Introduction
Most tumor cells have an unlimited replicative
potential, mainly due to the presence of the active telomerase
holoenzyme, showing it as an attractive target for cancer
therapies. Telomerase is a ribonucleoprotein complex that adds
telomeric sequences at the end of the chromosome, maintaining
telomere length in tumor cells (1).
The human holoenzyme telomerase is a ribonucleoprotein composed by
a catalytic subunit, (hTERT) and an RNA component (hTR) which acts
as a template for the addition of a short repetitive sequence
d(TTAGGG)n in the 3′ end of the telomeric DNA and species-specific
accessory proteins (2). Recently,
it has been demonstrated that dyskerin binds to hTR, giving it the
right conformation to ensemble with hTERT, being essential for the
conformation of an active enzyme. Additionally N0P10, NHP2 and GAR1
proteins provide greater stability to the complex while pontin and
reptin are two closed ATPases necessary for the stability of
dyskerin and hTR in vivo (3).
3′-Azido-2′,3′-dideoxythymidine [azidothymidine
(AZT) or zidovudine] is a thymidine analogue used in the treatment
of AIDS since it competitively inhibits reverse transcriptase (RT).
The main component of telomerase, hTERT, is a catalytically active
RT structurally similar to HIV-1 RT (4). Such similarity prompted studies on the
feasibility of telomerase inhibition with a known inhibitor of
viral RT such as AZT. In our laboratory, it was demonstrated for
the first time that AZT is incorporated preferentially in the
telomeric DNA of CHO cells (5). Few
years later, we demonstrated the inhibitory action of AZT on
telomere dynamics (6), and later we
found that the cells of an experimental mammary carcinoma (F3II)
treated for prolonged periods with no cytotoxic doses of AZT enter
into senescence and programmed cell death (7).
Besides its role in telomere maintenance (canonical
function), TERT has additional functions. They are related, with
regulation of cell behavior, but in a telomeric-independent manner
(non-canonical). For instance, TERT can function as a
transcriptional modulator of the signaling pathway Wnt/β-catenin
(8,9). In this manner, TERT acts as a cofactor
in a transcriptional complex of β-catenin through interactions with
a chromatin remodelling protein, allowing regulation of target
genes of this pathway (10). Good
examples are cyclin-D1 (Cyc-D1), which plays a key role in cell
cycle progression (11) and c-Myc
with a direct link to cell growth, differentiation and apoptosis
(12).
In order to increase insight into the mechanism of
action of AZT as an anticancer drug, the present study analyzed the
possible effects of AZT treatment on the transcription of genes
involved in the signaling pathway Wnt/β-catenin (TERT, cMyc and
Cyc-D1) its effect on telomerase activity, cytoskeleton structure,
tumor cell migration and cell cycle in a murine mammary
adenocarcinoma cell model (F3II).
Materials and methods
Cell line and culture conditions
The mammary carcinoma cell line F3II is a highly
aggressive and metastatic variant, established from a clonal
subpopulation of a spontaneous hormone-independent BALB/c mouse
mammary tumor (13). Cells were
grown in Dulbecco's modified Eagle's medium (DMEM) (Life
Technologies) supplemented with 10% heat-inactivated fetal bovine
serum (FBS), 2 mM glutamine and 80 µg/ml gentamicin at 37°C
in 5% CO2 atmosphere. Cell cultures were routinely
subculture by trypsinization using standard procedures.
Cell proliferation assay
Cells (1×104) were plated in 96-well
plates, and then treated with different concentration of AZT
(50–2,000 µM) for 72 h. Cell growth was measured by
colorimetric MTT assay (Sigma). The concentration producing 50%
inhibition (IC50) was determined by non-linear
regression function of GraphPad Prism 6®. Results shown
correspond to the average of three individual experiments.
Furthermore, in order to demonstrate that AZT was not toxic at this
dose, another control with AZT in parallel with the untreated
control cells was added on the experiments on doubling time,
telomerase activity and cell migration.
RNA extraction and cDNA synthesis
For each treatment, total RNA was isolated by
extraction with TRIzol reagent (Life Technologies) according to the
manufacturer's specifications. cDNA synthesis was performed using
SuperScript III First-Strand Synthesis System (Life Technologies)
from ~1 µg of total RNA with oligonucleotide dT18 (Life
Technologies) according to the manufacturer's instructions. All
cDNA samples were normalized by quantification at 260 nm in
NanoDrop 1000 (Thermo).
Analysis of Cyc-D1, c-Myc and TERT
transcription by real-time PCR
Specific primers were designed for each target using
the Primer Express® Software Version 3.0 (Life
Technologies). All primers designed presented efficiency values
between 90 and 100%, and suitable dynamic ranges, allowing its
proper use. The assays were carried out on One-Step Real-Time PCR
System (Life Technologies) using SYBR-Green detection reagent (Life
Technologies). β-actin transcript was used as a loading control.
Once optimized all the parameters for RT-qPCR, the analysis of
transcriptional expression was carried out by the ΔΔCt method.
Determination of telomerase activity
Telomerase activity was determined by TRAP assay,
using RT-qPCR method with SYBR-Green (StepOne™ System equipment).
The growing tumor cells were harvested and washed once with
phosphate-buffered saline (PBS). Cells (2×106) were
transferred to 1.5 ml conical tubes and centrifuged for 8 min at
450 × g. The pellet was lysed with 200 µl of buffer CHAPS
(RNaseOUT + Protease Inhibitor), quantified and stored at −20°C
until use.
Actin staining
Control and AZT-treated cells were grown in glass
coverslips in serum-free DMEM. Cells were fixed in 4% formaldehyde
in PBS and stained with Alexa Fluor 555-conjugated phalloidin
(Molecular Probes, Life Technologies) following the manufacturer's
instructions. Images were recorded by an inverted fluorescence
microscope (Nikon Eclipse T2000). Alternatively, a quantitative
analysis in which the intensity of emitted fluorescence of the same
reagent was determined by measurement in a fluorometer Synergy HT
(BioTek).
Cell migration
Cell migration was measured using an in vitro
wound healing assay as previously described (14). Briefly, in vitro 'scratch'
wounds were created by scraping confluent F3II (controls and 5, 10
and 15 AZT passages) monolayers with a sterile pipette tip. After
16 h incubation in DMEM with 10% FBS in the presence or absence of
AZT, cells were fixed and stained. Ten random micrographs/well were
obtained and migration area was quantified using NIS-Elements 3.0
(Nikon) software.
Cell cycle
For cell cycle analysis by flow cytometry, cells
were washed and incubated in serum-free DMEM for synchronization
for 24 h. Cells, both control and treated with AZT 600 µM
for passages 5, 10 and 15 were cultured, trypsinized and
centrifuged at 450 × g. Cells were fixed in 70% methanol in PBS and
stained with propidium bromide (1 mg/ml) (Life Technologies). Cell
cycle progression was analyzed in a BD FACSCalibur™ (BD
Biosciences) flow cytometer. Before recording 10,000 events, the
verification of the doublet discrimination function of the flow
cytometer was performed using DNA QC Particles kit (BD
Biosciences).
Doubling time assay
Cells (1,5×104) treated with AZT 600
µM for 5, 10 and 15 passages were plated in 12-well plates.
After incubation with DMEM 10% FBS and AZT treatment for 24, 48, 72
and 96 h, each plate was stained and fixed by 0.5% violet crystal
20% methanol, resuspended in 500 µl of ethanol: acetic acid
(3:1) and measured at 570 nm. A growth curve was built, where the
100% was defined as the 24 h value for each condition.
Statistical analysis
All data are presented as the mean ± SD. Significant
differences were determined by one-way ANOVA with Dunnett contrast,
and two-way ANOVA with a Bonferroni's multiple comparisons test.
The analyses were performed by GraphPad Prism 6 software (GraphPad
Software, La Jolla, CA, USA). The criterion for a statistical
significance is p<0.05.
Results
Effect of AZT on cell viability
In order to confirm the effect of AZT on cell
viability, we determined the IC50 of this molecule for
F3II cell line, obtaining a value of 1,195±37 µM. Regarding
the data, we determined 600 µM as the treatment dose,
equivalent to an IC25 value.
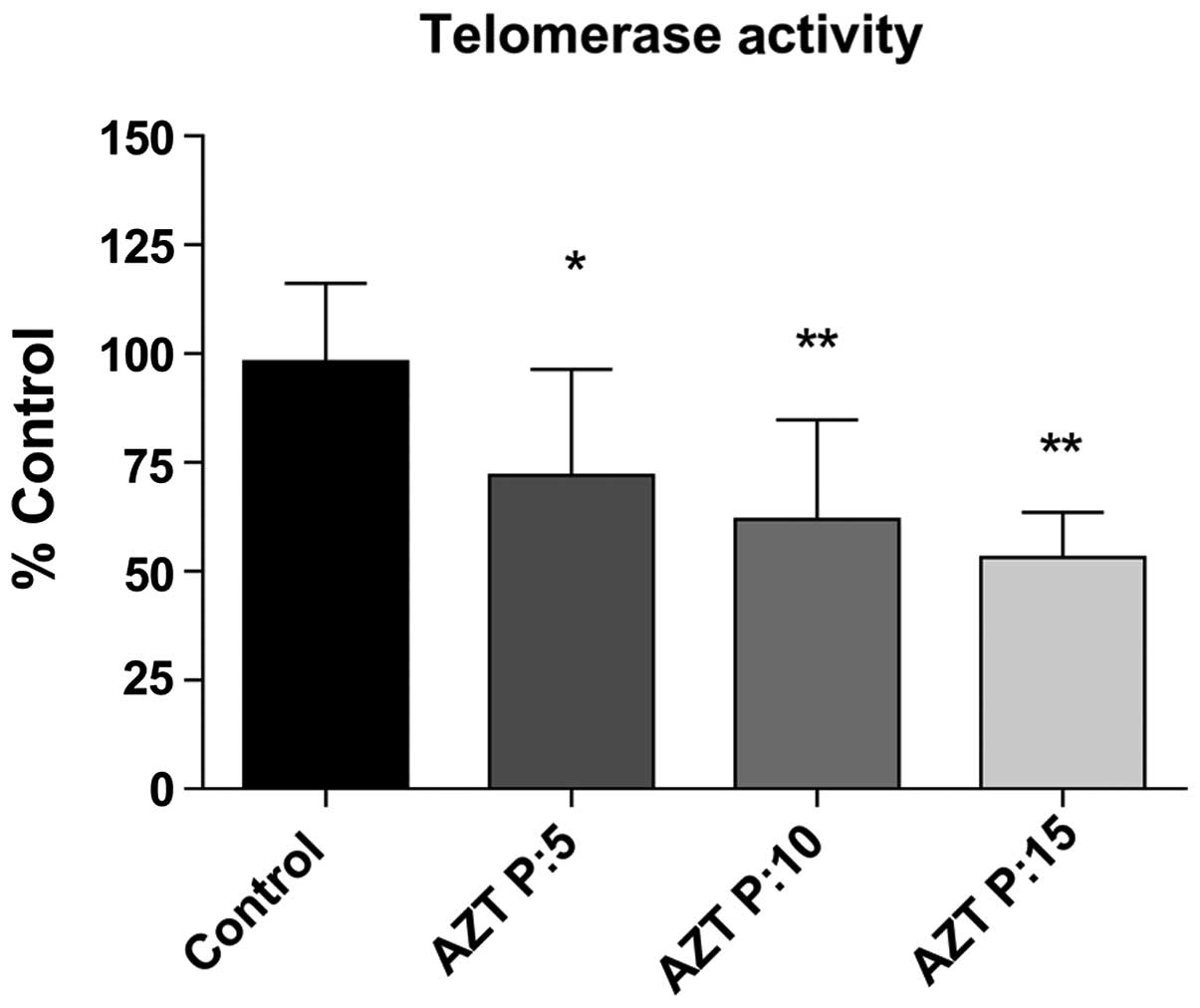
Determination of telomerase activity
Telomerase activity significantly decreased and was
evaluated progressively during the period. Telomerase activity
dropped 26.22% (p<0.05), 36.27% (p<0.01) and 45.08%
(p<0.001) after 5, 10 and 15 passages with AZT, respectively
(Fig. 1). In this manner,
corroborating the inhibitory function of AZT on telomerase
activity.
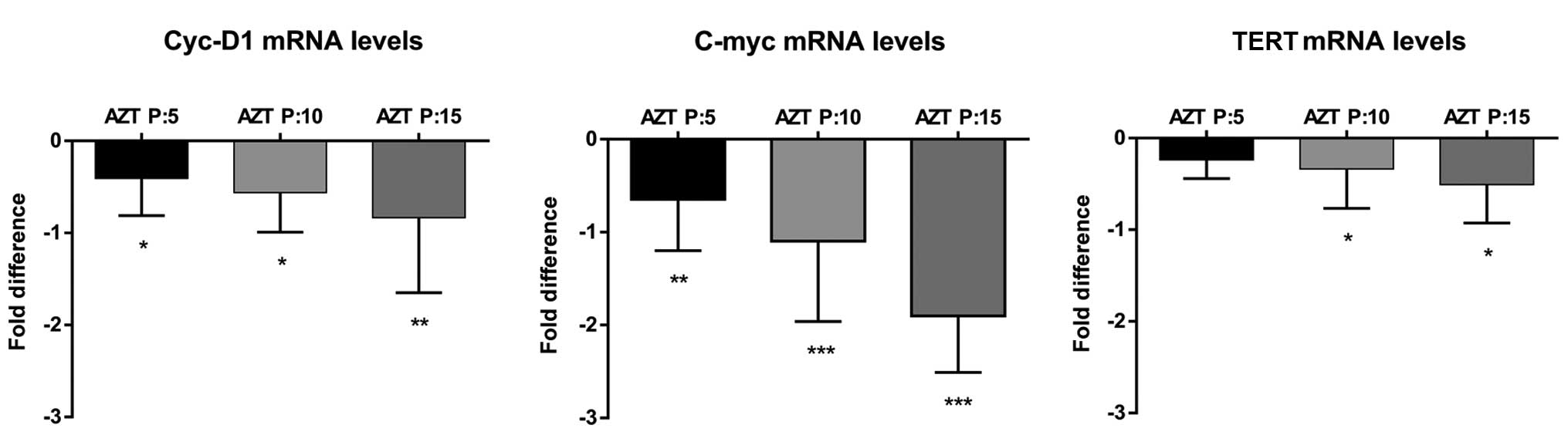
Analysis of TERT, c-Myc and Cyc-D1
transcription
We quantified relative expression by ΔΔCt method of
the expression of TERT, Cyc-D1 and c-Myc genes, where β-actin gene
was used as endogenous loading control.
We determined the relative expression of TERT and
confirmed its expression was influenced by the treatment, in a
passage-dependent manner. After 5 passages TERT expression
decreased by 17.1% (p<0.05), at 10 passages, expression reduced
by 20.28% (p<0.05) and at the end of treatment had declined by
30.91% (p<0.05) (Fig. 2C).
Regarding the expression of Cyc-D1 in cells treated
with AZT, we found a passage-dependent decrease. After 5, 10 and 15
passages, we observed a decline of 24.52% (p<0.05), 30.87%
(p<0.05) and 38.24% (p<0.01), respectively (Fig. 2A).
Analyzing c-Myc we found a more pronounced decline,
reaching 34.14% (p<0.01), 43.85% (p<0.001) and 64.2%
(p<0.01) of inhibition at 5, 10 and 15 treated passages,
respectively (Fig. 2B).
Anti-migratory effect of AZT
Due to the changes in the transcription of c-Myc, we
expected an effect on cell migration. Using the wound assay, we
analyzed this effect on cells treated with 600 µM AZT for 5,
10 and 15 passages. After 5 passages significant effects on cell
migration were not seen. However, after 10 and 15 passages, the
invaded area decreased 31% (p<0.01) and 47.79% (p<0.01)
(Fig. 3).
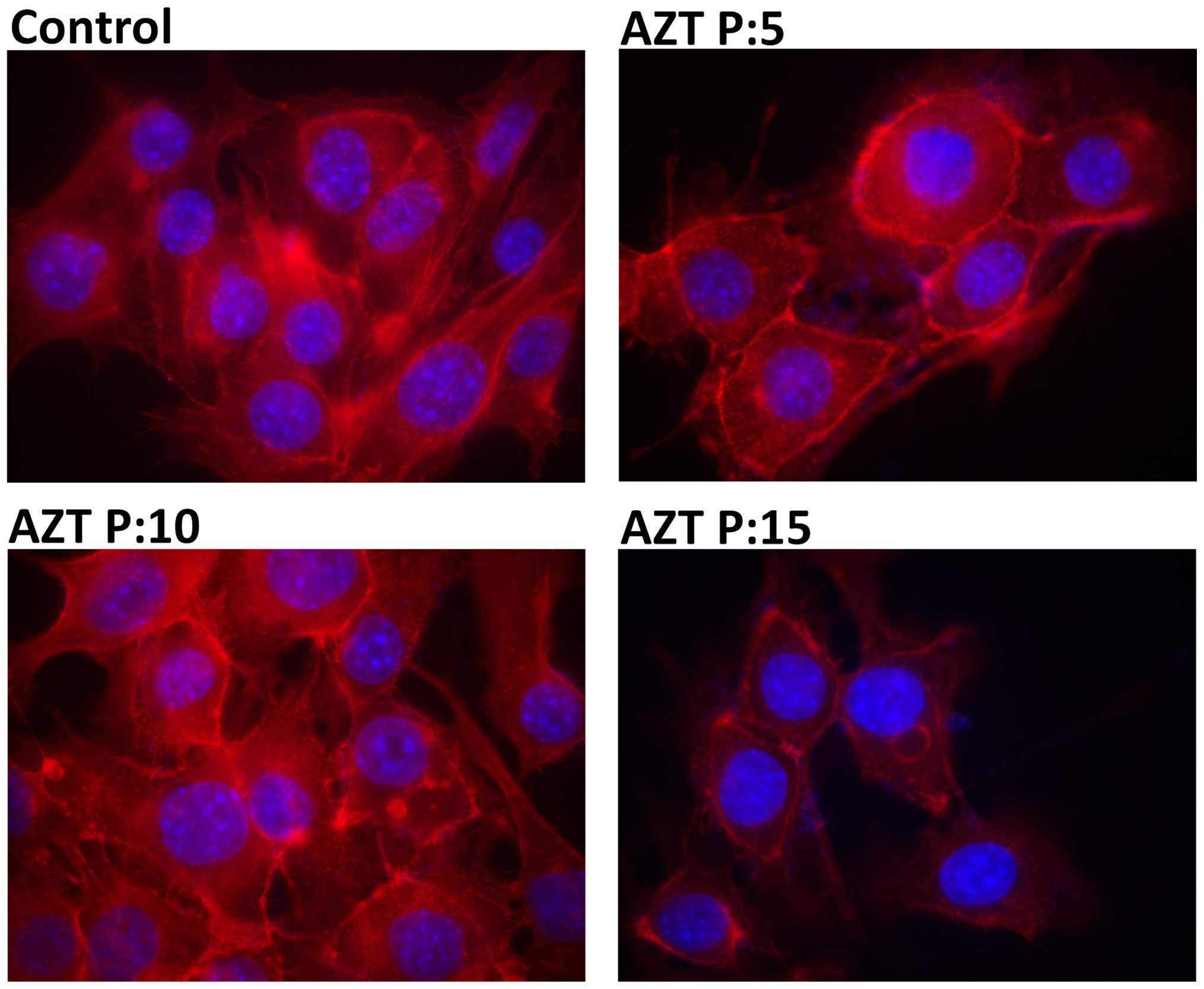
Evaluation of the actin cytoskeleton
Control cells showed normal actin fibers in most
cells with intense fluorescence on the whole cell area (Fig. 4A). At passage 5 treated cells
exhibited some fluorescence in the cell periphery and also actin
fibers like the untreated ones (Fig.
4B). Changes in the cytoskeleton were evident after passage 10.
Cells had less actin fibers whereas a greater amount of cortical
fluorescence was observed (Fig.
4C). Similar effect was observed at passage 15, where the
fluorescence was much more diffuse, actin fibers were no longer
present, and low fluorescence intensity was observed in the
cytoplasm (Fig. 4D).
Effect of AZT on growth and the cell
cycle
Cell populations were without significant
differences throughout all phases of the cell cycle (Fig. 5). There were slight differences in
the cell proportions in each cell cycle stage, but along the
treatment, cells growth rate was not the same between control and
treated cells. Once discarded effects on cell cycle arrest, we
proceeded to determine doubling times of cells during treatment
with AZT. Doubling time determination was performed by cell
counting after 24, 48, 72 and 96 h. The F3II cell line in normal
growth presented a doubling time of 22.82 h. At passage 5 after
treatment, the doubling time was 43.13 h, at 10 passages was 56.21
h, and finally after 15 passages was 67.22 h (Fig. 6). This information demonstrates a
clear and progressive increase in the doubling time, in relation
with the number of passages treated with AZT.
Discussion
3-Azido-2,3-dideoxythymidine [azidothymidine (AZT)
or zidovudine] was the first reported telomerase inhibitor. The
mechanism of AZT antitumor action still remains elusive. AZT is
able to transform an immortal cell in a mortal one. It has been
said that this was due to its action as a chain terminator.
Although the experimental results support this view, AZT by itself
could produce a specific antitumor action by other means,
independently of its action as a telomerase inhibitor. Lately, a
number of non-telomeric telomerase functions have been described.
For instance, TERT, the main component of telomerase, allows the
regulation of target genes of the signaling pathway Wnt/β-catenin
(15), highlighting Cyc-D1 and
C-myc. In the present study, we intend to demonstrate that
canonical and non-canonical functions of telomerase are affected by
AZT in the same cell line and with the same dose integrating the
different mechanisms of action underlying the role of AZT in tumor
biology (16).
Chronic treatment with a dose of 600 µM of
AZT progressively decreased the telomerase activity along 15
passages in F3II cell line without effects on cell viability. Our
group was the first to find this effect using 800 µM
(6). Later, many other authors
confirmed this finding. In the present study we found at 600
µM, that expression levels of TERT were significantly
reduced through the passages evaluated. Given that, it has been
reported that TERT acts as a modulator of β-catenin/BRG1
transcriptional complex, which acts on target genes of the
signaling pathway Wnt/β-catenin, it is evident to expect that a
decrease in the expression levels of TERT negatively impacts on
expression of C-Myc and Cyc-D1 genes. Our results revealed a
continuing decline, corresponding with the amount of passages, in
the expression of both genes, reaching expression levels up to
two-fold reduction for C-Myc and one-fold reduction to Cyc-D1. This
result allows us to infer that the decline in TERT expression due
to treatment with AZT, could be affecting its role as a positive
regulator of these genes, coinciding with studies in the literature
who analyze knockdown models of TERT (17). Ji et al demonstrated in 2005
that intermittent treatment with AZT initially suppressed hTERT and
then c-Myc. Similar results were found by Jin et al in 2012
(18).
Regarding variations in Cyc-D1 after AZT treatment,
we found a passage-dependent decrease reaching 38.24% at 15
passages. Downregulating expression of Cyc-D1 is a clear marker of
changes or modulation in the cell cycle, being the protein which is
required for G1/S cell cycle progress. The decrease of
this factor represents a cycle arrest in phase
G0/G1 cell cycle. Due to the evidence
collected regarding the expression of Cyc-D1, cell cycle analysis
was performed by flow cytometry. As a result of this assay, we
determined there was no such arrest. Similarly, Datta et al
showed that long-term AZT-treated MT-2 cells arrest in all phases
of the cell cycle (19). Fang et
al found that cyclin-A was increased at early times after AZT
treatment cyclin-dependent kinase 1 was decreased at later time
points, while it was found that AZT-treated compared to untreated
mice downregulated Cyc-D2 (p=0.0003) (20). However, some authors have found
different results in other models. Olivero et al found that
AZT induced an upregulation of Cyc-D1 accompanied by a
downregulation of the Cyc-D1-associated inhibitors P18 and P57
(21). Regarding the decline found
in a Cyc-D1 expression after treatment, it is important to
highlight the key role played by this protein in cell cycle entry.
The first approximation of this effect was based on the impact it
would have on this process expecting an increase in the proportion
of cells in state G0/G1 (22). The results obtained on the cell
cycle assay did not allow us to observe this phenomenon, showing a
decrease in the proportion of cells in G1 phase; a
constant proportion of cells in S phase and an increased
G2/M phase at the long-term AZT-treated cells. One
possible interpretation for this observed phenomenon could be based
on the fact that treatment with AZT increased levels of p21 mRNA
(23). This protein is known for
its role in controlling the cell cycle through inhibition of
complex E/cdk2 and A/cdk2, being able to stop the progression of
the cell cycle. In addition to this, during S phase, it is also
able to inhibit CDK1-cyclin-A and CDK2-cyclin-A, which are required
for progression through S and G2, respectively (24). Another possible alternative to this
alteration of the cell cycle, which also involves p21, is the
relationship between this protein and c-Myc, being the last one
presented as a repressor of p21 expression. At lower levels of
expression of c-Myc, p21 levels increase, leading the regulation of
different cell cycle cyclins. These facts could explain the
differences between the cell amount in each phases of the cell
cycle observed after treatment (25).
In addition, has been reported that c-Myc has an
important role in what concerns mitosis and DNA replication, being
a regulator of the target genes Cdc6, Cdt1, MCM3, 4 and 5 and other
genes associated with this processes (22). This explanation coincides with was
observed in our results of doubling time performed on cells at
different passages (0, 5, 10 and 15), where the average doubling
time is increased significantly along passages, suggesting that the
AZT could impact in mitosis and duplication of the treated
cells.
Chronic treatment with AZT decreases cell migration,
and substantially modifies the reorganization of actin
cytoskeleton, finding passage-dependent response in a high
aggressiveness model as F3II. The literature suggests that there is
a link between the expression of c-Myc and invasive phenotype of
the cell that correlates high expression of this gene with
increased migratory ability (26).
Our results agree with this evidence, observing that along the
treatment the expression of this gene is diminished as well as
migration and actin filament formation, indicating that the treated
cells have an epithelial phenotype which it was associated with a
less aggressive profile. This link between c-Myc, cytoskeleton and
migration is described in literature and it was related to the
effect of c-Myc on signaling via RhoA/ROCK, which is highly related
to the regulation of epithelial-mesenchymal transition, motility
cell signaling and cytoskeletal organization (27). Focussing on the results obtained, we
can postulate that AZT treatment has an effect on cell migration,
probably through the regulation of c-Myc and TERT by AZT, leading
tumor cells to a less aggressive phenotype, being a remarkable
event in the field of extratelomerics activities. To the best of
our knowledge no previous study has focused on cell migration after
AZT treatment.
In conclusion, the results and information presented
here, are intended to clarify and contribute to the study of how
AZT exerts its antitumoral effect. Telomerase stands at the
crossroads of multiple signaling pathways and its
upregulation/reactivation leads to the modulation of critical
cellular processes, including gene expression and metabolism.
Understanding AZT antitumoral action is of utmost importance, even
more since the recent report of Bhushan and Kush on the
pharmocophoric studies of anti-telomerase drugs. They found that
AZT and few non-nucleoside HIV-RTIS have exquisite selectivity, not
requiring bioactivation by kinases to triphosphate and not
attaching into growing DNA chain. Instead, binding to allosteric
site of the reverse transcriptase that is distinct from the
substrate binding site. Therefore, they bind reverse transcriptase
near catalytic site and instantly denature it by the
non-competitive mechanism (28).
Although most of the described effects were known,
this is the first time that the mentioned variables were analyzed
after treatment with AZT using the same treatment, in the same cell
line and at the same time, providing evidence that they are not
interfering among them, and that AZT inhibitory action may be due
to a mix of canonical and non-canonical effects.
Even knowing that more experiments are needed, we
started a differential display study with cells treated or not with
AZT. Preliminary results point toward the described dual
effect.
Acknowledgments
The authors wish to express their gratitude to Dr
Daniel Alonso for critical reading of the manuscript. The present
study was supported by grants from UNQ and ANPCyT (Argentina).
Daniel E. Gomez and Diego Mengual Gómez are members of the National
Research Council (CONICET, Argentina).
References
|
1
|
Teralı K and Yilmazer A: New surprises
from an old favourite: The emergence of telomerase as a key player
in the regulation of cancer stemness. Biochimie. 121:170–178. 2016.
View Article : Google Scholar
|
|
2
|
Martínez P and Blasco MA: Replicating
through telomeres: A means to an end. Trends Biochem Sci.
40:504–515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gomez DE, Armando RG, Farina HG, Menna PL,
Cerrudo CS, Ghiringhelli PD and Alonso DF: Telomere structure and
telomerase in health and disease (Review). Int J Oncol.
41:1561–1569. 2012.PubMed/NCBI
|
|
4
|
Hájek M, Matulová N, Votruba I, Holý A and
Tloust'ová E: Inhibition of human telomerase by diphosphates of
acyclic nucleoside phosphonates. Biochem Pharmacol. 70:894–900.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gomez D, Kassim A and Olivero O:
Preferential incorporation of 3′-azido-2′,3′-dideoxythymidine (azt)
in telomeric sequences of cho cells. Int J Oncol. 7:1057–1060.
1995.PubMed/NCBI
|
|
6
|
Gomez DE, Tejera AM and Olivero OA:
Irreversible telomere shortening by 3′-azido-2′,3′-dideoxythymidine
(AZT) treatment. Biochem Biophys Res Commun. 246:107–110. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tejera AM, Alonso DF, Gomez DE and Olivero
OA: Chronic in vitro exposure to 3′-azido-2′, 3′-dideoxythymidine
induces senescence and apoptosis and reduces tumorigenicity of
metastatic mouse mammary tumor cells. Breast Cancer Res Treat.
65:93–99. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koh CM, Khattar E, Leow SC, Liu CY, Muller
J, Ang WX, Li Y, Franzoso G, Li S, Guccione E, et al: Telomerase
regulates MYC-driven oncogenesis independent of its reverse
transcriptase activity. J Clin Invest. 125:2109–2122. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JI, Venteicher AS, Hong JY, Choi J,
Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, et al:
Telomerase modulates Wnt signalling by association with target gene
chromatin. Nature. 460:66–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martínez P and Blasco MA: Telomeric and
extra-telomeric roles for telomerase and the telomere-binding
proteins. Nat Rev Cancer. 11:161–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Leonardis F, Monti L, Gualeni B, Tenni
R, Forlino A and Rossi A: Altered signaling in the G1 phase
deregulates chondrocyte growth in a mouse model with proteoglycan
undersulfation. J Cell Biochem. 115:1779–1786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Casey SC and Felsher DW:
Inactivation of MYC reverses tumorigenesis. J Intern Med.
276:52–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alonso DF, Farías EF, Urtreger A, Ladeda
V, Vidal MC and Bal De Kier Joffé E: Characterization of F3II, a
sarcomatoid mammary carcinoma cell line originated from a clonal
subpopulation of a mouse adenocarcinoma. J Surg Oncol. 62:288–297.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Segatori VI, Otero LL, Fernandez LE, Gomez
DE, Alonso DF and Gabri MR: Antitumor protection by NGcGM3/VSSP
vaccine against transfected B16 mouse melanoma cells overexpressing
N-glycolylated gangliosides. In Vivo. 26:609–617. 2012.PubMed/NCBI
|
|
15
|
Chen YY, Wu XQ, Tang WJ, Shi JB, Li J and
Liu XH: Novel dihydropyrazole-chromen: Design and modulates hTERT
inhibition proliferation of MGC-803. Eur J Med Chem. 110:65–75.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gomez DE, Armando RG and Alonso DF: AZT as
a telomerase inhibitor. Front Oncol. 2:1132012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji HJ, Rha SY, Jeung HC, Yang SH, An SW
and Chung HC: Cyclic induction of senescence with intermittent AZT
treatment accelerates both apoptosis and telomere loss. Breast
Cancer Res Treat. 93:227–236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin RR, Chao R, Xi YM, Chen C, Chu HY, Li
M and Zhang H: Effects of AZT on leukemia cell line KG-1a
proliferation and telomerase activity. Zhongguo Shi Yan Xue Ye Xue
Za Zhi. 20:277–281. 2012.PubMed/NCBI
|
|
19
|
Datta A, Bellon M, Sinha-Datta U,
Bazarbachi A, Lepelletier Y, Canioni D, Waldmann TA, Hermine O and
Nicot C: Persistent inhibition of telomerase reprograms adult
T-cell leukemia to p53-dependent senescence. Blood. 108:1021–1029.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang JL, McGarrity LJ and Beland FA:
Interference of cell cycle progression by zidovudine and lamivudine
in NIH 3T3 cells. Mutagenesis. 24:133–141. 2009. View Article : Google Scholar :
|
|
21
|
Olivero OA, Tejera AM, Fernandez JJ,
Taylor BJ, Das S, Divi RL and Poirier MC: Zidovudine induces
S-phase arrest and cell cycle gene expression changes in human
cells. Mutagenesis. 20:139–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bretones G, Delgado MD and León J: Myc and
cell cycle control. Biochim Biophys Acta. 1849:506–516. 2015.
View Article : Google Scholar
|
|
23
|
Hassani S, Ghaffari SH, Zaker F, Mirzaee
R, Mardani H, Bashash D, Zekri A, Yousefi M, Zaghal A, Alimoghaddam
K, et al: Azidothymidine hinders arsenic trioxide-induced apoptosis
in acute promyelocytic leukemia cells by induction of p21 and
attenuation of G2/M arrest. Ann Hematol. 92:1207–1220. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Mannava S, Grachtchouk V, Zhuang
D, Soengas MS, Gudkov AV, Prochownik EV and Nikiforov MA: c-Myc
depletion inhibits proliferation of human tumor cells at various
stages of the cell cycle. Oncogene. 27:1905–1915. 2008. View Article : Google Scholar
|
|
26
|
Chen D, Huang J, Zhang K, Pan B, Chen J,
De W, Wang R and Chen L: MicroRNA-451 induces
epithelial-mesenchymal transition in docetaxel-resistant lung
adenocarcinoma cells by targeting proto-oncogene c-Myc. Eur J
Cancer. 50:3050–3067. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi JW, Liu W, Zhang TT, Wang SC, Lin XL,
Li J, Jia JS, Sheng HF, Yao ZF, Zhao WT, et al: The enforced
expression of c-Myc in pig fibroblasts triggers
mesenchymal-epithelial transition (MET) via F-actin reorganization
and RhoA/Rock pathway inactivation. Cell Cycle. 12:1119–1127. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bhushan B and Kush L: Pharmocophoric
studies of anti-telomerase drugs. Int J Innov Res Dev. 3:268–272.
2014.
|