Introduction
Brain tumor develops from glial or precursor cells.
There are several types of brain tumors including astrocytoma,
oligodendroglioma, glioblastoma (GBM), ependymoma, malignant and
mixed glioma, and not otherwise specified (NOS). GBM is one of the
neuroepithelial tissue tumors and sub-classified into gliosarcoma
and giant-cell GBM. In adults, GBM occurs most commonly in the
primary malignant brain tumor. In general, GBM represent 17% of all
types of brain tumors including both primary and metastatic state.
Adults from the age of 45 to 70 tend to be diagnosed with these
tumors. The average age of death from brain and other nervous
system cancer is at the of age of 64 years. GBM has an incidence of
3.19/100,000 in the US (1,2) and ~0.59–3.69 cases/100,000 are
annually diagnosed worldwide (3–7). It is
one of the most devastating glial tumors that typically results in
survival of only one-third of patients in the first year, and less
than 5% beyond 5 years after diagnosis (8). The median survival with GBM is ~12–15
months despite the aggressive surgical and conventional treatment
(9,10).
Interleukin (IL)4 acts through a multi-unit
transmembrane receptor, IL4R. The overexpression of IL4R has been
observed in brain, head and neck, lung, pancreatic, prostate
cancers, and AIDS-associated Kaposi's sarcoma (11–14),
whereas normal cells including immune cells express a low number of
this receptor (15,16). IL4R complex presented (in cells) are
of two different types. Type I IL4R consists of IL4Rα and IL2Rγ
chains; however, type II IL4R has IL4Rα and IL13Rα1 chains. The
signal transduction through type I or II IL4R is mediated by two
receptor chains (17). It was also
observed that the blockade of IL4R abrogates highly both
hematogenous and lymphatic metastases in vivo and therefore,
IL4R signaling pathway could be a target for inhibiting
rhabdomyosarcoma tumor progression and metastasis (18).
Although the detail biological function of IL4R
expression on brain tumors is not clearly understood, based on
previous studies, it seems that IL4R expression may play an
important role in brain tumor development and be one of the
potential candidate cell surface target proteins for a targeted
diagnostic and therapy. As one of the novel approach for this
purpose, peptide drugs have been applied to treat tumors with the
high IL4R expression since peptides can be relatively easily
synthesized by either biological recombinant method or chemical
solid phase synthesis. In addition, costs for production are less
than for antibody-based therapeutics (19). IL4Rα has been previously reported to
have high expression on the surface of a variety of human solid
tumors such as renal cell carcinoma, malignant melanoma and GBM
(20).
In the present study IL4R targeting peptide
ligand-conjugated liposomal microbubbles [(MBs)-Lipo doxorubicin
(Dox)-IL4RTP] were prepared as ultrasound contrast agents, and to
analyze the anticancer effect of the novel ultrasound contrast
agents containing MBs in vitro in the cell line model of
brain tumor, which is a clinically significant tumor to provide
experimental proof of concept for specific ultrasound imaging of
brain tumor at an early stage.
Materials and methods
Chemical reagents and antibodies
Dimethyl sulfoxide (DMSO), glycerol, glycine, sodium
chloride, thiazolyl blue tetrazolium bromide, Trizma-base and
Tween-20 were purchased from Sigma (St. Louis, MO, USA). IL4R
targeting peptide ligand (sequence: CRKRLDRN) was from Anygen Inc.
(Daejeon, Korea). Mouse anti-p53, mouse anti-PARP1, rabbit
anti-IL4R and mouse anti-β-actin antibodies were obtained from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit anti-Bax
(1:1,000 dilution), rabbit anti-phospho-p53 (Ser15), rabbit
anti-p21 and rabbit anti-γH2AX antibodies were purchased from Cell
Signaling Technology (Danvers, MA, USA). Goat anti-mouse and goat
anti-rabbit horseradish peroxidase-conjugated IgG were obtained
from Jackson ImmunoResearch (West Grove, PA, USA). ECL Western
Blotting detection reagents were obtained from GenDEPOT (Barker,
TX, USA).
Cell culture
U87MG, LN229, HS683, U138, MDA-MB-231 and H460 cells
[all from American Type Culture Collection (ATCC); Manassas, VA,
USA) were maintained in Dulbecco's modified Eagle's medium (DMEM)
media supplemented with 10% fetal bovine serum and 1%
streptomycin/penicillin at 37°C in a humidified incubator
containing 5% CO2 in air.
Preparation of MB-Lipo complex
The protocol for preparing of MB-Lipo complex was
based on our previous study (21).
Thiol-active MBs (0.67 ml) (13 mg/ml, 1.2×1,011 MB/ml), derived
from DSPE-PEG-PDP, were mixed with 2 ml thiolated Lipo (10 mg/ml)
by shaking for 2 h at room temperature. The reaction was monitored
to pyridine-2-thione releasing by UV-Vis spectra. The prepared Lipo
still had an amine functional group on the surface, despite
treatment with Traut's reagent, as shown by quantitative amine
analysis. The 30 mg MB-Lipo dispersion aqueous solution (2 ml),
containing an amine functional group, was reacted with
sulfosuccinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate
(Sulfo-SMCC; 5 mg; Sigma-Aldrich) for 3 h at 25°C after adjusting
the pH ~8.2 with 1 M NaHCO3, to activate maleimide
functional group. This particle solution was then linked to
appropriate antibodies using a general conjugation procedure.
Dox loading into MB-Lipo
Dox (Sigma-Aldrich) was incorporated into Lipo
particles by the thin-film hydration and remote-loading method with
an ammonium sulfate gradient. Lipid film (21.1 mg) was hydrated
with 2 ml ammonium sulfate solution (250 mM), and the liposomal
suspension was sequentially extruded 5 times through polycarbonate
filters with pore sizes of 200 nm. The ammonium sulfate was removed
by centrifugation (5 min, 13,000 rpm). At this point, ammonium
sulfate ions were located only inside of Lipo particles. The
liposomal dispersion and 440 μM Dox (1:1, v/v) were mixed
and incubated for 2 h at 60°C. The mixture was washed to remove
unloaded Dox, and the loading capacity of Dox in Lipo was
determined by measuring the concentration of Dox in the supernatant
by UV-Vis spectroscopy. LipoDox particles were then complexes with
MBs as described above.
Preparation and characterization of
MB-Lipo (Dox)-IL4RTP
MB-Lipo (Dox) complex containing NH2 group was
reacted with Sulfo-SMCC in phosphate-buffered saline (PBS; pH 8.2)
for 2 h to introduce maleimide group outside the MB-Lipo (Dox)
complex. Then, MB-Lipo (Dox)-maleimide was further conjugated with
IL4R targeting peptide-thiol group in PBS (pH 7.5) for 2 h to
produce MB-Lipo (Dox)-IL4RTP. For determining the conjugation
reaction of the IL4R targeting peptide into MB-Lipo complex,
analytical high-performance liquid chromatography (HPLC) was
adopted. Using water symmetry C18 column (4.6×220 nm), the eluting
solvent was used for the 40% acetonitrile. At flow rate of the
solvent 0.8 ml/min a single absorption peak at 250 nm was
identified.
Confocal microscopy analysis
The same quantity of IL4R targeting peptide ligand
conjugated MB (FITC)-Lipo (Cy5) were added to U87MG cells with or
without ultrasound flash (Sonidel SP-100; Sonidel, Boston, MA,
USA), and then observed using confocal laser scanning microscopy.
U87MG cells were grown on glass coverslips. After incubation with
MB (FITC)-Lipo (Cy5) for 3 h, cells were fixed with 4%
paraformaldehyde for 10 min and permeabilized with Triton X-100
(0.5%). Slide culture chambers were washed with PBS. Cells were
stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma) to show
the nuclei. Specific staining was visualized and images were
captured with confocal laser scanning microscope (Zeiss LSM510
microscope).
WST-1 cell viability assay
A 200 μl aliquot of cells (1×103
cells in media) was added to each well of a 96-well plate and
incubated for 18 h at 37°C in a humidified incubator containing 5%
CO2 in air. After incubation, MB-Lipo (Dox)-IL4RTP was
added with or without ultrasound flash (Sonidel SP-100) into each
well for 48 h. Control cultures were treated with PBS. After
incubation, a 20 μl WST-1 solution was added to each well
and the incubation continued for 4 h. The visible absorbance at 560
nm of each well was quantified using a microplate reader.
Western blot analysis
Cells were washed with PBS and lysed in lysis buffer
(50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.1% SDS,
pH 8.0) with protease and phosphatase inhibitors. Cell lysates were
centrifuged (10,000 × g, 4°C, 10 min) and the supernatants were
separated on 6 or 10% SDS-PAGE gels and blotted onto nitrocellulose
membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes
were blocked in 3% non-fat dry milk for 1 h at room temperature,
and probed with appropriate antibodies. Membranes were then probed
with HRP-tagged anti-mouse or anti-rabbit IgG antibodies diluted
1:5,000–1:15,000 in 3% non-fat dry milk for 1 h at room
temperature. Chemiluminescence was detected using enhanced
chemiluminescence (ECL).
Statistical analysis
Results are expressed as arithmetic mean ± SEM
(standard error of the mean). To compare the statistical
significance between the groups, two-sided unpaired Student's
t-test was used. All experiments were repeated three times and
representative data are shown. Statistical analyses were performed
using SPSS software (version 19.0; SPSS, Inc., Chicago, IL, USA).
Mean differences with P-values <0.05 were considered to indicate
a statistically significant result.
Results
Preparation and characterization of the
MB-Lipo (Dox)-IL4RTP complex
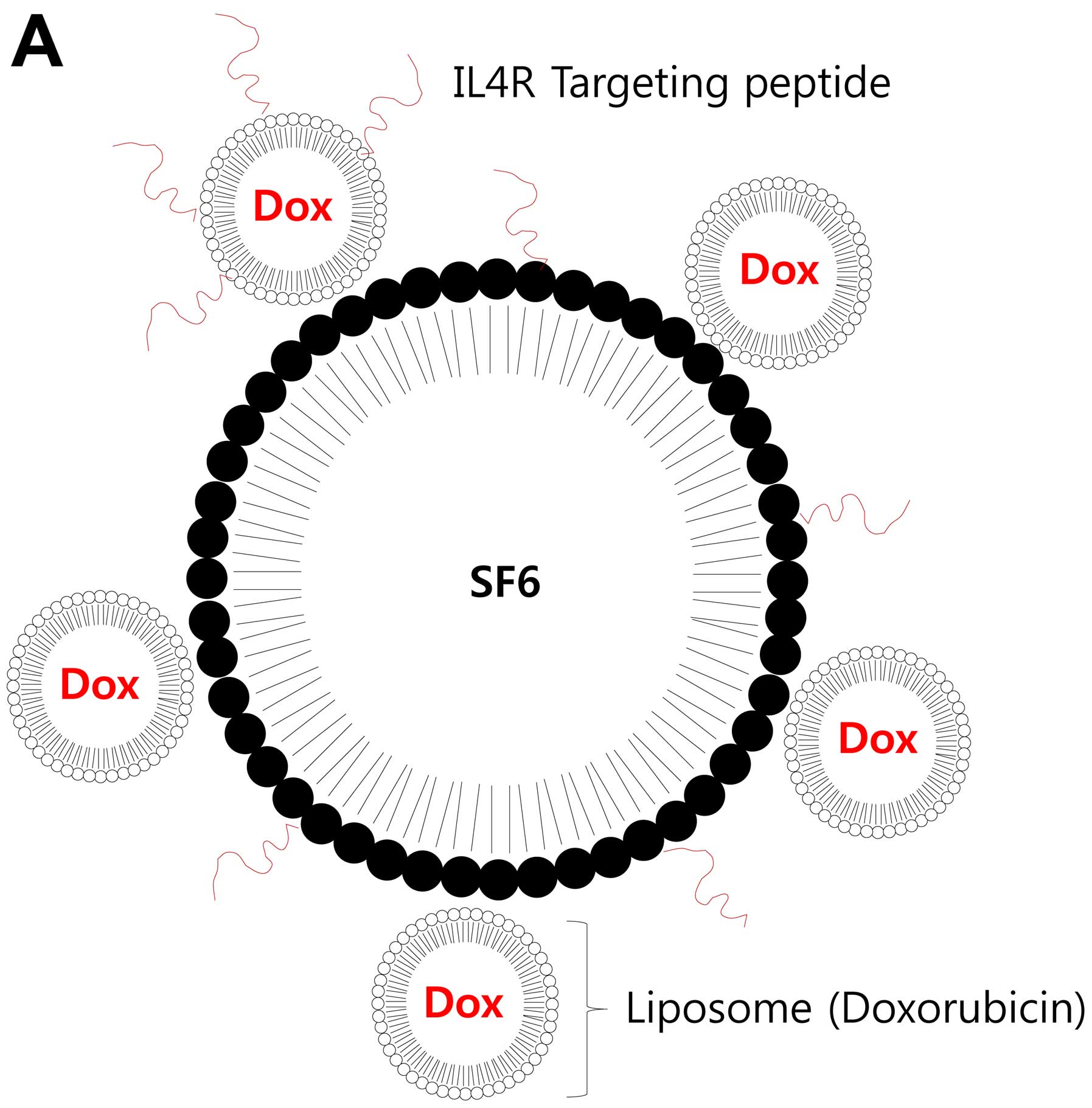
The gas (SF6)-filled MBs were prepared by emulsion
and solvent-evaporation method. The prepared MBs were further
conjugated with Dox-loaded nano-sized liposome and peptide ligands
to IL4R for targeting brain tumor cells (Fig. 1A). The suspensions of the MBs in PBS
appeared as milky white. Light microscopy revealed that the MBs
were uniformly distributed with no visible aggregation. A single
microbubble was round (Fig. 1C). As
shown in Fig. 1B, the particle size
ranged from 1,295–1,468 nm with a mean of 1,000 nm for
MB-Lipo-IL4RTP and 1,000 nm for MB-Lipo. HPLC data demonstrated the
incorporation of IL4R targeting peptide into MB-Lipo complex
(yield, 75.6%) (Fig. 1D).
Targeting ability of MB-Lipo-IL4RTP in
U87MG cells
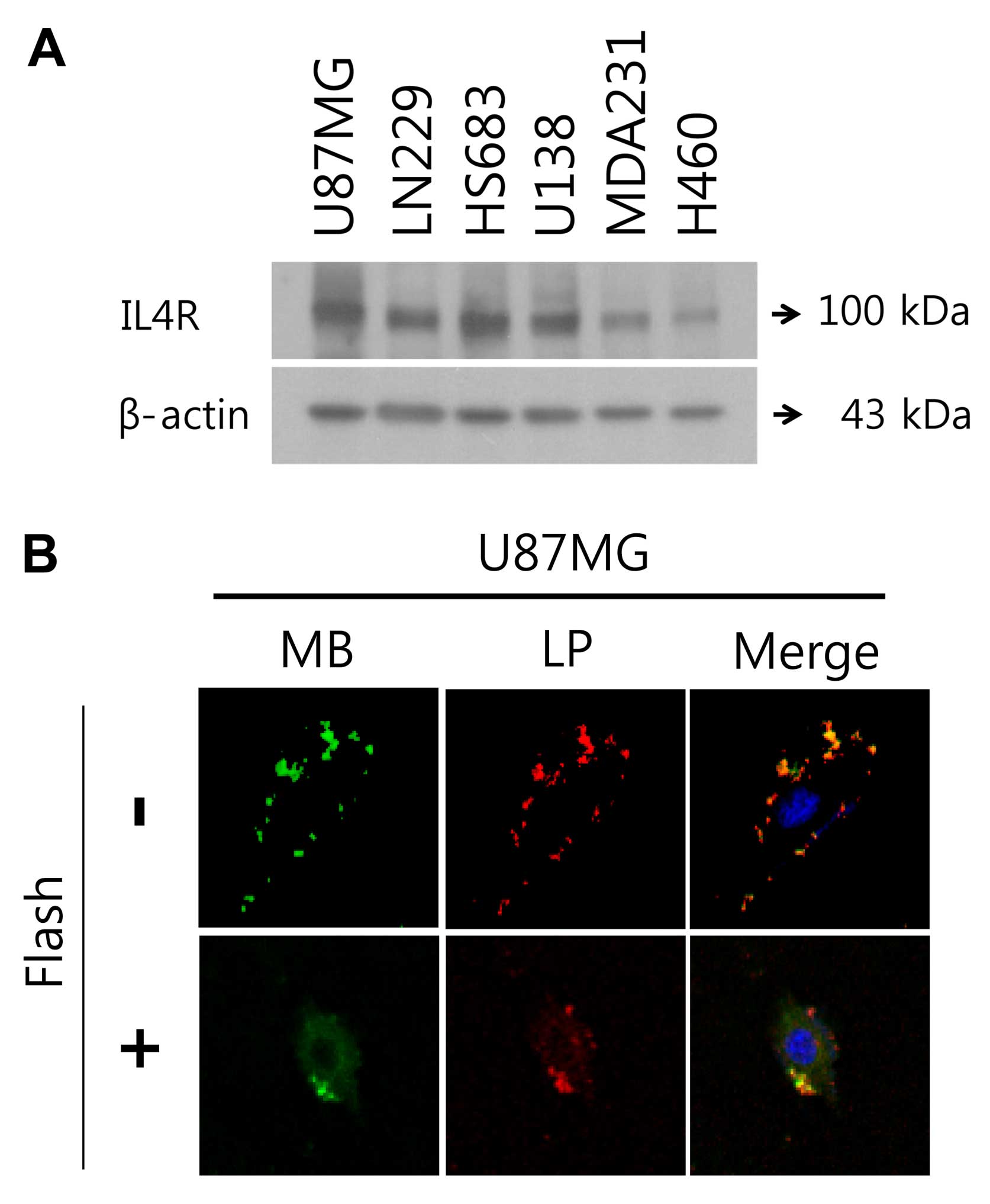
To select the most available brain tumor cell line
with high expression of IL4R, we performed western blotting of IL4R
in four brain tumor cell types (U87MG, LN229, HS683 and U138), one
breast tumor cell line (MDA-MB-231) as positive control, and in one
lung tumor cell line (H460). As shown in Fig. 2A, among brain tumor cell lines,
U87MG cells had the highest expression of IL4R and H460 had very
low expression of IL4R. MBs targeted to IL4R were synthesized and
binding specificity to IL4R was first tested in cell culture
experiments. Fig. 2B illustrates
binding of MB (FITC)-Lipo (Cy5) to IL4R-positive U87MG human brain
tumor cells. IL4R targeting peptide ligands on the surface of
MB-Lipo complex target and bind to IL4R in U87MG brain tumor cells,
which resulted in selective accumulation and long resident time in
U87MG brain tumor cells. The MB-Lipo (Dox)-IL4RTP exhibited
cellular uptake in U87MG brain tumor cells (one of brain tumor cell
line expressing strongly IL4R) with frequency ultrasound energy
suggesting that MB-Lipo (Dox)-IL4RTP provided effective targeting
ability for brain tumor cells.
Cell growth inhibition effect of MB-Lipo
(Dox)-IL4RTP against U87MG cells
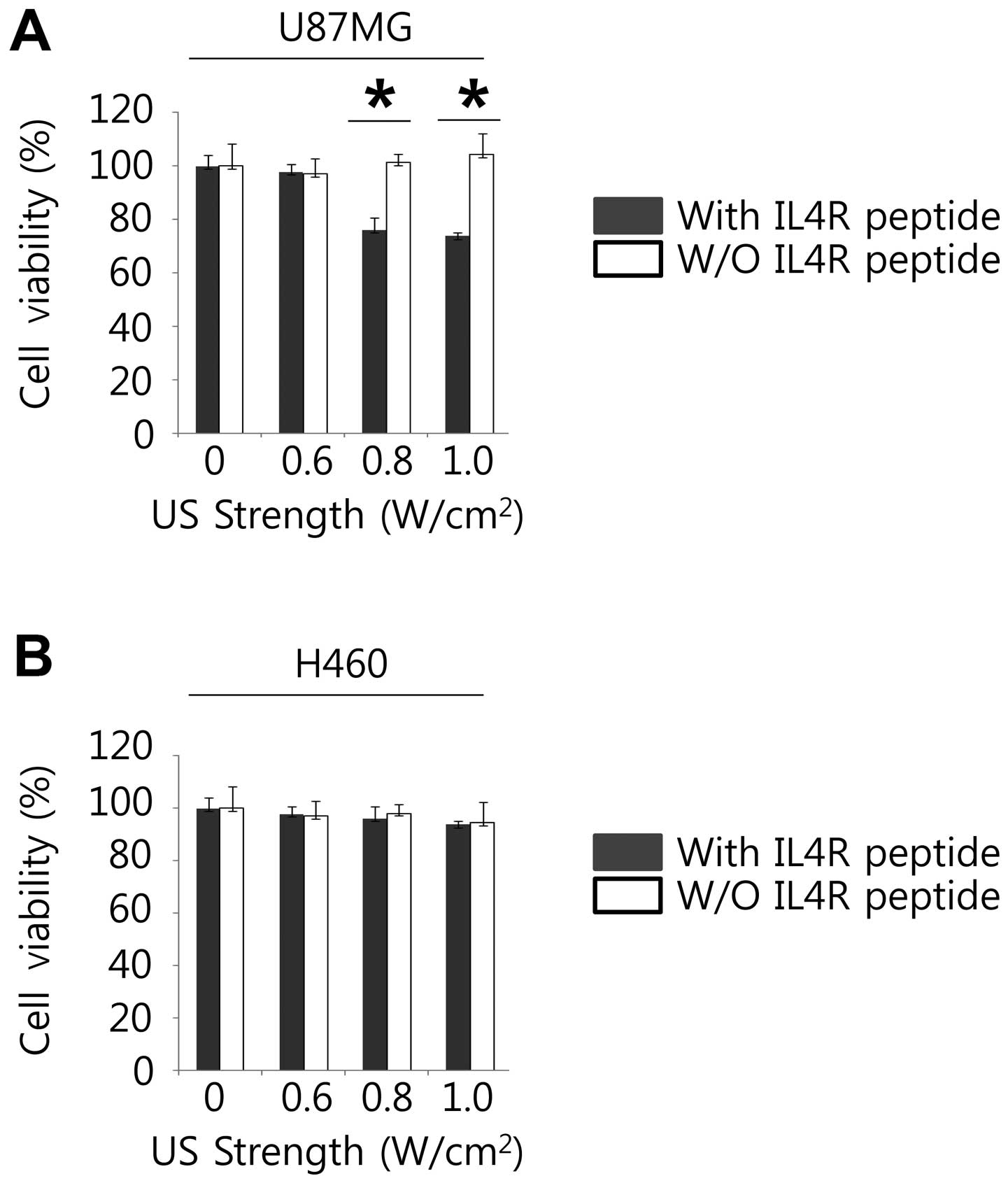
For analysis of the effects of anticancer
chemotherapy containing the ultrasound contrast agents to target
cancer cells, and to evaluate the toxicity of the ultrasound
contrast agents for ultrasound contrast media in the cells, WST-1
assay was performed (Fig. 3A and
B). The cell viability measurements of U87MG (high IL4R
expression) with MB-Lipo (Dox)-IL4RTP (with IL4R targeting peptide
ligands) were lower than MB-Lipo (Dox) (without IL4R targeting
peptide ligands) (P<0.01) at the ultrasound strength of 0.8 and
1.0 w/cm2. However, the cell viability measurements of
H460 (low IL4R expression) with MB-Lipo (Dox)-IL4RTP (with IL4R
targeting peptide ligands) were almost the same as MB-Lipo (Dox)
(without IL4R targeting peptide ligands) at the respective
ultrasound strength of 0, 0.6, 0.8 and 1.0 w/cm2. These
results suggest that MB-Lipo (Dox)-IL4RTP may play an essential
role in inhibiting cancer cell growth in an IL4R dependent
manner.
DNA damaging response-related cell cycle
arrest induced by MB-Lipo (Dox)-IL4RTP in U87MG cells
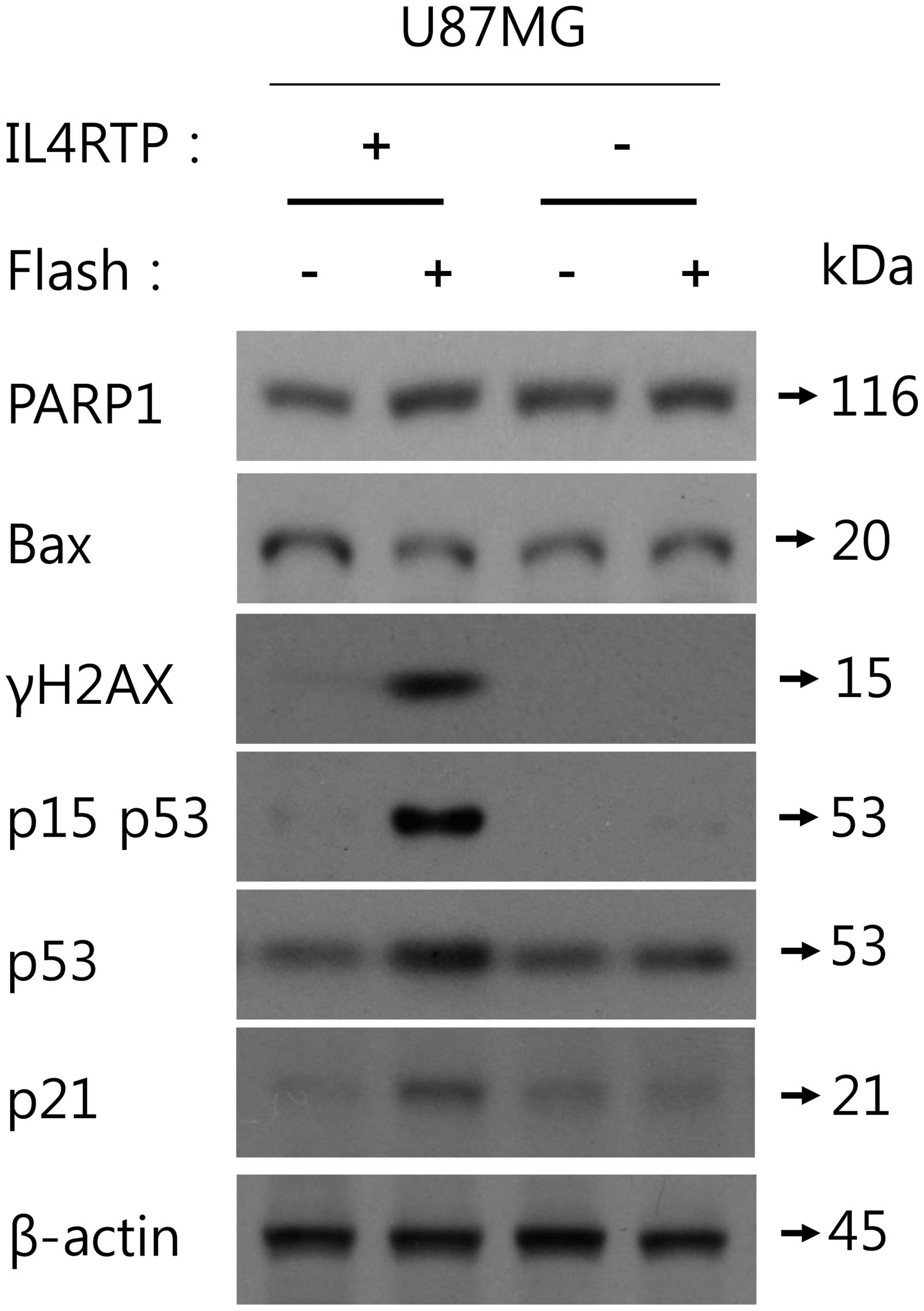
To examine the role of IL4R in MB-Lipo
(Dox)-IL4RTP-induced DNA damaging response-related cell cycle
arrest in U87MG cells, we incubated U87MG cells with MB-Lipo (Dox)
with IL4R targeting peptide or MB-Lipo (Dox) without IL4R targeting
peptide and analyzed the protein status of PARP1, Bax, phospho-p53
(Ser15), p53, p21 and γH2AX using western blot assays. MB-Lipo
(Dox)-IL4RTP did not cause apoptosis in U87MG cells since PARP1 was
not cleaved and Bax expression level was not changed. As shown in
Fig. 4, MB-Lipo (Dox)-IL4RTP
significantly augmented phospho-p53 (Ser15) and γH2AX, two of the
representative DNA damaging-related markers after ultrasound
exposure in U87MG cells but MB-Lipo (Dox) could not affect them. In
addition, MB-Lipo (Dox)-IL4RTP upregulated p53 and p21 expression
that may activate cell cycle arrest. In contrast, MB-Lipo (Dox) did
not change either p53 or p21 expression. Collectively, these
results suggest that MB-Lipo (Dox)-IL4RTP may play an essential
role in promoting IL4R-dependent cell cycle arrest after treatment
and ultrasound exposure in U87MG cells.
Discussion
Ultrasound molecular imaging has great potential to
impact early disease diagnosis, evaluation of disease progression
and the development of target-specific therapy. In the present
study, IL4R targeting peptide was conjugated onto the surface of
microbubbles (MBs) to evaluate molecular imaging of brain tumors
using U87MG cell line model. There have been studies on the high
expression of IL4R in the various human solid tumors including
brain tumors which promotes tumor development via upregulation of
anti-apoptotic proteins and prevention of chemotherapy-induced
cancer cell death. Thus, it would be a good therapeutic treatment
to target IL4R on the brain tumor by developing a novel drug
delivery system with IL4R targeted ligands. In addition, targeted
therapy against cancer provides various biological advantages such
as increase in the pharmacokinetic profile of drugs and
minimization of the unexpected side-effect caused by the
chemotherapy with high specificity.
In the present study, we designed an IL4R targeting
peptide that was described as an atherosclerotic plaque and breast
tumor tissue homing peptide screened by phage display screening
method. According to the study using this peptide ligand (22), polymer conjugated with IL4R
targeting peptide could play an important role in increasing in
vitro tumor-specific targeting and uptake efficiency in both
H226 and MDA-MB-231 cancer cell lines, and has excellent homing and
longer retention in tumor tissues in MDA-MB-231 xenograft mouse
model. These results suggested that IL4R targeting peptide ligands
can be adapted to a novel tool for selective delivery of
therapeutic drugs to tumors.
As one of the clinically most challenging fields,
brain tumor exhibits various distinct characteristics. One of the
obstacles to cure brain tumor is the blood-brain barrier (BBB) that
is a blockade that blocks not only the external infectious
organisms, but also the treatment for disease from entering deeply
into the brain (23–28). Thus, various studies have been
developing possible approaches to enhance the penetration activity
of the helpful pharmaceutical options through BBB. For this
purpose, a new technique using ultrasound and MBs has been actively
applied to degenerative brain disease and brain cancers, which can
lead the drug-loaded MBs by the focused ultrasound to enter
properly into the brain (29).
Recently, Wei et al reported that they had invented a novel
liposomal formulation to overcome BBB conjugated with dual peptide
ligands that are stable from protease in the blood using D-form
amino acids. One of the peptide ligands was for nicotine
acetylcholine receptors on the BBB and other ligands were for
integrin highly expressed on both of BBB and tumor cells (30). According to the above studies, their
liposome loaded with doxorubicin (Dox) could effectively penetrate
BBB and target brain tumor in vitro and in vivo.
Although we proved that our novel microbubble
complex containing Dox-loaded nano-sized liposome can target brain
tumor cells and inhibit tumor cell growth with IL4R-dependent
manner, the present study still has a few limitations. First, the
targeting ability of MB (FITC)-Lipo (Cy5)-IL4RTP to U87MG brain
tumor cells was not performed by considering the real flow
conditions in blood vessels since it may be important to reflect
in vivo exposure of MB (FITC)-Lipo (Cy5)-IL4RTP to shear
stress as well as the dose-dependence of MB (FITC)-Lipo
(Cy5)-IL4RTP. Secondly, in vivo ultrasound imaging and
therapeutic effect of MB-Lipo-IL4RTP should be assessed using both
xenograft and orthotopic mouse models with brain tumor cell lines
that have different levels of IL4R expression. In conclusion, the
present study contribute to the effort of development of
methodology to measure the contrast enhancement effect of the new
ultrasound contrast agent to breast tumor, and to target the breast
cancer cell line in vitro and in vivo studies.
Acknowledgments
The present study was supported by grant no.
13–2014–006 from the SNUBH Research Fund. This study was supported
by the Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education,
Science and Technology (NRF-2014R1A6A3A 04054307).
References
|
1
|
Ostrom QT, Gittleman H, Liao P, Rouse C,
Chen Y, Dowling J, Wolinsky Y, Kruchko C and Barnholtz-Sloan J:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2007–2011. Neuro Oncol.
16(Suppl 4): iv1–iv63. 2014. View Article : Google Scholar :
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohgaki H and Kleihues P: Population-based
studies on incidence, survival rates, and genetic alterations in
astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol.
64:479–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arora RS, Alston RD, Eden TO, Estlin EJ,
Moran A and Birch JM: Age-incidence patterns of primary CNS tumors
in children, adolescents, and adults in England. Neuro Oncol.
11:403–413. 2009. View Article : Google Scholar :
|
|
5
|
Lee CH, Jung KW, Yoo H, Park S and Lee SH:
Epidemiology of primary brain and central nervous system tumors in
Korea. J Korean Neurosurg Soc. 48:145–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dobes M, Khurana VG, Shadbolt B, Jain S,
Smith SF, Smee R, Dexter M and Cook R: Increasing incidence of
glioblastoma multiforme and meningioma, and decreasing incidence of
Schwannoma (2000–2008): Findings of a multicenter Australian study.
Surg Neurol Int. 2:1762011. View Article : Google Scholar
|
|
7
|
Gigineishvili D, Shengelia N, Shalashvili
G, Rohrmann S, Tsiskaridze A and Shakarishvili R: Primary brain
tumour epidemiology in Georgia: First-year results of a
population-based study. J Neurooncol. 112:241–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dunn GP, Rinne ML, Wykosky J, Genovese G,
Quayle SN, Dunn IF, Agarwalla PK, Chheda MG, Campos B, Wang A, et
al: Emerging insights into the molecular and cellular basis of
glioblastoma. Genes Dev. 26:756–784. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Husain SR, Kreitman RJ, Pastan I and Puri
RK: Interleukin-4 receptor-directed cytotoxin therapy of
AIDS-associated Kaposi's sarcoma tumors in xenograft model. Nat
Med. 5:817–822. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Joshi BH, Leland P, Asher A, Prayson RA,
Varricchio F and Puri RK: In situ expression of interleukin-4
(IL-4) receptors in human brain tumors and cytotoxicity of a
recombinant IL-4 cytotoxin in primary glioblastoma cell cultures.
Cancer Res. 61:8058–8061. 2001.PubMed/NCBI
|
|
13
|
Joshi BH, Leland P, Lababidi S, Varrichio
F and Puri RK: Interleukin-4 receptor alpha overexpression in human
bladder cancer correlates with the pathological grade and stage of
the disease. Cancer Med. 3:1615–1628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimamura T, Royal RE, Kioi M, Nakajima A,
Husain SR and Puri RK: Interleukin-4 cytotoxin therapy synergizes
with gemcitabine in a mouse model of pancreatic ductal
adenocarcinoma. Cancer Res. 67:9903–9912. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Husain SR, Gill P, Kreitman RJ, Pastan I
and Puri RK: Interleukin-4 receptor expression on AIDS-associated
Kaposi's sarcoma cells and their targeting by a chimeric protein
comprised of circularly permuted interleukin-4 and Pseudomonas
exotoxin. Mol Med. 3:327–338. 1997.PubMed/NCBI
|
|
16
|
Kawakami K, Leland P and Puri RK:
Structure, function, and targeting of interleukin 4 receptors on
human head and neck cancer cells. Cancer Res. 60:2981–2987.
2000.PubMed/NCBI
|
|
17
|
Murata T, Taguchi J and Puri RK:
Interleukin-13 receptor alpha' but not alpha chain: A functional
component of interleukin-4 receptors. Blood. 91:3884–3891.
1998.PubMed/NCBI
|
|
18
|
Hosoyama T, Aslam MI, Abraham J, Prajapati
SI, Nishijo K, Michalek JE, Zarzabal LA, Nelon LD, Guttridge DC,
Rubin BP, et al: IL-4R drives dedifferentiation, mitogenesis, and
metastasis in rhabdomyosarcoma. Clin Cancer Res. 17:2757–2766.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kohno M, Horibe T, Haramoto M, Yano Y,
Ohara K, Nakajima O, Matsuzaki K and Kawakami K: A novel hybrid
peptide targeting EGFR-expressing cancers. Eur J Cancer.
47:773–783. 2011. View Article : Google Scholar
|
|
20
|
Garland L, Gitlitz B, Ebbinghaus S, Pan H,
de Haan H, Puri RK, Von Hoff D and Figlin R: Phase I trial of
intravenous IL-4 pseudomonas exotoxin protein (NBI-3001) in
patients with advanced solid tumors that express the IL-4 receptor.
J Immunother. 28:376–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoon YI, Kwon YS, Cho HS, Heo SH, Park KS,
Park SG, Lee SH, Hwang SI, Kim YI, Jae HJ, et al:
Ultrasound-mediated gene and drug delivery using a
microbubble-liposome particle system. Theranostics. 4:1133–1144.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sarangthem V, Cho EA, Bae SM, Singh TD,
Kim SJ, Kim S, Jeon WB, Lee BH and Park RW: Construction and
application of elastin like polypeptide containing IL-4 receptor
targeting peptide. PLoS One. 8:e818912013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Serwer LP and James CD: Challenges in drug
delivery to tumors of the central nervous system: An overview of
pharmacological and surgical considerations. Adv Drug Deliv Rev.
64:590–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao H and Jiang X: Progress on the
diagnosis and evaluation of brain tumors. Cancer Imaging.
13:466–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao H, Pang Z and Jiang X: Targeted
delivery of nano-therapeutics for major disorders of the central
nervous system. Pharm Res. 30:2485–2498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhan C, Wei X, Qian J, Feng L, Zhu J and
Lu W: Co-delivery of TRAIL gene enhances the anti-glioblastoma
effect of paclitaxel in vitro and in vivo. J Control Release.
160:630–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sarin H, Kanevsky AS, Wu H, Sousa AA,
Wilson CM, Aronova MA, Griffiths GL, Leapman RD and Vo HQ:
Physiologic upper limit of pore size in the blood-tumor barrier of
malignant solid tumors. J Transl Med. 7:512009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Groothuis DR: The blood-brain and
blood-tumor barriers: A review of strategies for increasing drug
delivery. Neuro Oncol. 2:45–59. 2000.
|
|
29
|
Papademetriou IT and Porter T: Promising
approaches to circumvent the blood-brain barrier: Progress,
pitfalls and clinical prospects in brain cancer. Ther Deliv.
6:989–1016. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei X, Gao J, Zhan C, Xie C, Chai Z, Ran
D, Ying M, Zheng P and Lu W: Liposome-based glioma targeted drug
delivery enabled by stable peptide ligands. J Control Release.
218:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|