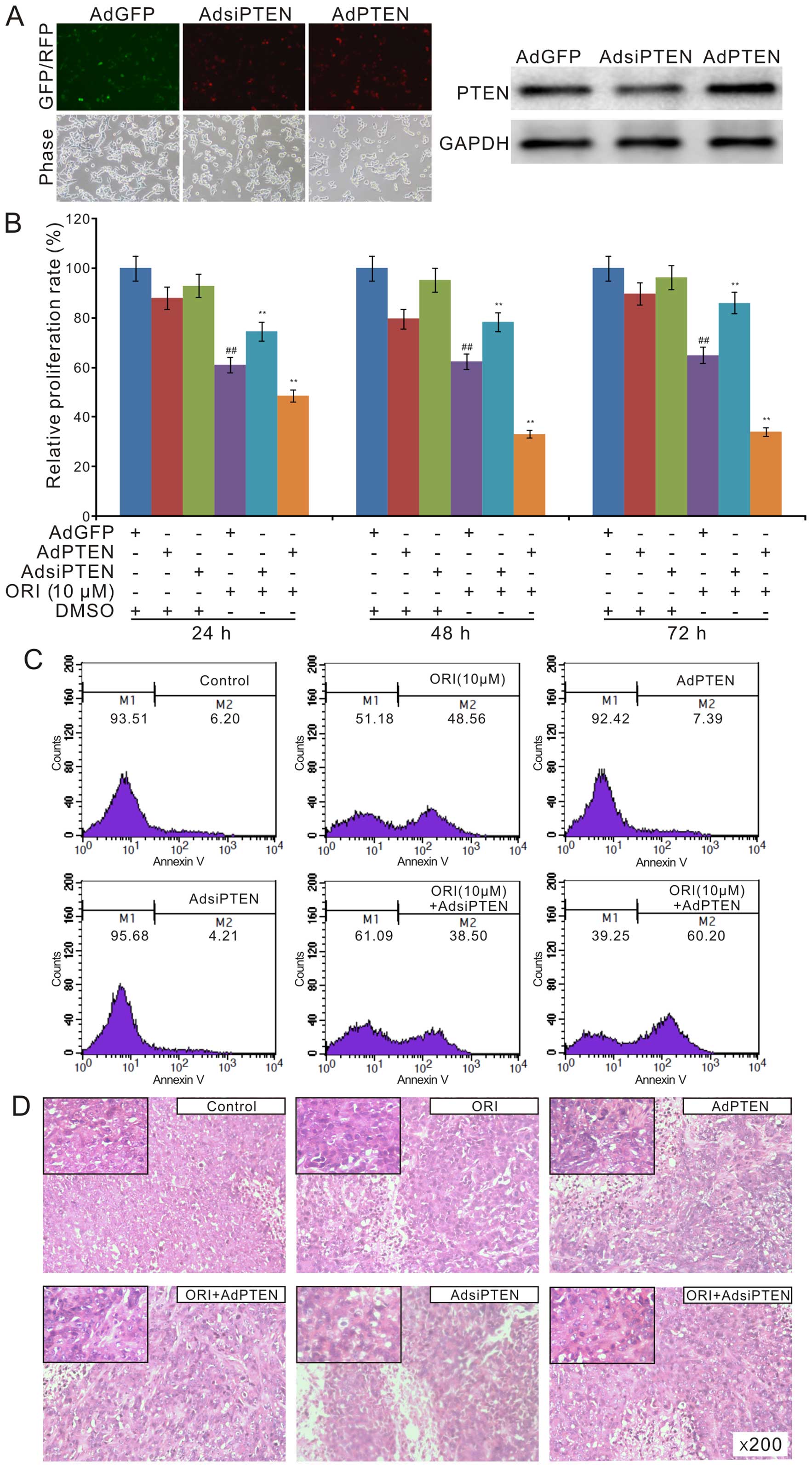
antiproliferation effect ORI in colon cancer cells. We constructed
recombinant adenoviruses of PTEN and siRNA fragments for PTEN.
Western blot results showed that all the recombinant adenoviruses
exert their function well (Fig.
5A). The crystal violet staining analysis showed that exogenous
expression of PTEN substantially enhances the antiproliferation of
ORI, while knockdown of PTEN reduces this effect of ORI in HCT116
cells (Fig. 5B). The flow cytometry
assay results showed that exogenous expression of PTEN increases
the apoptotic cell rate induced by ORI in HCT116 cells. On the
contrary, PTEN knockdown reduces ORI-induced apoptosis (Fig. 5C). The H&E staining results
showed that the anticancer effect of ORI was enhanced by exogenous
expression of PTEN, but decreased by PTEN knockdown (Fig. 5D). These data suggested that the
anticancer effect of ORI may be partly mediated by PTEN
upregulation.
ORI upregulates PTEN by activating p38
MAPK in HCT116 cells
Although PTEN can partly mediate the
antiproliferation of ORI in HCT116, the mechanism of how ORI
upregulates PTEN remains unknown. With further analysis, we found
that ORI increases the phosphorylation of p38 MAPK,
concentration-dependently (Fig.
6A), increases the level of PTEN, but decreases the
phosphorylation of PTEN; p38 MAPK inhibitor shows no substantial
effect on the level of PTEN, but obviously increases its
phosphorylation. When ORI was combined with p38 MAPK inhibitor, the
level of p-PTEN was clearly increased, although no substantial
effect on the level of PTEN was observed (Fig. 6B). Crystal violet staining analysis
results showed that p38 MAPK inhibitor partly reverses the
antiproliferation effect of ORI in HCT116 cells (Fig. 6C), which is similar with the effect
of PTEN knockdown on the antiproliferation effect of ORI (Fig. 5B). These data suggested that
ORI-induced upregulation of PTEN may be resulted from activating
p38 MAPK, which may block the phosphorylation of PTEN in colon
cancer cells.
Discussion
In the present study, we demonstrated that ORI can
be used as a potential antiproliferation drug for colon cancer
cells. Mechanistically, we found that the anticancer effect of ORI
may be partly mediated by upregulating PTEN through activating p38
MAPK to reduce PTEN phosphorylation.
ORI has been used as a drug for antitumor,
anti-microbial, anti-inflammatory and antioxidant for many years
(18). It has been reported that
ORI can inhibit proliferation and induce apoptosis in various
cancer cells, such as breast, pancreatic, lung, gastric and
prostate cancer (8,19–23),
as well as induce apoptosis and senescence in colon cancer cells
(12,24). Our data also confirmed that ORI can
inhibit proliferation and induce apoptosis in HCT116 cells
concentration-dependently, as well as inhibit the tumor growth of
colon cancer. Therefore, ORI may be a promising natural product as
chemotherapy drug for colon cancer treatment. As reported, ORI is a
multi-target natural product (18),
and the anticancer effect of ORI may be mediated by upregulating
p53 and p21, decreasing Bcl-2 and epidermal growth factor receptor,
inhibiting Wnt/β-catenin and PI3K/Akt signaling (25–29).
Although the anticancer activity of ORI in colon cancer may also be
associated with upregulating p21 and inhibiting Wnt/β-catenin
signaling (12,24), the precise mechanism underlying this
process remains unclear.
PI3K/Akt signal is an essential pathway regulating
proliferation, apoptosis and survival. It has been targeted for
many anticancer drugs (29–31). PI3K/Akt signaling can be negatively
regulated by PTEN, another important tumor suppressor. The mutation
and/or function loss of PTEN will lead to PI3K/Akt signaling
over-activation and uncontrolled proliferation. Hence, PTEN is an
important target for cancer control, and some promising
chemotherapy drugs have been used to target PTEN (32). In the present study, we found that
ORI can reduce p-Akt1/2 (Fig. 4A),
which suggested that the anticancer activity of ORI in colon cancer
cells may result from inactivating PI3K/Akt signaling. Although
PTEN is one of the major negative regulators for PI3K/Akt
signaling, we found ORI exerts no significant change on the mRNA
level of PTEN. Further analysis indicated that ORI can
substantially increase the protein level of PTEN and reduce the
level of p-PTEN. Thus, our data indicated that the anticancer
activity of ORI in colon cancer may be mediated by inactivating
PI3K/Akt signaling through upregulating PTEN, which may be the
result of decreasing the phosphorylation of PTEN.
PTEN acts as a lipid phosphatase to catalyse PIP3
dephosphorylation resulting in the production of PIP2. One of its
main physiological function is to negatively regulate PI3K/Akt
signaling (33). For colon cancer,
the reduction or mutation of PTEN plays an essential role at the
early stage of sporadic colorectal carcinogenesis (34), and the highly expressed PTEN is also
associated with the chemosensitivity of colon cancer (35). Therefore, PTEN may be a potential
therapeutical target for colon cancer treatment. PTEN is well
regulated by various mechanisms, such as transcriptional regulation
and post-transcriptional regulation, and phosphorylation (36). However, the detail of each
regulation process still need to be fully unveiled. Our results
showed that ORI may increase the level of PTEN by reducing its
phosphorylation (Fig. 4C). p38 MAPK
is a critical mediator for signaling transduction, which responds
to a wide range of extracellular stresses such as UV radiation,
hypoxia and oxidative stress (37).
It has been reported that the activation of p38 MAPK mediates the
anticancer activity of ORI in breast cancer (38). Our previous study indicated that
bone morphogenetic protein 9 (BMP9), the most potent osteogenic
protein, can activate p38 MAPK and downregulates PTEN in
mesenchymal stem cells (39).
However, the relationship between p38 MAPK and PTEN in cancer
remains unknown. Thus, we hypothesized that p38 MAPK may interact
with PTEN directly or indirectly in cancer. Using western blot
assay, we found that ORI can activate p38 MAPK in HCT116 cells, as
well as upregulate PTEN (Fig. 6A).
With further analysis, we found that ORI can reduce the level of
p-PTEN, which can be partly reversed by p38 MAPK inhibitor
(Fig. 6B). Inhibition of p38 MAPK
can partly reverse the antiproliferation effect of ORI in HCT116
cells (Fig. 6C). These results
indicated that upregulation of PTEN and activation of p38 MAPK are
both involved in the antiproliferation effect of ORI in HCT116
cells. Therefore, our data strongly suggested that ORI-induced
upregulation of PTEN may be the result of reducing the
phosphorylation of PTEN, which may be mediated by the ORI-induced
activation of p38 MAPK.
In summary, we demonstrated that ORI can be used as
an effective chemotherapy drug for human colon cancer. The
anticancer activity of ORI in colon cancer may be mediated by
increasing the level of PTEN, which may result from the ORI-induced
activation of p38 MAPK. Future studies should be carried out to
investigate the possible molecular mechanism of ORI on the
activation of p38 MAPK, and decipher the possible interaction
between p38 MAPK and PTEN phosphorylation in colon cancer
cells.
Acknowledgments
We thank Professor Tong-Chuan He of the University
of Chicago Medical Center (Chicago, IL, USA) for his kind provision
of the recombinant adenoviruses. The present study was supported by
the Research Grant from the National Natural Science Foundation of
China (grant nos. NSFC 81372120 and 81572226 to Bai-Cheng He).
References
|
1
|
Chemotherapy of metastatic colorectal
cancer. Prescrire Int. 19:219–224. 2010.PubMed/NCBI
|
|
2
|
Tran NH, Cavalcante LL, Lubner SJ,
Mulkerin DL, LoConte NK, Clipson L, Matkowskyj KA and Deming DA:
Precision medicine in colorectal cancer: The molecular profile
alters treatment strategies. Ther Adv Med Oncol. 7:252–262. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Binefa G, Rodríguez-Moranta F, Teule A and
Medina-Hayas M: Colorectal cancer: From prevention to personalized
medicine. World J Gastroenterol. 20:6786–6808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng QY, Wei Y, Chen JW, Chang WJ, Ye LC,
Zhu DX and Xu JM: Anti-EGFR and anti-VEGF agents: Important
targeted therapies of colorectal liver metastases. World J
Gastroenterol. 20:4263–4275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Banjerdpongchai R, Chanwikruy Y,
Rattanapanone V and Sripanidkulchai B: Induction of apoptosis in
the human Leukemic U937 cell line by Kaempferia parviflora
Wall.ex.Baker extract and effects of paclitaxel and camptothecin.
Asian Pac J Cancer Prev. 10:1137–1140. 2009.PubMed/NCBI
|
|
6
|
Weaver BA: How Taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Y, Xie L, Chen G, Wang H and Zhang R:
Effects of oridonin on proliferation of HT29 human colon carcinoma
cell lines both in vitro and in vivo in mice. Pharmazie.
62:439–444. 2007.PubMed/NCBI
|
|
8
|
Liu YQ, Mu ZQ, You S, Tashiro S, Onodera S
and Ikejima T: Fas/FasL signaling allows extracelluar-signal
regulated kinase to regulate cytochrome c release in
oridonin-induced apoptotic U937 cells. Biol Pharm Bull.
29:1873–1879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsieh TC, Wijeratne EK, Liang JY,
Gunatilaka AL and Wu JM: Differential control of growth, cell cycle
progression, and expression of NF-kappaB in human breast cancer
cells MCF-7, MCF-10A, and MDA-MB-231 by ponicidin and oridonin,
diterpenoids from the Chinese herb Rabdosia rubescens. Biochem
Biophys Res Commun. 337:224–231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou GB, Kang H, Wang L, Gao L, Liu P, Xie
J, Zhang FX, Weng XQ, Shen ZX, Chen J, et al: Oridonin, a
diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion
protein and shows potent antitumor activity with low adverse
effects on t(8;21) leukemia in vitro and in vivo. Blood.
109:3441–3450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu HZ, Yang YB, Xu XD, Shen HW, Shu YM,
Ren Z, Li XM, Shen HM and Zeng HT: Oridonin induces apoptosis via
PI3K/Akt pathway in cervical carcinoma HeLa cell line. Acta
Pharmacol Sin. 28:1819–1826. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao FH, Liu F, Wei W, Liu LB, Xu MH, Guo
ZY, Li W, Jiang B and Wu YL: Oridonin induces apoptosis and
senescence by increasing hydrogen peroxide and glutathione
depletion in colorectal cancer cells. Int J Mol Med. 29:649–655.
2012.PubMed/NCBI
|
|
13
|
Cheng Y, Qiu F, Ye YC, Tashiro S, Onodera
S and Ikejima T: Oridonin induces G2/M arrest and
apoptosis via activating ERK-p53 apoptotic pathway and inhibiting
PTK-Ras-Raf-JNK survival pathway in murine fibrosarcoma L929 cells.
Arch Biochem Biophys. 490:70–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bu HQ, Liu DL, Wei WT, Chen L, Huang H, Li
Y and Cui JH: Oridonin induces apoptosis in SW1990 pancreatic
cancer cells via p53- and caspase-dependent induction of p38 MAPK.
Oncol Rep. 31:975–982. 2014.
|
|
15
|
Luo J, Deng ZL, Luo X, Tang N, Song WX,
Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al: A protocol
for rapid generation of recombinant adenoviruses using the AdEasy
system. Nat Protoc. 2:1236–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu K, Zhou M, Wu QX, Yuan SX, Wang DX, Jin
JL, Huang J, Yang JQ, Sun WJ, Wan LH, et al: The role of IGFBP-5 in
mediating the anti-proliferation effect of tetrandrine in human
colon cancer cells. Int J Oncol. 46:1205–1213. 2015.
|
|
17
|
He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim
SH, Huang E, Gao Y, Yang K, Wagner ER, et al: Tetrandrine inhibits
Wnt/β-catenin signaling and suppresses tumor growth of human
colorectal cancer. Mol Pharmacol. 79:211–219. 2011. View Article : Google Scholar :
|
|
18
|
Owona BA and Schluesener HJ: Molecular
insight in the multifunctional effects of oridonin. Drugs RD.
15:233–244. 2015. View Article : Google Scholar
|
|
19
|
Wang S, Zhong Z, Wan J, Tan W, Wu G, Chen
M and Wang Y: Oridonin induces apoptosis, inhibits migration and
invasion on highly-metastatic human breast cancer cells. Am J Chin
Med. 41:177–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu DL, Bu HQ, Jin HM, Zhao JF, Li Y and
Huang H: Enhancement of the effects of gemcitabine against
pancreatic cancer by oridonin via the mitochondrial
caspase-dependent signaling pathway. Mol Med Rep. 10:3027–3034.
2014.PubMed/NCBI
|
|
21
|
Liu Y, Liu JH, Chai K, Tashiro S, Onodera
S and Ikejima T: Inhibition of c-Met promoted apoptosis, autophagy
and loss of the mitochondrial transmembrane potential in
oridonin-induced A549 lung cancer cells. J Pharm Pharmacol.
65:1622–1642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao SY, Li J, Qu XY, Zhu N and Ji YB:
Downregulation of Cdk1 and cyclinB1 expression contributes to
oridonin-induced cell cycle arrest at G2/M phase and growth
inhibition in SGC-7901 gastric cancer cells. Asian Pac J Cancer
Prev. 15:6437–6441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Li X, Wang J, Ye Z and Li JC:
Oridonin up-regulates expression of P21 and induces autophagy and
apoptosis in human prostate cancer cells. Int J Biol Sci.
8:901–912. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao FH, Hu XH, Li W, Liu H, Zhang YJ, Guo
ZY, Xu MH, Wang ST, Jiang B, Liu F, et al: Oridonin induces
apoptosis and senescence in colorectal cancer cells by increasing
histone hyperacetylation and regulation of p16, p21, p27 and c-myc.
BMC Cancer. 10:6102010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cui Q, Tashiro S, Onodera S, Minami M and
Ikejima T: Oridonin induced autophagy in human cervical carcinoma
HeLa cells through Ras, JNK, and P38 regulation. J Pharmacol Sci.
105:317–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng FF, Zhang DR, Tian KL, Lou HY, Qi XL,
Wang YC, Duan CX, Jia LJ, Wang FH, Liu Y, et al: Growth inhibition
and induction of apoptosis in MCF-7 breast cancer cells by oridonin
nanosuspension. Drug Deliv. 18:265–271. 2011. View Article : Google Scholar
|
|
27
|
Li D, Wu LJ, Tashiro S, Onodera S and
Ikejima T: Oridonin induces human epidermoid carcinoma A431 cell
apoptosis through tyrosine kinase and mitochondrial pathway. J
Asian Nat Prod Res. 10:77–87. 2008. View Article : Google Scholar
|
|
28
|
Huang HL, Weng HY, Wang LQ, Yu CH, Huang
QJ, Zhao PP, Wen JZ, Zhou H and Qu LH: Triggering Fbw7-mediated
proteasomal degradation of c-Myc by oridonin induces cell growth
inhibition and apoptosis. Mol Cancer Ther. 11:1155–1165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar
|
|
32
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Milella M, Falcone I, Conciatori F, Cesta
Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S,
Cognetti F, et al: PTEN: Multiple functions in human malignant
tumors. Front Oncol. 5:242015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Waniczek D, Śnietura M, Młynarczyk-Liszka
J, Pigłowski W, Kopeć A, Lange D, Rudzki M and Arendt J: PTEN
expression profiles in colorectal adenocarcinoma and its
precancerous lesions. Pol J Pathol. 64:15–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hsu CP, Kao TY, Chang WL, Nieh S, Wang HL
and Chung YC: Clinical significance of tumor suppressor PTEN in
colorectal carcinoma. Eur J Surg Oncol. 37:140–147. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012.PubMed/NCBI
|
|
37
|
Tormos AM, Taléns-Visconti R, Nebreda AR
and Sastre J: p38 MAPK: A dual role in hepatocyte proliferation
through reactive oxygen species. Free Radic Res. 47:905–916. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cui Q, Tashiro S, Onodera S, Minami M and
Ikejima T: Autophagy preceded apoptosis in oridonin-treated human
breast cancer MCF-7 cells. Biol Pharm Bull. 30:859–864. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang J, Yuan SX, Wang DX, Wu QX, Wang X,
Pi CJ, Zou X, Chen L, Ying LJ, Wu K, et al: The role of COX-2 in
mediating the effect of PTEN on BMP9 induced osteogenic
differentiation in mouse embryonic fibroblasts. Biomaterials.
35:9649–9659. 2014. View Article : Google Scholar : PubMed/NCBI
|