Introduction
Endometrial cancer (EC) is one of the most common
cancers worldwide. In 2015, in the US alone, 54,870 women were
newly diagnosed with EC and 10,170 may die of this disease
(1). Hysterectomy with or without
adjuvant whole pelvic radiation is the standard treatment; however,
30% of EC cases are not diagnosed until regional or distant
metastasis has developed, resulting in a poorer outcome (2). Therefore, identification of additional
molecular mechanisms associated with tumorigenesis and progression
of EC, and the discovery of novel diagnostic and prognostic markers
for EC are critical for making informed treatment decisions.
Forkhead-box A1 (FOXA1) is a member of the FOX
family of transcription factors, which are involved in
carcinogenesis as oncogenes and/or tumor-suppressor genes (3). We have previously shown that FOXA1 is
a tumor-suppressor in EC, although the mechanism by which FOXA1
affects the progression of EC is incompletely understood (4).
Cell senescence is characterized by a series of
distinctive phenotypes and biomarkers, such as large and flattened
morphology, increased senescence-associated β-galactosidase
activity (SABG), accumulation of senescence-associated
heterochromatic foci (SAHF) and permanent G1 phase arrest (5). Cell senescence is the result of
telomere shortening and damage accumulation, which have both
anti-oncogenic and pro-oncogenic effects (6), and is a beneficial compensatory
response to damage, representing a potent tumor-suppressor
mechanism (7). In contrast, there
is also increasing evidence that the incidence of cancer increases
with age, indicating that the senescence of non-cancerous cells in
aging tissues may exert pro-carcinogenic effects.
p16INK4a is a tumor-suppressor protein
that functions as an inhibitor of cyclin-dependent kinase (CDK)4/6.
As a key regulator of G1 phase cell cycle arrest and senescence,
p16 on chromosome 9p21 is inactivated in a wide range of human
cancers (8,9). In view of the pivotal role of
p16INK4a in both tumor suppression and cell senescence,
numerous investigations have been conducted to elucidate the
genetic or epigenetic mechanisms regulating its activation in
senescence or its inactivation in carcinogenesis. It has been
reported that in senescence, FOXA1 binds to the promoter region of
p16, stimulates chromatin remodeling and enables long-range
promoter-enhancer communication to promote p16 expression (10).
Alterations in DNA methylation, chromatin structure,
histone modifications, and epigenetic regulatory mechanisms are
critical hallmarks of both senescence and cancer (7,11).
Given by the close association between senescence and tumors
(12), we focused on the role of
FOXA1 in EC in the present study. Our results provide further
support to the mounting evidence showing that FOXA1 is a favorable
prognostic marker in EC and mediates p16INK4a activation
to promote cell senescence.
Materials and methods
Patients and tissues
Tissues samples for immunohistochemistry (IHC) were
obtained from 61 patients with EC and 15 patients with normal
endometrium who underwent hysterectomy as treatment for other
diseases such as adenomyosis or myoma at the Shanghai First
Maternity and Infant Hospital, Tongji University School of Medicine
from 2013 to 2015. Primary fresh tissues were obtained from
patients immediately after surgical removal and were snap-frozen in
liquid nitrogen for storage prior to further use. The IHC protocol
was performed as previously described (4).
The present study was approved by the Human
Investigation Ethics Committee of Shanghai First Maternity and
Infant Hospital, Tongji University School of Medicine. The samples
of normal endometrial tissues and endometrial carcinoma were
collected after written informed consent was obtained from the
patients.
Laser capture microdissection (LCM)
Sections (thickness, 8-µm) were prepared from
the frozen endometrial tissues. Approximately 5,000 cells were
selected and isolated by laser capture using a light microscope
(LMD 7000; Leica, Solms, Germany). Total RNA was extracted
according to an RNA microisolation protocol previously described by
Niino et al (13). A reverse
transcription kit (Invitrogen, Carlsbad, CA, USA) was used for the
synthesis of cDNA.
Cell culture and transfections
Human EC cell lines HEC-1B and RL95-2 were obtained
from the Chinese Academy of Sciences Committee Type Culture
Collection (Shanghai, China). The cells were cultured in Dulbecco's
modified Eagle's medium/nutrient mixture F-12 (DMEM/F12)
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin (Gibco, Auckland, New Zealand) at 37°C in a
humidified atmosphere containing 5% CO2. HEC-1B and
RL95-2 cells stably expressing ectopic FOXA1 or FOXA1-specific
short hairpin RNA (shRNA) were generated as previously described
(4). Where relevant, the AKT
pathway agonist LY 294002 (10 µM; Beyotime, Shanghai, China)
was added to the cells 30 min prior to co-treatment with the shRNA
plasmid.
Treatment of cells
Cells (2×105) were cultured in 6-well
dishes. For hypoxia experiments, the cells were cultured in
vitro in a hypoxic (<0.1% O2) container system
(BD Diagnostics, Sparks, MD, USA). Protein and RNA were collected
immediately after cells were removed from the hypoxic container.
For energy restriction experiments, the cells were cultured in
serum-free medium for 72 h in a 5% CO2 humidified
incubator at 37°C.
Senescence-associated β-gal staining
(SABG)
Cells (1×105) were seeded in 6-well
dishes (≥triplicate wells). After treatment, cells were fixed with
0.2% glutaraldehyde + 2% formaldehyde in phosphate-buffered saline
(PBS) at room temperature for 15 min. After washing in PBS, the
cells were stained with a solution containing 1 mg/ml X-gal
(Beyotime) and incubated in a CO2-free incubator at 37°C
for 16 h. Green cells were counted and scanned with an Aperio
ScanScope system (Aperio Technologies, Vista, CA, USA). The
percentage of senescent cells was determined by counting cells in
three randomly selected fields of images.
Immunofluorescence analysis
Cells cultured in 48-well plates were washed in PBS
and fixed with 4% paraformaldehyde (Beyotime) for 15 min. The cells
were then incubated with PBS supplemented with 0.1% Triton X-100
for 10 min at room temperature, blocked in 5% normal goat serum for
30 min and washed in PBS. Nuclei were then stained with
4′,6-diamidino-2-phenylindole (DAPI) (1:10,000; Beyotime) to
visualize the SAHF. Three randomly selected fields were captured
under a fluorescence microscope (Leica 126 DMI 3000B; Leica) and a
total of 50 cells were counted in each sample to analyze the
percentage of positively stained cells.
Cell proliferation assay
Cells (3×103 cells/well) were plated into
96-well plates. The cells were counted at 24 h intervals using a
colorimetric assay with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma) performed as previously described (4). For the plate colony formation assay,
1,000 cells/well were seeded in 6-well plates. Following the
formation of clearly defined cell clones, the cells were fixed and
stained with crystal violet and counted under a light microscope.
Each experiment was repeated at least three times and assessed in
triplicate.
Migration and invasion assays
Cell migration activity was evaluated using Boyden
chambers containing polycarbonate filters (8-µm pore size;
Millipore, Billerica, MA, USA). Cell invasive activity was assessed
using a BD BioCoat Matrigel invasion chamber (BD Biosciences,
Franklin Lakes, NJ, USA) following the manufacturer's
recommendations. Cells were essentially treated as previously
described (4). Each experiment was
conducted, treated in triplicate and repeated at least three
times.
Quantitative RT-PCR
Total RNA from the HEC-1B and RL95-2 cells was
isolated using TRIzol (Invitrogen) and cDNA was prepared with the
PrimeScript RT reagent kit (Takara, Otsu, Japan). PCR amplification
of target genes was performed in a 50-µl reaction volume
using single-stranded cDNA as the template. The primer sequences
used in the present study are listed in Table I. The following protocol was used
for PCR amplifications: 40 cycles of denaturation (94°C) for 60
sec, annealing (55°C) for 30 sec and elongation (72°C) for 30 sec
using SYBR Premix Ex Taq (Takara). All data were obtained in
triplicate from three independent experiments and analyzed using
the 2−ΔΔCt method.
 | Table IPrimer sequences for real-time PCR
analysis. |
Table I
Primer sequences for real-time PCR
analysis.
| mRNA | | Primer
sequence |
|---|
| FOXA1 | F |
5′-AGGTGTGTATTCCAGACCCG-3′ |
| R |
5′-TTGACGGTTTGGTTTGTGTG-3′ |
|
p16INK4a | F |
5′-GAAGGTCCCTCAGACATCCCC-3′ |
| R |
5′-CCCTGTAGGACCTTCGGTGAC-3′ |
|
p14ARF | F |
5′-CCCTCGTGCTGATGCTACTG-3′ |
| R |
5′-ACCTGGTCTTCTAGGAAGCGG-3′ |
|
p15INK4b | F |
5′-CATTCCATGGATGCACAAAG-3′ |
| R |
5′-GCAATGGGAAGAAAAGCAAG-3′ |
|
p18INK4c | F |
5′-GGACCCAGGACTATCCCTTC-3′ |
| R |
5′-TTTAGGGTCCCTTGTTCACG-3′ |
|
p19INK4d | F |
5′-CCAAGGGCAGAGCATTTAAG-3′ |
| R |
5′-AAGCAACGTGCACACTTCAG-3′ |
| p53 | F |
5′-ACCACCATCCACTACAACTACAT-3′ |
| R |
5′-CACAAACACGCACCTCAAA-3′ |
| PTEN | F |
5′-TCACCAACTGAAGTGGCTAA AGA-3′ |
| R |
5′-CTCCATTCCCCTAACCCGA-3′ |
| ACTB | F |
5′-CAGCCATGTACGTTGCTATCCAGG-3′ |
| R |
5′-AGGTCCAGACGCAGGATGGCATG-3′ |
Western blot analysis and antibodies
Western blot assays were performed as previously
described (4). The following
antibodies were used in the present study: anti-FOXA1 (1:1,000;
Abcam, Cambridge, MA, USA), anti-p16INK4a (1:2,000),
anti-β-actin (1:75,00) (both from Epitomics, Burlingame, CA, USA),
anti-AKT and anti-phospho-AKT (1:2,000; Cell Signaling Technology,
Danvers, MA, USA).
Statistical analysis
Each experiment was repeated for at least three
independent occasions. All statistical analysis was performed with
the Statistical Package for the Social Science (SPSS) software
version 17.0 (SPSS, Inc., Chicago, IL, USA). Data represent the
mean ± standard deviation (SD). Data were compared using two-tailed
Student's t-test or Mann-Whitney U test for multiple comparisons.
Differences with a probability of P<0.05 were regarded as
statistically significant.
Results
FOXA1 overexpression is associated with
good prognosis in EC
In our previous study, we found that FOXA1 was
significantly downregulated in EC tissues compared to that noted in
normal endometrium (4). To further
assess the role of FOXA1 in EC, we compared FOXA1 expression with
prognostic factors in normal endometrium and EC tissues using
initial reverse transcription-quantitative PCR (RT-qPCR) and IHC
analyses. RT-qPCR analysis of normal endometrium and EC cells
captured by LCM revealed that FOXA1 mRNA levels were higher in the
normal endometrium than levels in the EC tissues (P<0.001;
Fig. 1A), and indicated that there
were significant differences in FOXA1 expression among tumor grades
(P<0.001; Fig. 1B). Furthermore,
the IHC results showed that FOXA1 was negatively associated with
the depth of myometrial invasion (P<0.001; Fig. 1C) and lymph node metastasis of EC
(P<0.01; Fig. 1D), which is
consistent with the results previously described by Abe et
al (14). The pattern of FOXA1
expression was correlated with ERα expression (P<0.001; Fig. 1E), but showed no significant
correlation with the expression of PR and p53 (P>0.05; Fig. 1F and G). These data suggest that
FOXA1 expression is correlated with EC and the prognostic ability
of FOXA1 in low-risk EC may be useful in making clinical treatment
decisions.
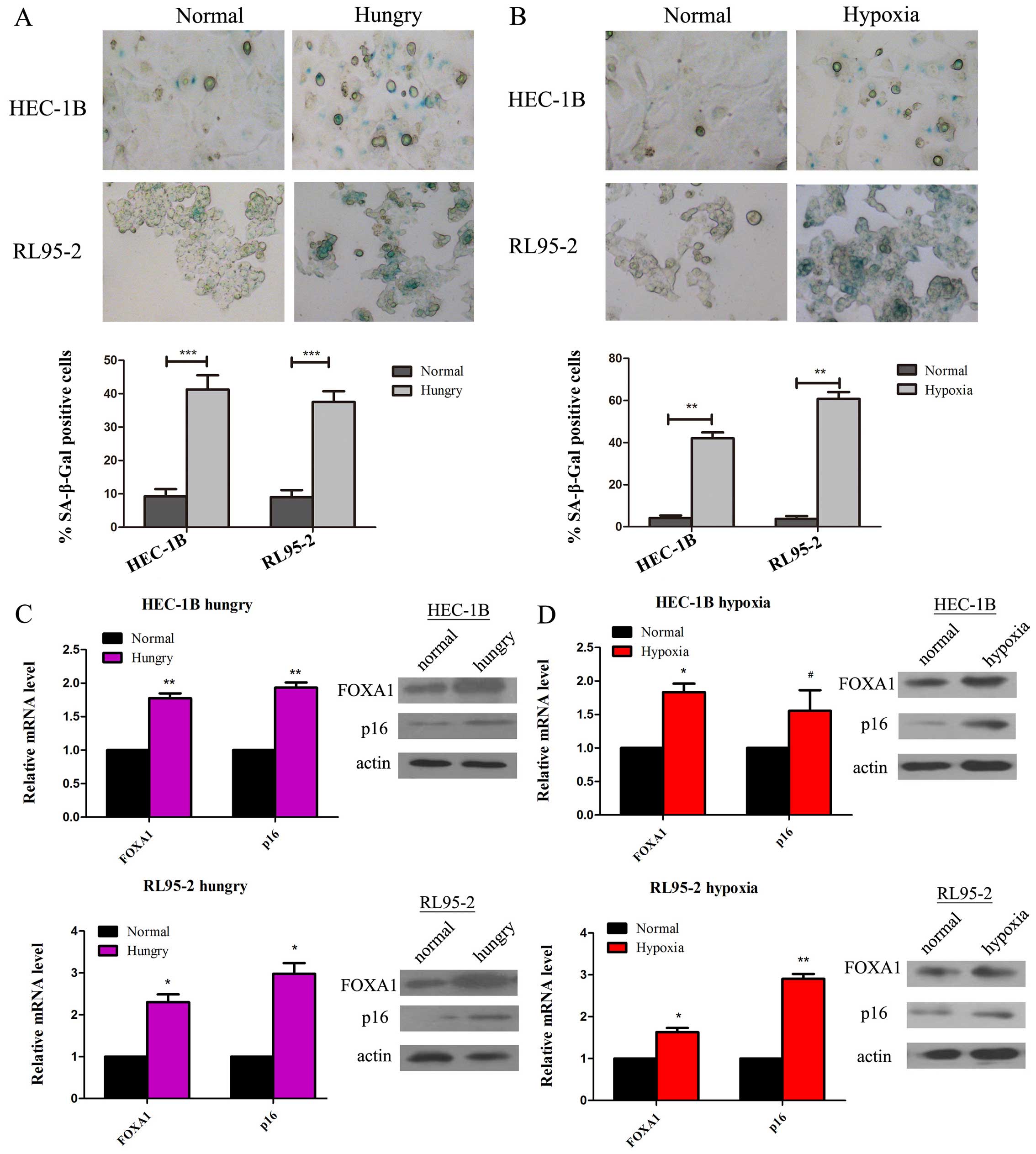
FOXA1 is upregulated in senescent EC
cells
Previously, we demonstrated that FOXA1 suppresses
the progression of EC and evidence is emerging that inhibition of
FOXA1 is essential for EC cell growth and survival. We hypothesized
that FOXA1 contributes to the activation of tumor suppressor
networks in senescence. EC HEC-1B and RL95-2 cell lines were
subjected to conditions of hypoxia or energy restriction (hunger)
to promote cell senescence (7).
Cells were then stained for β-galactosidase activity, a widely used
biomarker for senescent cells (P<0.01; Fig. 2A) (P<0.001; Fig. 2B). Compared to the untreated group,
robust induction of FOXA1 was observed in the senescent EC cells at
both the mRNA and protein levels, and was correlated with increased
p16INK4a expression (P<0.05; Fig. 2C) (P<0.05; Fig. 2D).
FOXA1 triggers multiple steps of cellular
senescence in EC cells and activates p16INK4a
expression
Several lines of evidence have linked FOXA1 with
cell senescence. To further characterize the functional role of
FOXA1 in EC cells, we restored FOXA1 expression in HEC-1B cells
(Fig. 3A). MTT and plate colony
formation assays revealed that HEC-1B cells transiently transfected
with a FOXA1 expression plasmid showed significant growth
inhibition and limited colony formation compared with the cells
transfected with the negative control vector (P<0.001; Fig. 3A). Furthermore, restoring FOXA1
expression induced morphological features of senescence, including
accumulation of granular cytoplasmic inclusions and elevated
β-galactosidase activity.
As a comparison with the effects of FOXA1
overexpression, we transiently transfected RL95-2 cells with a
vector encoding a short hairpin RNA (shRNA) against FOXA1 or its
corresponding negative control (Fig.
3B). FOXA1 depletion promoted a statistically significant
induction of cell proliferation and the cells transfected with
shFOXA1 formed more colonies than those transfected with the
control vector (P<0.01; Fig.
3B). All of these results suggest that FOXA1 triggers multiple
steps of cellular senescence in EC cells.
There are several senescence-associated genes and
key cell cycle regulators in senescence networks. We, therefore,
investigated the association of the restoration of FOXA1 expression
in HEC-1B cells with these key genes. Expression of
p16INK4a and p18INK4c was increased
(P<0.05; Fig. 3C) while
expression of the other genes such as phosphate and tensin homology
(PTEN), p53 or p27kip1 showed no marked changes. These
observations provided further evidence of the association of FOXA1
with senescence in EC.
FOXA1 promotes senescence in EC cells via
the AKT pathway
A recent study showed that inhibition of AKT results
in increased cellular senescence in uterine leiomyoma cells,
accompanied by increased levels of several senescence-associated
genes (including p16, p53 and p21) (15). These findings raised the possibility
that FOXA1-induced senescence is associated with the AKT pathway in
EC. To test this hypothesis, HEC-1B cells were treated with
different concentrations of LY 294002, a selective PI3K inhibitor,
which inhibits cell proliferation and induces apoptosis (16,17).
Western blot analysis of the protein levels of p(Ser473)-AKT and
AKT revealed almost complete inhibition of p-AKT expression in
cells treated with LY 294002 at 1, 10 and 20 µM, with
optimal inhibition achieved at 10 µM (P<0.001; Fig. 4A). SABG and SAHF assays were
performed to investigate whether inhibition of the AKT pathway
induces senescence in EC. Treatment with LY 294002 (10 µM)
resulted in a significant increase in β-galactosidase stained cells
(P<0.001; Fig. 4B). Furthermore,
~40% of cells exhibited SAHF when treated with LY 294002 compared
to 15% in the negative control group (P<0.001; Fig. 4C). Additionally, following treatment
with LY 294002, expression of the senescence-associated genes
p16INK4a and p27kip1 was markedly increased,
while PTEN gene expression was decreased (P<0.05; Fig. 4D). All of these results demonstrated
that inhibition of AKT in EC cells induced senescence.
Next, we examined the relationship between FOXA1 and
the AKT pathway. We found that in HEC-1B cells, FOXA1
overexpression abolished the p-AKT expression at the protein level
(Fig. 4E) and in RL95-2 cells,
decreased FOXA1 expression was correlated with increased levels of
p-AKT (Fig. 4E), with no obvious
changes in AKT levels. All of these results indicated a reverse
role for FOXA1 in mediating the AKT pathway in EC.
Discussion
Endometrial cancer (EC) is the most prevalent
malignancy in women, although the mechanism of EC tumorigenesis and
metastasis remain to be clarified. In our previous study, we found
that FOXA1 suppressed the progression of estrogen receptor
(ER)-positive EC, which was consistent with other studies (14,18).
We also found that FOXA1 promoted cell proliferation via the
androgen receptor (AR) and activated Notch pathway in ER-negative,
AR-positive EC (19). Thus, FOXA1
is a bifunctional cancer-associated gene, which is oncogenic or
tumor suppressive in a context-dependent manner. However, in the
present study, our data support the hypothesis that FOXA1 is
associated with senescence in EC, playing a role in addition to its
function as a pioneer factor.
Our findings showed that FOXA1 is downregulated in
EC compared to normal endometrium and low-FOXA1 expression is
significantly associated with markers of a poorer outcome as well
as poor survival. The positive correlation between FOXA1 and
p16INK4a was observed in EC tissues, which is consistent
with the recent studies that positive regulation of
p16INK4a by FOXA1 counteracts its tumorigenic repression
of by EZH2 in cancers (20). This
indicated that FOXA1 may have a role in EC that is not linked to
ERα, and that the function of FOXA1 could be organ specific. In
accordance with our results, recent findings in EC have revealed
that gene expression related to FOXA1 levels do not overlap with
the pattern of ERα expression (18).
In the present study, we initially found that FOXA1
was upregulated in senescent EC cell lines, which was correlated
with increased p16INK4a expression. FOXA1 can mimic
linker histones and bind directly to compacted chromatin (21), functioning as a pivotal regulator of
chromatin remodeling of the p16INK4a promoter, which is
the key senescence-associated gene (10,20).
To understand the interplay of chromatin, senescence and EC,
further studies are required to elucidate the functional and causal
roles of senescence-associated chromatin features in preventing
tumor phenotype and to delineate the underlying mechanisms. Our
data support the hypothesis that FOXA1 is a transcription factor
that is induced during senescence in EC. Li et al showed
that FOXA1 was significantly upregulated in senescent human 2BS
diploid fibroblast cells (10);
therefore, we proposed that FOXA1 is also associated with
senescence in EC, the functional role of FOXA1 in tumor progression
should not be restricted to its ̔pioneering̓ role associated with
hormone receptors.
Since cancer and senescence may share certain
molecular processes (22), it is
plausible that FOXA1 prevented EC by acting on the senescence
process. In the present study, further phenotypic and biochemical
investigations showed that inhibition of FOXA1 resulted in
decreased growth of EC cells and promoted cell senescence. Cell
senescence can be defined as a stable arrest of the cell cycle
coupled to stereotypical phenotypic change (5). On this basis, we proposed that the
accumulation of senescent cells in EC tissue suppresses the
progression of cancer. In accordance with previous reports that
p16INK4a expression is upregulated in endometrial polyps
compared to endometrial hyperplasia due to the p16-induced cellr
senescence (23), our functional
investigations indicated that p16INK4a acts downstream
of FOXA1 to regulate carcinogenesis. Recent studies also suggest
that p16INK4a is one of the most differentially
expressed genes associated with FOXA1 protein expression in
metastatic EC lesions (18). Thus,
we speculated that cell senescence is the barrier to EC, and is
regulated by FOXA1-induced p16INK4a expression.
It has been shown in uterine leiomyomas that AKT
inhibition results in a transient regulation of specific mechanisms
that ultimately drive cells into cellular senescence or death
(15). In the present study, we
have demonstrated that upregulation of FOXA1 blocks AKT pathway
activation and is accompanied by changes in the expression of
several key senescence-associated genes (p16, p27 and PTEN) in EC
cells. Although our knowledge of specific mechanism of cell
senescence in EC reported is limited, AKT has been shown to protect
against RAS-induced senescence in other cell types (24), and several classical transcription
factors related to senescence have been identified, including p16,
p21 and p53 (25–27). Based on our findings, we suggest
that FOXA1 promotes cell senescence in EC by interaction with
p16INK4a, possibly via the AKT pathway, which may be a
newly identified regulatory mechanism of cell senescence in EC.
Despite our understanding of FOXA1-mediated
p16INK4a activation in senescence, the deregulation of
the FOXA1/p16INK4a axis in EC cancer biology remains to
be clarified. FOXA1 is downregulated in numerous cancer tissues
compared to their normal counterparts (28–30)
and a positive correlation between FOXA1 and p16INK4a is
observed in breast cancers with lower EZH2 (epigenetic repressor
for p16INK4a) (20).
However, further studies are required to determine whether the
downregulation of FOXA1 expression is associated with epigenetic
inactivation. BRCA1 has been proposed to maintain FOXA1 expression
in breast cancer by suppressing FOXA1 gene methylation (31), although little is known regarding
the methylation status of the FOXA1 promoter in EC. This
information may further our understanding of the mechanisms of
EC.
In summary, the results of the present study
indicate that FOXA1 expression may be a characteristic of EC and
constitute a useful prognostic marker. FOXA1 is upregulated in
senescent EC cells and mediates p16INK4a activation to
promote cell senescence.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81272885), the Foundation
Project of Shanghai Science and Technology Commission (no.
15ZR1433400 and no. 16ZR1427100) and the Young Talents Training
Programs Foundation of Tongji University (no. 1400811).
Abbreviations:
|
EC
|
endometrial cancer
|
|
FOXA1
|
forkhead-box A1
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan X, Wang LU, Xue J, Li LI and Zhang J:
Endocrine MPA enhances the effects of TAC chemotherapy on
improvement of prognosis and increase in long-term survival rates
for patients with endometrial cancer. Oncol Lett. 10:1902–1906.
2015.PubMed/NCBI
|
|
3
|
Bernardo GM and Keri RA: FOXA1: A
transcription factor with parallel functions in development and
cancer. Biosci Rep. 32:113–130. 2012. View Article : Google Scholar
|
|
4
|
Wang J, Bao W, Qiu M, Liao Y, Che Q, Yang
T, He X, Qiu H and Wan X: Forkhead-box A1 suppresses the
progression of endometrial cancer via crosstalk with estrogen
receptor α. Oncol Rep. 31:1225–1234. 2014.PubMed/NCBI
|
|
5
|
Kuilman T, Michaloglou C, Mooi WJ and
Peeper DS: The essence of senescence. Genes Dev. 24:2463–2479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Falandry C, Bonnefoy M, Freyer G and
Gilson E: Biology of cancer and aging: A complex association with
cellular senescence. J Clin Oncol. 32:2604–2610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
López-Otín C, Blasco MA, Partridge L,
Serrano M and Kroemer G: The hallmarks of aging. Cell.
153:1194–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kool J, Uren AG, Martins CP, Sie D, de
Ridder J, Turner G, van Uitert M, Matentzoglu K, Lagcher W,
Krimpenfort P, et al: Insertional mutagenesis in mice deficient for
p15Ink4b, p16Ink4a, p21Cip1, and
p27Kip1 reveals cancer gene interactions and
correlations with tumor phenotypes. Cancer Res. 70:520–531. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McLaughlin-Drubin ME, Park D and Munger K:
Tumor suppressor p16INK4A is necessary for survival of
cervical carcinoma cell lines. Proc Natl Acad Sci USA.
110:16175–16180. 2013. View Article : Google Scholar
|
|
10
|
Li Q, Zhang Y, Fu J, Han L, Xue L, Lv C,
Wang P, Li G and Tong T: FOXA1 mediates p16INK4a
activation during cellular senescence. EMBO J. 32:858–873. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Collado M, Blasco MA and Serrano M:
Cellular senescence in cancer and aging. Cell. 130:223–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niino YS, Irie T, Takaishi M, Hosono T,
Huh N, Tachikawa T and Kuroki T: PKCtheta II, a new isoform of
protein kinase C specifically expressed in the seminiferous tubules
of mouse testis. J Biol Chem. 276:36711–36717. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abe Y, Ijichi N, Ikeda K, Kayano H,
Horie-Inoue K, Takeda S and Inoue S: Forkhead box transcription
factor, forkhead box A1, shows negative association with lymph node
status in endometrial cancer, and represses cell proliferation and
migration of endometrial cancer cells. Cancer Sci. 103:806–812.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu X, Lu Z, Qiang W, Vidimar V, Kong B,
Kim JJ and Wei JJ: Inactivation of AKT induces cellular senescence
in uterine leiomyoma. Endocrinology. 155:1510–1519. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaussade C, Rewcastle GW, Kendall JD,
Denny WA, Cho K, Grønning LM, Chong ML, Anagnostou SH, Jackson SP,
Daniele N, et al: Evidence for functional redundancy of class IA
PI3K isoforms in insulin signalling. Biochem J. 404:449–458. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Semba S, Itoh N, Ito M, Harada M and
Yamakawa M: The in vitro and in vivo effects of
2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific
inhibitor of phosphatidylinositol 3′-kinase, in human colon cancer
cells. Clin Cancer Res. 8:1957–1963. 2002.PubMed/NCBI
|
|
18
|
Tangen IL, Krakstad C, Halle MK, Werner
HM, Oyan AM, Kusonmano K, Petersen K, Kalland KH, Akslen LA, Trovik
J, et al: Switch in FOXA1 status associates with endometrial cancer
progression. PLoS One. 9:e980692014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiu M, Bao W, Wang J, Yang T, He X, Liao Y
and Wan X: FOXA1 promotes tumor cell proliferation through AR
involving the Notch pathway in endometrial cancer. BMC Cancer.
14:782014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y and Tong T: FOXA1 antagonizes
EZH2-mediated CDKN2A repression in carcinogenesis. Biochem Biophys
Res Commun. 453:172–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hirai H, Tani T and Kikyo N: Structure and
functions of powerful transactivators: VP16, MyoD and FoxA. Int J
Dev Biol. 54:1589–1596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Serrano M: Unraveling the links between
cancer and aging. Carcinogenesis. 37:1072016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moritani S, Ichihara S, Hasegawa M,
Iwakoshi A, Murakami S, Sato T, Okamoto T, Mori Y, Kuhara H and
Silverberg SG: Stromal p16 expression differentiates endometrial
polyp from endometrial hyperplasia. Virchows Arch. 461:141–148.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kennedy AL, Morton JP, Manoharan I, Nelson
DM, Jamieson NB, Pawlikowski JS, McBryan T, Doyle B, McKay C, Oien
KA, et al: Activation of the PIK3CA/AKT pathway suppresses
senescence induced by an activated RAS oncogene to promote
tumorigenesis. Mol Cell. 42:36–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goswami A, Shah BA, Kumar A, Rizvi MA,
Kumar S, Bhushan S, Malik FA, Batra N, Joshi A and Singh J:
Antiproliferative potential of a novel parthenin analog P16 as
evident by apoptosis accompanied by down-regulation of PI3K/AKT and
ERK pathways in human acute lymphoblastic leukemia MOLT-4 cells.
Chem Biol Interact. 222C:60–67. 2014. View Article : Google Scholar
|
|
26
|
Kipkeew F, Kirsch M, Klein D, Wuelling M,
Winterhager E and Gellhaus A: CCN1 (CYR61) and CCN3 (NOV) signaling
drives human trophoblast cells into senescence and stimulates
migration properties. Cell Adh Migr. 10:163–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu J, Chen C, Hu X, Cai X, Guan Y, Hu H,
Wang Q, Chen X, Cai B and Jing X: Suppressing cyclooxygenase-2
prevents nonalcoholic steatohepatitis and inhibits apoptosis of
hepatocytes that are involved in the Akt/p53 signal pathway.
Biochem Biophys Res Commun. 469:1034–1040. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren H, Zhang P, Tang Y, Wu M and Zhang W:
Forkhead box protein A1 is a prognostic predictor and promotes
tumor growth of gastric cancer. Onco Targets Ther. 8:3029–3039.
2015.PubMed/NCBI
|
|
29
|
Song Y, Washington MK and Crawford HC:
Loss of FOXA1/2 is essential for the epithelial-to-mesenchymal
transition in pancreatic cancer. Cancer Res. 70:2115–2125. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tachi K, Shiraishi A, Bando H, Yamashita
T, Tsuboi I, Kato T, Hara H and Ohneda O: FOXA1 expression affects
the proliferation activity of luminal breast cancer stem cell
populations. Cancer Sci. 107:281–289. 2016. View Article : Google Scholar :
|
|
31
|
Gong C, Fujino K, Monteiro LJ, Gomes AR,
Drost R, Davidson-Smith H, Takeda S, Khoo US, Jonkers J, Sproul D,
et al: FOXA1 repression is associated with loss of BRCA1 and
increased promoter methylation and chromatin silencing in breast
cancer. Oncogene. 34:5012–5024. 2015. View Article : Google Scholar :
|