Introduction
The transcription factor NF-κB was discovered in
1986 as a nuclear factor that binds to the enhancer element of the
immunoglobulin kappa light-chain of activated B cells (thereby
coining the abbreviation NF-κB) (1). NF-κB represents a family of eukaryotic
transcription factors participating in the regulation of various
cellular genes involved in the immediate early processes of immune,
acute phase and inflammatory responses as well as genes involved in
cell survival (2). A commonly known
NF-κB consists of a RelA (p65)/p50 heterodimer and RelA (p65)
contains a C-terminal transactivation domain in addition to the
N-terminal Rel-homology domain, thus, serving as a critical
transactivation subunit of NF-κB (3). In the resting state, the inactive
NF-κB is retained in the cytoplasm by an inhibitory subunit called
IκB. The phosphorylation of IκB by the IκB-kinase (IKK) containing
IKKα, IKKβ and the regulatory protein NF-κB essential modifier
(NEMO) is a key step in NF-κB activation in response to various
stimuli such as tumor necrosis factor-α (TNF-α) (3,4). In
response to stimulation, IκBs are rapidly ubiquitinated and
degraded by 26S proteasome complex and the release of IκB unmasks
the nuclear localization signal and results in the translocation of
NF-κB to the nucleus where it can bind to κB sites, followed by the
activation of specific target genes (5).
It is reported that NF-κB regulates several hundreds
of genes, including those involved in immunity and inflammation,
anti-apoptosis, cell proliferation, tumorigenesis and the negative
feedback of the NF-κB signal (6).
NF-κB regulates major inflammatory cytokines, including interleukin
8 (IL-8), monocyte chemotactic protein 1 (MCP1), many of which are
potent activators for NF-κB. NF-κB has been shown to regulate the
expression of several genes whose products are involved in
tumorigenesis (2), including
cyclooxygenase-2 (COX-2), cyclin D1, c-Myc, apoptosis suppressor
proteins such as cellular inhibitor of apoptosis 1 (cIAP-1),
cellular inhibitor of apoptosis 2 (cIAP-2), cellular FLICE
inhibitory protein (FLIP), B-cell lymphoma-2 (BCL-2) and genes
required for invasion and angiogenesis such as matrix
metalloproteinase (MMP-9) and vascular endothelial growth factor
(VEGF).
Baicalein is a naturally occurring flavonoid which
is an active component of Scutellaria baicalensis (7). Scutellaria baicalensis is one
of the most popular traditional Chinese medicine herbal remedies
used in China and several oriental countries for treatment of
inflammation, bacterial and viral infections, and have been shown
to possess anticancer activities in vitro and in vivo
in mouse tumor models (8). Previous
investigations also showed that baicalein have multiple
pharmacological activities including anti-oxidant effects,
chemo-preventive effects against several types of cancer and
anti-inflammatory effect (9–11).
However, the molecular mechanism of anti-inflammatory and
anticancer effects has not been sufficiently explained. In the
present study, whether baicalein exerts its anti-inflammatory and
anticancer effects through suppression of the NF-κB pathway was
investigated. Our data demonstrated baicalein downregulates the
expression of target genes involved in antiapoptosis (cIAP-1,
cIAP-2, BCL-2 and FLIP), proliferation (cyclin D1, COX-2 and
c-Myc), invasion (MMP-9), angiogenesis (VEGF) and major
inflammatory cytokines (IL-8 and MCP1). Taken together, these
findings support further studies of baicalein as candidate for
treatment of inflammation and cancer.
Materials and methods
Cell culture and reagents
HeLa cells were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA). Cells were cultured
in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS; (Gibco, Grand Island, NY, USA) and 1%
penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C
with 5% CO2 atmosphere in a humidified incubator. TNF-α
was obtained from R&D Systems (Minneapolis, MN, USA). Baicalein
was from Sigma-Aldrich (St. Louis, MO, USA) and its structure is
shown in Fig. 1A. The purity of
baicalein was over 99% in HPLC analysis.
MTT assay
HeLa cells were seeded in 96-well plates at a
density of 1×105 cells/ml and cultured overnight.
Following cell treatment with different concentrations of baicalein
(10–100 µM) for 12 h, 10 µl MTT solution (5 mg/ml)
was added into each well and incubated with cells for 4 h at 37°C.
Then, DMSO was added to dissolve the formazan crystals. The
absorbance at 570 nm was measured by Multiskan GO.
Plasmids, transfections and luciferase
reporter assay
A pNF-κB-Luc plasmid for NF-κB luciferase reporter
assay was obtained from Strategene (La Jolla, CA, USA).
Transfections were performed as previously described (12). NF-κB-dependent luciferase activity
was measured using the Dual-luciferase reporter assay system.
Briefly, HeLa cells (1×105 cells/well) were seeded in a
96-well plate for 24 h. The cells were then transfected with
plasmids for each well and then incubated for a transfection period
of 24 h. After that, the cell culture medium was removed and
replaced with fresh medium containing various concentrations of
baicalein for 6 h, followed by treatment with 10 ng/ml of TNF-α for
6 h. Luciferase activity was determined in MicroLumat plus
luminometer (EG&G Berthold, Bad Wildbad, Germany) by injecting
100 µl of assay buffer containing luciferin and measuring
light emission for 10 sec. Co-transfection with pRL-CMV (Promega,
Madison, WI, USA), which expresses Renilla luciferase, was
performed to enable normalization of data for transfection
efficiency.
Western blot analysis
HeLa cells were cultured in 10 cm-dishes and allowed
to adhere for 24 h. After treatment with various concentrations of
baicalein in the presence or absence of TNF-α (10 ng/ml), then,
cells were harvested and lysed. An equal amount of protein was
separated by SDS-polyacrylamide gels and transferred to
polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA,
USA). The membrane was blocked with 5% non-fat dried milk for 1 h,
the membrane was incubated with the primary antibodies. Antibodies
for IκBα, phosphor (Ser32)-specific IκBα, p65, PARP, caspase-8,
cIAP-1, cIAP-2, phospho-ERK, phospho-JNK, phospho-p38, ERK, JNK and
P38 were purchased from Cell Signaling Technology (Beverly, MA,
USA). Antibodies for COX-2, MMP-9, VEGF, BCL-2, FLIP, and Topo-I
were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Antibody for α-tubulin was from Sigma-Aldrich. After binding of an
appropriate secondary antibody coupled to horseradish peroxidase.
Then the immunoreactive bands were visualized by enhanced
chemiluminescence according to the manufacturer's instructions
(Amersham Pharmacia Biotech, Buckinghamshire, UK).
Immunofluorescence of NF-κB p65
HeLa cells were seeded into 24-well plates at
1×104 cells/well. Twenty-four hours later, cells were
pretreated with baicalein (100 µM) for 12 h, followed by
treatment with TNF-α (10 ng/ml) for 30 min. Cells pretreated with
DMSO and TNF-α (10 ng/ml) alone were used as negative and positive
controls, respectively. Subsequently, the cells were washed in PBS,
fixed at room temperature with 4% paraformaldehyde, and
permeabilized with 0.2% Triton X-100. Immunofluorescence staining
was performed according to the standard procedures. Briefly, the
treated cells were first stained with the anti-p65 antibody
followed by incubation with FITC conjugated anti-rabbit IgG
secondary antibody and nuclei were counterstained with DAPI. The
staining was examined using a fluorescence microscope.
Apoptosis assays
Apoptosis assays were performed as previously
described (13). Annexin V-staining
was performed using Annexin V-FITC apoptosis detection kit (BD
Biosciences, San Jose, CA, USA) following the instructions of the
manufacturer. Briefly, after incubation, detached cells were
collected with the supernatant, pelleted by centrifugation. The
adherent cells were rinsed twice with medium before harvesting.
Then cells were harvested in trypsin without EDTA. Detached and
adherent cells were finally pooled and were resuspended in binding
buffer (10 mM Hepes, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2)
to a final concentration of 1×l05 cells/ml. The pooled
cells were stained with Annexin V-FITC and 2 µg/ml propidium
iodide for 15 min at 37°C in the dark. To the samples was added 400
µl binding buffer before analyzed by flow cytometry. The
CellQuest software was used to analyze the data (Becton-Dickinson,
Franklin Lakes, NJ, USA).
RT-PCR analysis
Reverse transcription-PCR (RT-PCR) was performed to
determine NF-κB target gene expression as previously described
(14). In brief, HeLa cells were
preincubated with the indicated concentrations of baicalein at 37°C
for 12 h and then followed by treatment with 10 ng/ml of TNF-α for
12 h. Cells were harvested and washed twice with ice-cold PBS, and
then total RNA was isolated from cells using RNeasy Mini kits
according to the manufacturer's instructions (Qiagen, Valencia, CA,
USA). Complementary DNA was synthesized from 1 µg of total
RNA in a 20 µl reverse transcription reaction mixture
according to the manufacturer's protocol (Takara Bio, Kyoto,
Japan). The PCR primers for interleukin-8 (IL-8),
5′-TCTGCAGCTCTGTGTGAAGG-3′ and 5′-ACTTCTCCACAACCCTCTG-3′; for MCP1,
5′-CCCCAGTCACCTGCTGTTAT-3′ and 5′-AGATCTCCTTGGCCACAATG-3′; for
c-Myc, 5′-CTCTCAACGACAGCAGCCCG-3′ and 5′-CCAGTCTCAGACCTAGTGGA-3′;
for GAPDH, 5′-ACCAGGTGGTCTCCTCT-3′ and 5′-TGCTGTAGCCAAATTCGTTG-3′.
The mRNA levels of all genes were normalized to that of GAPDH. PCR
products were separated on 3% agarose gel and then stained with
ethidium bromide. Stained bands were visualized under UV light and
photographed.
Cell cycle assay
HeLa cells were cultured in 6-well plates until
70–80% confluent. The cells were then treated with baicalein at
indicated concentrations in serum-free medium. Cells were then
washed with PBS, fixed in ice-cold 70% ethanol and stained with PI
buffer (0.1% Triton X-100, 0.2 mg/ml RNaseA, and 0.05 mg/ml PI) for
30 min. The DNA content was measured using a FACSCalibur flow
cytometer with CellQuest software (Becton-Dickinson). For all
assays, 10,000 events were counted. The ModFit LT v4.0 software
package (Verity Software House, Inc., Topsham, ME, USA) was used to
analyze the data.
Statistical analysis
All values are expressed as mean ± SD. A comparison
of the results was performed with one-way ANOVA and Tukey's
multiple comparison tests (GraphPad Software, Inc., San Diego, CA,
USA) and the Student's t-test. P-values of <0.05 were considered
statistically significant.
Results
Baicalein inhibits TNF-α-induced NF-κB
activation
We first investigated the effect of baicalein on
TNF-α-induced NF-κB activation by NF-κB-dependent reporter gene
assay. HeLa cells were transiently transfected with the
NF-κB-regulated luciferase reporter vector. When the HeLa cells
were pretreated with various concentration of baicalein,
TNF-α-induced NF-κB-reporter gene expression was inhibited in a
dose-dependent manner (Fig. 1B). We
evaluated the cytotoxic effects of baicalein on HeLa cell survival
by MTT assay. The results showed that up to 100 µM of
baicalein had no cellular toxicity on HeLa cells (Fig. 1C).
Baicalein inhibits TNF-α-induced IκBα
phosphorylation and degradation, and p65 nuclear translocation
Transcriptional activity of NF-κB is dependent on
IκBα phosphorylation. To determine whether baicalein inhibition of
TNF-α-induced NF-κB activation, total cell extracts were prepared
with baicalein and then exposed to TNF-α for various time periods,
phosphorylation and degradation of IκBα was analyzed by western
blot analysis. The results showed that baicalein potently inhibited
the TNF-α-induced phosphorylation and degradation of IκBα in a
dose-dependent manner (Fig. 2A). In
addition, TNF-α-induced phosphorylation and degradation of IκBα
were occurred as quickly as 15 min (Fig. 2B). Next, we examined whether
baicalein modulates TNF-α-induced nuclear translocation of p65.
Nuclear extracts were pretreated with baicalein and then exposed to
TNF-α for various time periods and analyzed p65 nuclear
translocation by western blot analysis. The results showed that
baicalein also potently inhibited TNF-α-induced nuclear
translocation of p65 in a dose-dependent manner (Fig. 2C), and the earliest inhibition also
occurred within 15 min after TNF-α addition (Fig. 2D). To further confirm these results,
the immunofluorescence staining assay was performed.
Immunofluorescence images showed that in untreated, p65 was
localized in the cytoplasm. In TNF-α alone treated, p65 was
translocated to the nucleus. Followed by inhibited nuclear
translocation of p65 with baicalein pretreatment (Fig. 2E).
Baicalein inhibits TNF-α-induced
NF-κB-regulated gene products
NF-κB regulates the expression of anti-apoptotic
gene products cIAP-1, cIAP-2, BCL-2 and FLIP, proliferation gene
products COX-2 and cyclin D1, invasion and angiogenesis gene
products MMP-9 and VEGF, which are known to be induced by TNF-α. We
used western blotting to determine whether baicalein inhibits the
expression of these gene products in HeLa cells. Our results showed
that baicalein markedly downregulated TNF-α-induced expression of
all these proteins in a dose-dependent manner (Fig. 3A). NF-κB regulates major
inflammatory cytokines and proliferation, including IL-8, MCP1 and
c-Myc. Thus, we also investigated whether baicalein can modulate
TNF-α-induced expression of these genes by RT-PCR analysis. The
results showed that baicalein blocked TNF-α-induced mRNA expression
of IL-8, MCP1 and c-Myc in a dose-dependent manner (Fig. 3B).
Baicalein potentiates TNF-α-induced
apoptosis
HeLa cells were sequentially treated with baicalein
and TNF-α, then stained with Annexin V-FITC and propidium iodide
and analyzed using a flow cytometer. As shown in Fig. 4A, treatment of HeLa cells with
vehicle only, TNF-α alone, and baicalein alone induced apoptosis of
4.5, 9.1 and 17.2% respectively. However, combined treatment of the
cells with TNF-α and baicalein resulted in a significant
potentiated apoptosis of HeLa cells (39.8%). To assess whether
baicalein can enhance the TNF-α-induced apoptosis, the activation
of caspases-8 and PARP was also investigated. Our results showed
that baicalein alone had little effect on caspases-8 and PARP
cleavage, However, combined treatment of TNF-α with baicalein
potentiated their activation (Fig.
4B). These results together indicate that baicalein enhances
the apoptotic effects of HeLa cells by TNF-α.
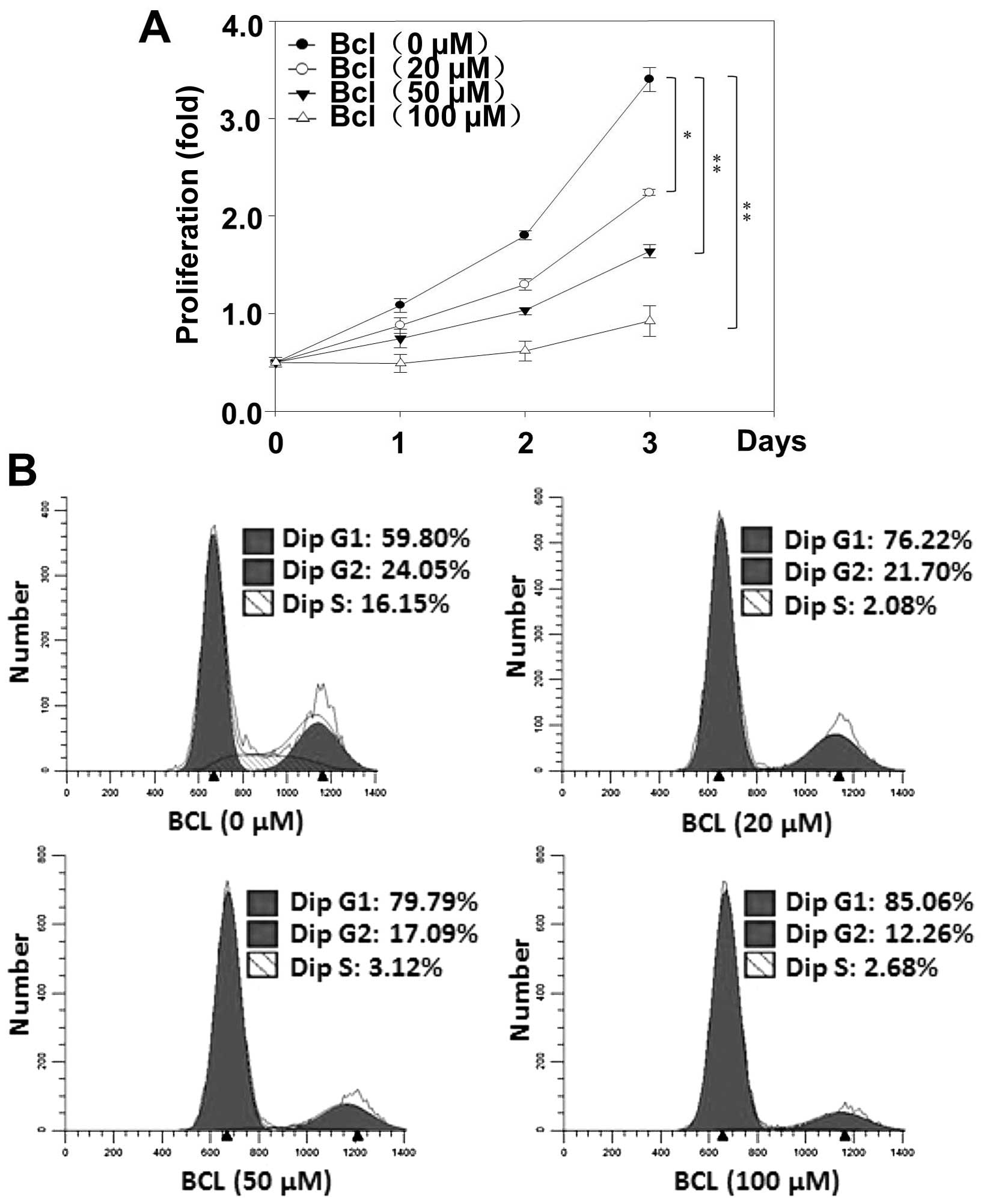
Baicalein inhibits the proliferation of
HeLa cells via blocking cell cycle progression in the G1 phase
Next, in order to investigate the effects of
baicalein on HeLa cell proliferation, the proliferation assay were
performed. Indeed, as in MTT experiments, the strongest growth
inhibitory effect was observed at 72 h of baicalein (100 µM)
incubation (Fig. 5A). In order to
elucidate if impairment of cell cycle participate in the reduction
of the HeLa cell growth rate induced by baicalein, the flow
cytometric analyses of cell cycle were performed. Our results
showed that baicalein increased the population of G1 phase cells.
These results suggest that baicalein inhibits cell proliferation
through blocking cell cycle progression in G1 phase in HeLa
cells.
Baicalein inhibits TNF-α-induced
phosphorylation of ERK1/2 and p38
The inflammatory response can be activated through
the MAP kinase pathway. Thus, we determined whether baicalein can
inhibition TNF-α-induced inflammatory responses through MAPK
signaling. Since the MAPK pathway is phosphorylation-dependent, the
phosphorylated proteins were easily detectable by western blot
analysis. The results showed that baicalein decreased TNF-α-induced
ERK1/2 and p38 by inhibiting their phosphorylation (Fig. 6).
Discussion
NF-κB is normally retained in the cytoplasm through
interaction with its inhibitor IκB. IκB exerts its inhibitory
effects by associating with the Rel homology domain of NF-κB
proteins, effectively masking their nuclear localization signals
(15–17). Our results determined that baicalein
suppresses TNF-α-induced NF-κB activation through the inhibition of
IκB phosphorylation and degradation, p65 nuclear translocation. Our
studies also determined that baicalein inhibits TNF-α-induced
NF-κB-regulated target gene products that are associated with
inflammation, apoptosis, tumor cell proliferation, cell cycle,
invasion and angiogenesis.
Apoptosis is an important mechanism to eliminate
unwanted cells, and deregulation of this process is implicated in
the pathogenesis of cancer development (18). Our results showed that baicalein
inhibits TNF-α-induced expression of antiapoptotic proteins such as
cIAP-1, cIAP-2, FLIP and BCL-2, which are known to be regulated by
NF-κB. Furthermore, the flow cytometric analysis showed that
baicalein enhanced TNF-α-induced apoptosis. The loss of caspase
activation appears to be central to the prevention of most cell
death events in cancer. Finding strategies to overcome caspase
inhibition will be valuable for the development of novel cancer
treatments (19). We also found
that baicalein potentiated TNF-α-induced activition of caspases-8
and PARP, which suggested that baicalein enhances cell apoptosis
signaling by TNF-α. Moreover, our results demonstrated that
baicalein suppressed TNF-α-induced expression of MMP-9, VEGF and
COX-2, which are major mediators involved in tumor invasion,
metastasis and proliferation (20–22).
Flow cytometric analysis with PI staining indicated that baicalein
can suppress cell proliferation via blocking cell cycle progression
in the G1 phase. Cyclin D1 is a protein that is expressed
relatively early in the cell cycle and is crucial for control of G1
phase (8). We also observed
baicalein suppressed TNF-α-induced expression of cyclin D1 protein
in HeLa cells.
MAP kinases are another signaling pathway that plays
a critical role in inflammation through activation of NF-κB
(23). This kinase family is
composed of several subgroups, such as ERK, JNK and p38. Therefore,
experiments were performed to determine whether baicalein regulates
TNF-α-stimulated expression of MAP kinases in HeLa cells. Our
results showed that baicalein prevented the activation of p38 and
ERK1/2. The anti-inflammatory effects of baicalein have been
determined via investigation of several major inflammatory
cytokines, such as IL-8 and MCP1, which are regulated by NF-κB and
are also potent activators for NF-κB. NF-κB-binding sites have been
identified in the promoter of over 300 different genes, and these
genes are known to regulate a wide variety of cellular responses
affected by baicalein. Overall, our results provide the molecular
basis through which baicalein mediates its anti-inflammatory and
anticancer effects. We conclude that baicalein is a potent
inhibitor of NF-κB and NF-κB-regulated gene products, and may be a
valuable new drug candidate for the treatment of inflammation and
cancer.
Acknowledgments
The present study was partially supported by the
National Natural Science Foundation of China (no. 81360496). This
study also received assistance from the Jilin Province Science and
Technology Development Plan item (20150101229JC) and the Jilin
Province Department of Education (2016.281).
Abbreviations:
|
NF-κB
|
nuclear factor-κB
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IκBα
|
inhibitor of NF-κB alpha
|
|
IL-8
|
interleukin 8
|
|
cIAP1
|
cellular inhibitor of apoptosis
protein 1
|
|
cIAP2
|
cellular inhibitor of apoptosis
protein 2
|
|
FLIP
|
cellular FLICE inhibitory protein
|
|
BCL-2
|
B-cell lymphoma-2
|
|
MCP1
|
monocyte chemotactic protein 1
|
|
COX-2
|
cyclooxygenase-2
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
VEGF
|
vascular endothelial growth factor
|
|
c-Myc
|
cellular-myelocytomatosis viral
oncogene
|
References
|
1
|
Sen R and Baltimore D: Multiple nuclear
factors interact with the immunoglobulin enhancer sequences. Cell.
46:705–716. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi H, Ma J, Mi C, Li J, Wang F, Lee JJ
and Jin X: Amorfrutin A inhibits TNF-α-induced NF-κB activation and
NF-κB-regulated target gene products. Int Immunopharmacol.
21:56–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li M, Song S, Li S, Feng J and Hua Z: The
Blockade of NF-κB activation by a specific inhibitory peptide has a
strong neuroprotective role in a Sprague-Dawley rat kernicterus
model. J Biol Chem. 290:30042–30052. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar
|
|
6
|
Sethi G, Sung B and Aggarwal BB: Nuclear
factor-kappaB activation: From bench to bedside. Exp Biol Med
(Maywood). 233:21–31. 2008. View Article : Google Scholar
|
|
7
|
Wang Y, Han E, Xing Q, Yan J, Arrington A,
Wang C, Tully D, Kowolik CM, Lu DM, Frankel PH, et al: Baicalein
upregulates DDIT4 expression which mediates mTOR inhibition and
growth inhibition in cancer cells. Cancer Lett. 358:170–179. 2015.
View Article : Google Scholar
|
|
8
|
Li-Weber M: New therapeutic aspects of
flavones: The anticancer properties of Scutellaria and its main
active constituents Wogonin, Baicalein and Baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar
|
|
9
|
Ku SK and Bae JS: Baicalin, baicalein and
wogonin inhibits high glucose-induced vascular inflammation in
vitro and in vivo. BMB Rep. 48:519–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma GZ, Liu CH, Wei B, Qiao J, Lu T, Wei
HC, Chen HD and He CD: Baicalein inhibits DMBA/TPA-induced skin
tumorigenesis in mice by modulating proliferation, apoptosis, and
inflammation. Inflammation. 36:457–467. 2013. View Article : Google Scholar
|
|
11
|
Shieh DE, Liu LT and Lin CC: Antioxidant
and free radical scavenging effects of baicalein, baicalin and
wogonin. Anticancer Res. 20(5A): 2861–2865. 2000.PubMed/NCBI
|
|
12
|
Hwangbo C, Kim J, Lee JJ and Lee JH:
Activation of the integrin effector kinase focal adhesion kinase in
cancer cells is regulated by crosstalk between protein kinase
Calpha and the PDZ adapter protein mda-9/Syntenin. Cancer Res.
70:1645–1655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin HR, Jin SZ, Cai XF, Li D, Wu X, Nan
JX, Lee JJ and Jin X: Cryptopleurine targets NF-κB pathway, leading
to inhibition of gene products associated with cell survival,
proliferation, invasion, and angiogenesis. PLoS One. 7:e403552012.
View Article : Google Scholar
|
|
14
|
Jin X, Jin HR, Jung HS, Lee SJ, Lee JH and
Lee JJ: An atypical E3 ligase zinc finger protein 91 stabilizes and
activates NF-kappaB-inducing kinase via Lys63-linked
ubiquitination. J Biol Chem. 285:30539–30547. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huxford T, Huang DB, Malek S and Ghosh G:
The crystal structure of the IkappaBalpha/NF-kappaB complex reveals
mechanisms of NF-kappaB inactivation. Cell. 95:759–770. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Napetschnig J and Wu H: Molecular basis of
NF-κB signaling. Annu Rev Biophys. 42:443–468. 2013. View Article : Google Scholar :
|
|
17
|
Jacobs MD and Harrison SC: Structure of an
IkappaBalpha/NF-kappaB complex. Cell. 95:749–758. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SY, Cho JS, Yuk DY, Moon DC, Jung JK,
Yoo HS, Lee YM, Han SB, Oh KW and Hong JT: Obovatol enhances
docetaxel-induced prostate and colon cancer cell death through
inactivation of nuclear transcription factor-kappaB. J Pharmacol
Sci. 111:124–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hunter AM, LaCasse EC and Korneluk RG: The
inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis.
12:1543–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeelenberg IS, Ruuls-Van Stalle L and Roos
E: The chemokine receptor CXCR4 is required for outgrowth of colon
carcinoma micrometastases. Cancer Res. 63:3833–3839.
2003.PubMed/NCBI
|
|
21
|
Alexiou D, Karayiannakis AJ, Syrigos KN,
Zbar A, Sekara E, Michail P, Rosenberg T and Diamantis T: Clinical
significance of serum levels of E-selectin, intercellular adhesion
molecule-1, and vascular cell adhesion molecule-1 in gastric cancer
patients. Am J Gastroenterol. 98:478–485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuniyasu H1, Troncoso P, Johnston D,
Bucana CD, Tahara E, Fidler IJ and Pettaway CA: Relative expression
of type IV collagenase, E-cadherin, and vascular endothelial growth
factor/vascular permeability factor in prostatectomy specimens
distinguishes organ-confined from pathologically advanced prostate
cancers. Clin Cancer Res. 6:2295–2308. 2000.PubMed/NCBI
|
|
23
|
Vanden Berghe W, Plaisance S, Boone E, De
Bosscher K, Schmitz ML, Fiers W and Haegeman G: p38 and
extracellular signal-regulated kinase mitogen-activated protein
kinase pathways are required for nuclear factor-kappaB p65
transactivation mediated by tumor necrosis factor. J Biol Chem.
273:3285–3290. 1998. View Article : Google Scholar : PubMed/NCBI
|