Introduction
Hepatocellular carcinoma (HCC) represents the third
leading cause of cancer-related death worldwide and its incidence
has been continuously increasing in the past several decades
(1). Due to delayed diagnosis and
high prevalence of HCC invasion and metastasis, the 5-year
postoperative survival rate of HCC is only 30–40% (2). Presently, although many different
treatments including surgery and chemotherapy have been widely
used, the therapeutic effect on patients with HCC is still limited
(3) and the detailed pathological
processes underlying HCC occurrence and development remain poorly
understood (4). Therefore, it is
urgent to clarify the molecular mechanisms of HCC development, and
apply them to develop new strategies for therapy to improve HCC
patient overall survival.
The poly(C)-binding proteins (PCBPs) are
characterized by their high affinities and sequence-specific
interactions with polycytosine. The PCBP family has central roles
in transcriptional and translational regulation, including mRNA
stabilization, translational silencing and translational
enhancement (5–7). Through these function, it has been
proven that PCBPs play an important role in the development and
processes of tumors, including apoptosis (8), proliferation (9), invasion (10) and epithelial-mesenchymal transition
(EMT) (11). However, the
physiological significance of PCBPs in the generation and
development of HCC has received less intensive attention.
PCBP2, belonging to the PCBP family, is one of the
least studied protein in human cancers among the PCBPs. Most of the
studies on PCBP2 have focused on its post-transcriptional and
translational controls in RNA viruses (12,13).
Recently, the carcinogenic effect of PCBP2 has attracted research
attention and some discoveries have revealed that PCBP2 may
participate in the development of human tumors. For example,
Molinaro et al showed that 2′,5′-oligoadenylate synthetase
(OAS) activation may occur in prostate cancer cells in vivo
when stimulated by cellular mRNAs for PCBP2 (14). In human glioma, PCBP2 is
overexpressed in tumor tissues and predicts adverse survival. It
has been reported to promote glioma cell growth, migration and
invasion (15,16). PCBP2 was also upregulated in human
gastric cancer and promoted gastric carcinoma development by
regulating the level of miR-34a (17). Our previous study also identified
that PCBP2 was significantly upregulated in pancreatic ductal
adenocarcinoma (PDAC) and knockdown of PCBP2 attenuated cell
proliferation and colony formation through triggering c-Myc
expression (18). Moreover, PCBP2
has been reported to participate in the replication and translation
of hepatitis C virus (HCV) (19–21),
and silencing of PCBP2 expression reversed the alcohol-induced
pro-fibrogenic effects in hepatic stellate cells (22). More importantly, Leidgens et
al found that PCBP2 was expressed in Huh7 cells, one of the HCC
cell lines (23). However, whether
PCBP2 participates in human HCC development and clinical
significance remains largely elusive.
In the present study, we showed that PCBP2
expression was significantly increased in human HCC specimens and
cell lines. In addition, we evaluated the correlation between PCBP2
expression and biological and clinical indices, as well as the
prognostic value of PCBP2 for predicting the HCC patient survival
rate. Furthermore, we discovered that depletion of PCBP2 critically
attenuated the proliferation of HCC cells. In addition, we found
that high expression of PCBP2 may contribute to sorafenib
resistance in HCC cells. These results showed that PCBP2 may be a
novel prognostic marker of HCC and targeting PCBP2 may have
therapeutic implications for the treatment of HCC in clinical
practice.
Materials and methods
Patients and tissue specimens
The fresh-frozen human HCC tissues and adjacent
normal tissues were obtained from eight patients who underwent
surgery without preoperative systemic chemotherapy between 2003 and
2011 at the Department of Surgery, the Affiliated Hospital of
Nantong University. All tissues were from patients with newly
diagnosed HCC who had received no therapy before sample collection,
and were snap-frozen in liquid nitrogen immediately after surgery
and stored at −80°C until use. The liver cancer tissue microarrays
containing 65 matched primary HCC and adjacent non-tumorous tissues
were obtained from the Department of Pathology of the Affiliated
Hospital of Nantong University. The study population consisted of
40 males and 25 females, and the age ranged from 21 to 69 years.
The total cases of HCC patients had been approved by the Ethics
Committee of the Affiliated Hospital of Nantong University. The
main clinical and pathologic variables are summarized in Table I.
 | Table I.Clinicopathological features of HCC
in relation to the PCBP2 expression pattern. |
Table I.
Clinicopathological features of HCC
in relation to the PCBP2 expression pattern.
|
|
| PCBP2 |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | Total | Low | High | P-value |
|---|
| Age (years) |
|
|
|
|
|
<45 | 21 | 8 | 13 | 0.829 |
|
≥45 | 44 | 18 | 26 |
|
| Gender |
|
|
|
|
|
Female | 25 | 13 | 12 | 0.118 |
|
Male | 40 | 13 | 27 |
|
| Serum AFP level
(ng/ml) |
|
|
|
|
|
<50 | 14 | 4 | 10 | 0.324 |
|
≥50 | 51 | 22 | 29 |
|
| Cirrhosis |
|
|
|
|
|
Absent | 19 | 7 | 12 | 0.738 |
|
Present | 46 | 19 | 27 |
|
| HBsAg |
|
|
|
|
|
Negative | 27 | 15 | 12 | 0.031a |
|
Positive | 38 | 11 | 27 |
|
| Tumor no. |
|
|
|
|
|
Single | 43 | 18 | 25 | 0.669 |
|
Multiple | 22 | 8 | 14 |
|
| Maximal tumor size
(cm) |
|
|
|
|
|
<4.5 | 31 | 12 | 19 | 0.839 |
|
≥4.5 | 34 | 14 | 20 |
|
| Tumor
metastasis |
|
|
|
|
|
Absent | 46 | 17 | 29 | 0.436 |
|
Present | 19 | 9 | 10 |
|
| Microvascular
invasion |
|
|
|
|
|
Absent | 38 | 12 | 26 | 0.100 |
|
Present | 27 | 14 | 13 |
|
| Tumor
differentiation |
|
|
|
|
|
Well | 27 | 16 | 11 | 0.028a |
|
Moderate | 19 | 5 | 14 |
|
|
Poor | 19 | 5 | 14 |
|
| Capsular
formation |
|
|
|
|
|
Present | 36 | 11 | 25 | 0.083 |
|
Absent | 29 | 15 | 14 |
|
| Ki67
expression |
|
|
|
|
|
Negative | 30 | 16 | 14 | 0.042a |
|
Positive | 35 | 10 | 25 |
|
Immunohistochemistry
In order to analyze PCBP2 and Ki67, serial sections
(5 µm thick) were mounted on glass slides coated with 10%
polylysine. These sections were dewaxed in xylene and rehydrated
through graded alcohol. Immunoreactivity was enhanced by high
temperature and pressure, and these sections were boiled in 0.01 M
citrate buffer (pH 6.0) for 20 min in an autoclave to retrieve the
antigen. Thereafter, endogenous peroxidase activity was blocked
using hydrogen peroxide (0.3%). Anti-PCBP2 antibody (1:200; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C and
anti-Ki67 antibody (1:500; Millipore Corp., Bedford, MA, USA) for 4
h at room temperature were incubated in the sections after rinsing
in phosphate-buffered saline (PBS) (pH 7.2). Negative control
slides were incubated in parallel using a nonspecific
immunoglobulin IgG (Sigma-Aldrich, St. Louis, MO, USA) at the same
concentration as the primary antibody. All slides were processed
using the peroxidase-anti-peroxidase method (Dako, Hamburg,
Germany). Finally, slides were counterstained with hematoxylin,
dehydrated, and mounted in resin mount. Stained sections were
observed under a microscope (24).
Immunohistochemistry evaluation
The immunostaining results were evaluated by two
independent pathologists to avoid possible technical errors. Five
high-power fields were randomly chosen for assessment of PCBP2, and
at least 500 cells were counted per field. Each tumor section was
assigned a score according to the intensity of the cytoplasmic
staining and the proportion of stained tumor cells. The intensity
of staining was scored as 0 (negative), 1 (weak), 2 (moderate), or
3 (strong). The extent of staining was scored based on the
percentage of positive tumor cells: 0 (<10%), 1 (10–30%), 2
(>30–50%), 3 (>50–70%), and 4 (>70%). The immunostaining
score was counted as the percentage positive score × the staining
intensity score and ranged from 0 to 12. A score of 0 was
considered negative; 1–4, weak; 5–9, moderate; and 10–12, strong.
For statistical analysis, 0–4 was counted as low expression, while
5–12 was counted as high expression.
Cell culture and transfection
The human HCC cell lines, HepG2, Huh7, SMMC-7721 and
Hep3B and the normal liver cell line (LO2) were obtained from
Shanghai Institute of Cell Biology, Academia Sinica, and maintained
in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS), streptomycin 100 µg/ml, and penicillin
100 U/ml at 37°C in 5% CO2. The siRNA oligos of
PCBP2 were synthesized by GeneChem Co., Ltd. (Shanghai,
China). The targeted sequences of PCBP2 siRNA were as
follows: siRNA#1, CATCACTATTGCTGGCATT; siRNA#2,
CACTAATGCCATCTTCAAA; siRNA#3, CGGATTCAGT GGCATTG; and control,
TTCTCCGAATGTCACGT. Cell transfection was performed using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions.
Western blot analysis
Western blot analysis was conducted as previously
described (25). Briefly, frozen
liver tissues and harvested cells were promptly homogenized in a
homogenization buffer (50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1%
NP-40, 5 mM EDTA, 5 mM EGTA, 15 mM MgCl2, 60 mM
b-glycerophosphate, 0.1 mM sodium orthovanadate, 0.1 mM NaF) and
then centrifuged at 10,000 × g for 30 min to collect the
supernatant. Protein concentrations were determined using a Bio-Rad
BCA protein assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Equal amounts of protein samples were separated by 10%
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred
to polyvinylidene fluoride (PVDF) filter membranes (Millipore
Corp.). The membranes were blocked with 5% dried skim milk in TBST
(20 mM Tris-Cl, 150 mM NaCl, 0.05% Tween-20) and incubated with
primary antibodies overnight at 4°C. The primary antibodies used in
the study were as follows: anti-PCBP2 (1:1,000), anti-PCNA
(1:1,000), cyclin D1 (1:500), anti-p27 (1:1,000), anti-Bax (1:500);
anti-Bcl-2 (1:500), anti-active caspase-3 (1:500) (all from Santa
Cruz Biotechnology, Inc.), and anti-GAPDH (1:1,000; Sigma-Aldrich).
The membranes were washed with TBST for 5 min for three times.
Then, horseradish peroxidase-linked IgG was used as the secondary
antibody (1:5,000; Pierce Biotechnology, Inc., Rockford, IL, USA)
for 2 h at room temperature according to the manufacturer's
instructions. The detection of immunoreactive bands was performed
using an enhanced chemiluminescence system (NEN Life Science
Products, Inc., Boston, MA, USA).
Cell Counting Kit-8
Cell proliferation was measured using Cell Counting
Kit-8 (CCK-8) assay (Dojindo Laboratories, Kumamoto, Japan)
following the manufacturer's instructions. Briefly, cells were
plated into a 96-well plate at a density of 1×104
cells/well in a volume of 100 µl. For proliferation measurement, 10
µl of CCK-8 reagent plus 90 µl of DMEM complete medium was added to
each well for a 2-h incubation at 37°C. The absorbance of cells was
read in a microplate reader (Bio-Rad Laboratories, Inc.) at 450 nm
with a reference wavelength of 650 nm. The detection of cell
absorbance was performed every 24 h. The experiments were repeated
at least three times (26).
Plate colony formation assay
After transfection, HepG2 and Huh7 cells were plated
into a 6-well plate (500 cells/well). Fifteen days later, the
colonies were washed with PBS, fixed with paraformaldehyde for 20
min, and then stained with 0.5% crystal violet for 30 min. Cell
colonies (0.5 mm in diameter) were counted after staining.
Annexin-V/PI apoptotic analysis
The apoptosis of HCC cells was evaluated by
FITC-Annexin V and propidium iodide (PI) assay using a commercial
kit (BD Biosciences, Shanghai, China) as previously described
(27). The upper right quadrant
(UR) indicates the late apoptotic and necrotic cells, the lower
right quadrant (LR) represents the early apoptotic cells, the upper
left quadrant (UL) represents the debris and damaged cells, while
the lower left quadrant (LL) represents the negative control cells.
In this study, we consider UR and LR as apoptotic cells.
Statistical analysis
The results are expressed as mean ± SEM. Statistical
analysis was performed using the Stata 7.0 software package. The
association between PCBP2 expression and clinicopathological
features was analyzed using the χ2 test. For analysis of
survival data, Kaplan-Meier curves were constructed, and the
log-rank test was performed. For multivariate analysis, Cox's
proportional hazards model was used and p<0.05 was considered to
be statistically significant.
Results
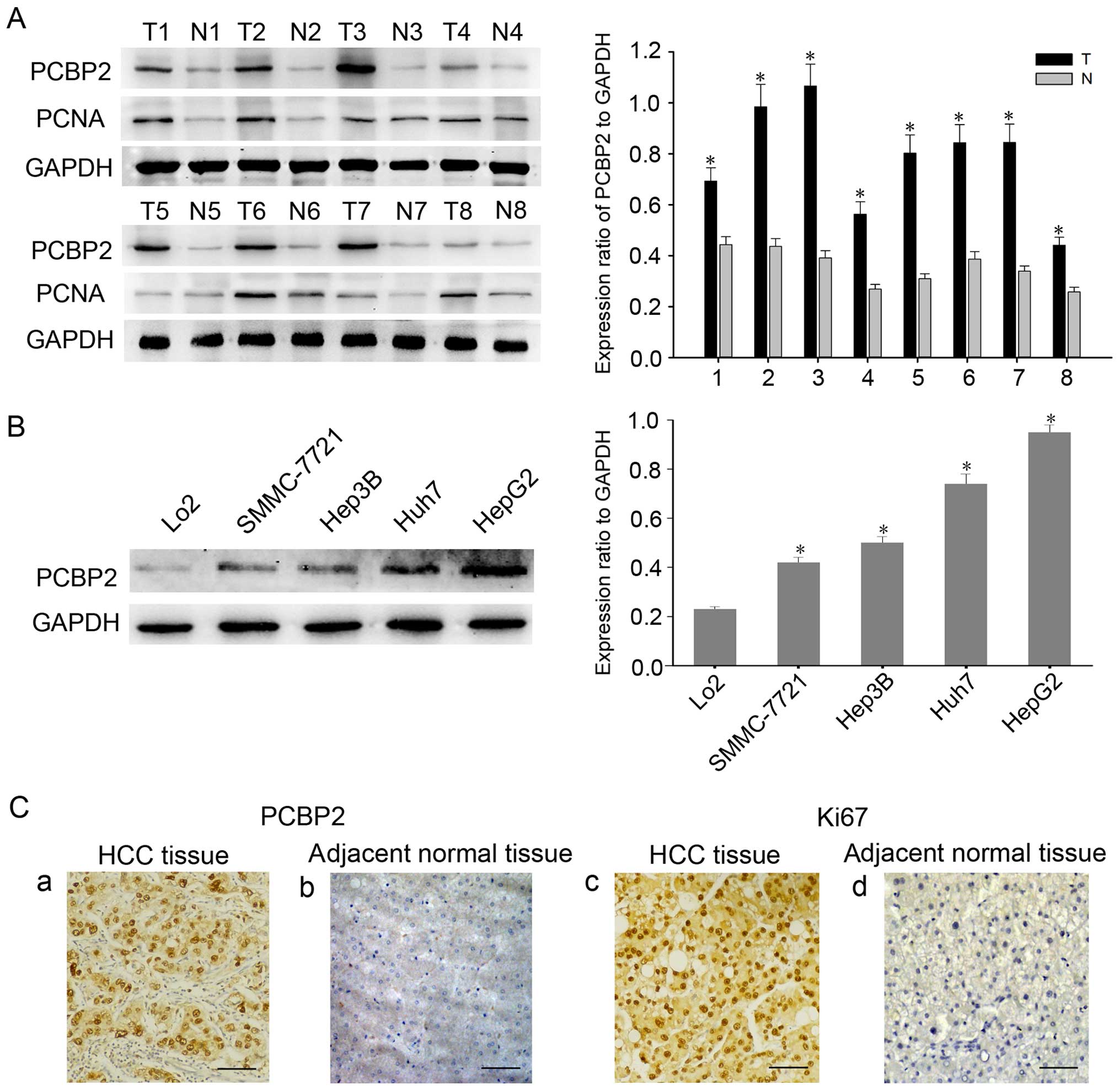
PCBP2 is highly expressed in HCC
tissues and cell lines
To determine the expression of PCBP2 in HCC tissues,
we first studied the expression of PCBP2 in HCC tissues and the
adjacent non-tumor liver tissues by western blot analysis. As shown
in Fig. 1A, we found that the
expression of PCBP2 was significantly elevated in the HCC tissues,
compared with that in the adjacent normal ones. Moreover, the
expression profile of PCBP2 was detected in the HCC cell lines
(HepG2, Huh7 and SMMC-7721) and the normal liver cell line (LO2).
Similarly, we found that HCC cell lines had a higher PCBP2 level
compared with that noted in the normal liver LO2 cells (Fig. 1B). Then, we conducted
immunohistochemical staining to confirm the expression of PCBP2 and
Ki67 in the HCC specimens. Following the different histological
stage, representative samples of PCBP2 and Ki67 are shown in
Fig. 1C. In most specimens, PCBP2
was highly expressed in the cytoplasm and nucleus of the HCC
tissues, whereas an obvious low signal of PCBP2 was observed in the
adjacent non-tumor tissue samples (Fig.
1C-a and -b), which is in line with the expression of Ki-67
(Fig. 1C-c and -d). These findings
indicated that PCBP2 may contribute to malignant progression of
HCC.
Relationship between PCBP2 expression
and clinicopathological parameters of HCC
To further investigate the significance and
prognostic value of PCBP2, we analyzed the correlations between
clinicopathological characteristics and the expression of PCBP2 in
65 patients with HCC. The results are summarized in Table I. We found that the expression of
PCBP2 was correlated with tumor differentiation (p=0.028), HBsAg
(p=0.031) and Ki67 expression (p=0.042), but not with other
clinicopathological factors, such as age, gender, tumor size, tumor
number, tumor metastasis, serum AFP level, cirrhosis, microvascular
invasion and capsular formation.
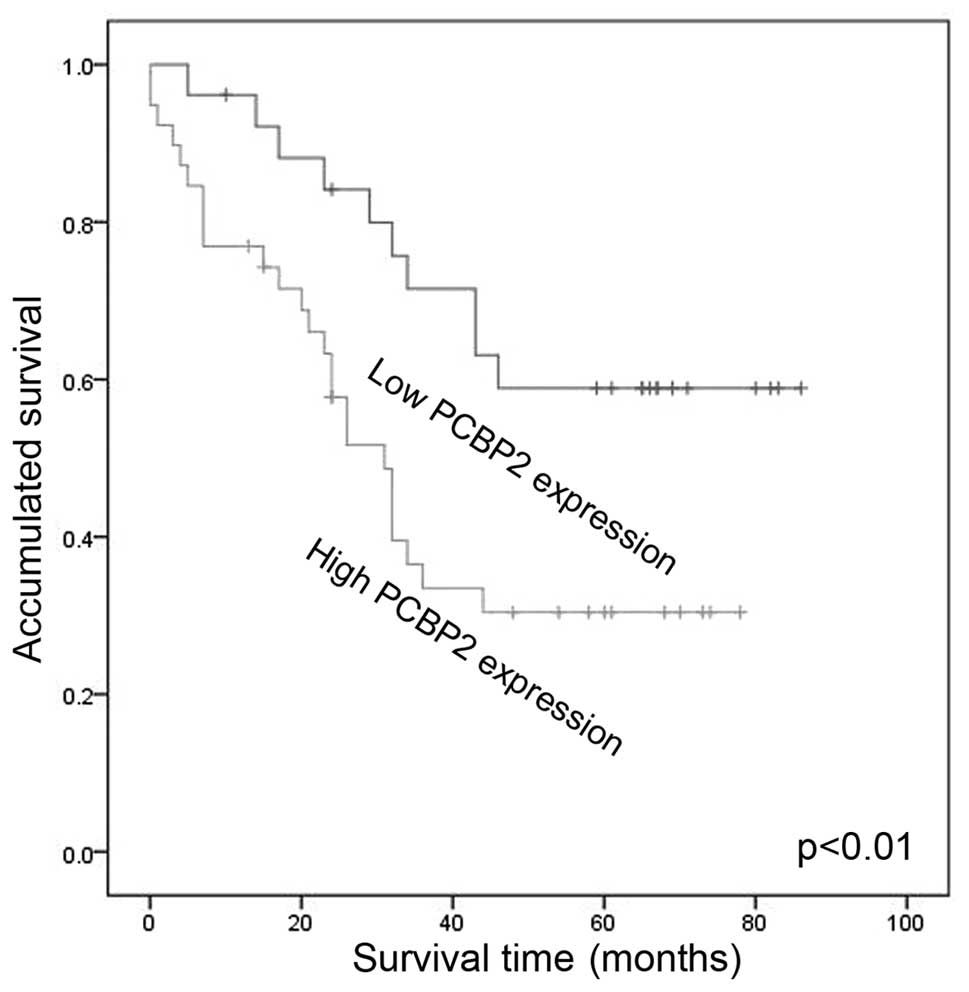
The prognostic significance of PCBP2
expression
Next, we performed Kaplan-Meier analysis to examine
whether PCBP2 could serve as an independent indicator to predict
the prognosis of HCC patients. Our data showed that patients with
high PCBP2 expression had significantly worse prognosis compared
with that of patients with low PCBP2 expression (Fig. 2). Based on the results of univariate
analyses, we identified that PCBP2 expression (p=0.016), Ki67
(p=0.018), tumor differentiation (p=0.007) and HBsAg (p=0.036) were
associated with patient survival. Furthermore, multivariate Cox
proportional hazard analysis showed that PCBP2 (p=0.043) could be
an independent prognostic indicator of overall survival (Table II).
 | Table II.Contribution of various potential
prognostic factors to survival by Cox regression analysis in HCC
speciments. |
Table II.
Contribution of various potential
prognostic factors to survival by Cox regression analysis in HCC
speciments.
|
|
| Univariate
analysis |
| Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Parameters | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years) | 1.030 | 0.494–2.148 | 0.937 |
|
|
|
| Gender | 0.587 | 0.302–1.144 | 0.118 |
|
|
|
| Serum AFP level
(ng/ml) | 0.562 | 0.269–1.171 | 0.124 |
|
|
|
| Cirrhosis | 1.406 | 0.658–3.002 | 0.379 |
|
|
|
| Tumor no. | 0.979 | 0.479–2.000 | 0.954 |
|
|
|
| Maximal tumor size
(cm) | 0.913 | 0.470–1.773 | 0.787 |
|
|
|
| Tumor
metastasis | 1.077 | 0.517–2.245 | 0.843 |
|
|
|
| Microvascular
invasion | 1.161 | 0.594–2.269 | 0.662 |
|
|
|
| HBsAg | 2.197 | 1.051–4.592 | 0.036a | 1.141 | 0.287–4.529 | 0.851 |
| Tumor
differentiation | 1.784 | 1.174–2.712 | 0.007a | 1.532 | 0.877–2.675 | 0.134 |
| Capsular
formation | 0.685 | 0.353–1.330 | 0.264 |
|
|
|
| Ki67 | 2.370 | 1.158–4.858 | 0.018a | 1.231 | 0.277–5.461 | 0.880 |
| PCBP2 | 2.471 | 1.181–5.172 | 0.016a | 2.169 | 1.024–4.592 | 0.043a |
PCBP2 is highly expressed in
proliferating HCC cells
Based on our data that PCBP2 expression is
associated with PCNA and Ki67, we considered that PCBP2 may promote
HCC development by regulating the proliferation of HCC cells.
Therefore, we analyzed the expression of PCBP2 during cell cycle
progression in HCC cells. HepG2 and Huh7 cells were arrested in G1
phase by serum deprivation for 72 h. After serum-refeeding, the
cells were released from G1 phase and entered into the S phase
(Fig. 3A and B). Western blot
analysis showed that the expression of PCBP2 increased gradually
with time following the release of cells from serum starvation in
both cell lines. At the same time, we found that the expression of
cyclin D1 was upregulated (Fig.
3C-F). These results suggest that PCBP2 may play a key role in
regulating HCC cell proliferation.
Depletion of PCBP2 inhibits cell
proliferation in HCC
To determinate the effect of PCBP2 in HCC cell
proliferation, we firstly transfected HepG2 and Huh7 cells with
PCBP2 siRNA oligos to knockdown endogenous PCBP2. Western blot
analysis was carried out at 48 h after transfection, which
confirmed a significant reduction in PCBP2 expression in the
PCBP2-siRNA#3-transfected cells (Fig.
4A). Therefore, PCBP2-siRNA#3 was used for further experiments.
At the same time, we discovered that the silencing of PCBP2
expression obviously downregulated the levels of cyclin D1 and
PCNA, with the upregulated levels of cyclin-dependent kinase
inhibitor p27 (Fig. 4B).
Furthermore, CCK-8 assay was used to confirm the effect of PCBP2 on
HCC cell proliferation. The HepG2 and Huh7 cells transfected with
PCBP2 siRNA#3 exhibited a marked decreased in the cell
proliferation, as compared with the control siRNA-transfected ones
(Fig. 4C). Likewise, colony
formation assay revealed that depletion of PCBP2 markedly inhibited
the colony formation capacity of the HepG2 and Huh7 cells (Fig. 4D). According to the above results,
we further demonstrated whether PCBP2 expression could affect the
cell cycle distribution in HCC cells by flow cytometric analysis.
The results showed that depletion of PCBP2 caused G0/G1 phase
arrest, accompanying a marked reduction in the cell population in
the S phase (Fig. 4E). These
results suggest that depletion of PCBP2 inhibits the proliferation
of HCC cells.
High expression of PCBP2 in HCC cells
contributes to sorafenib resistance
Sorafenib (Nexavar), a multiple kinase inhibitor,
has shown survival benefits in patients with advanced HCC and has
become the first approved drug for HCC (28,29).
However, the clinical response of sorafenib was seriously limited
by drug resistance and thus we tested the possibility that high
expression of PCBP2 may be involved in sorafenib resistance in HCC.
We firstly investigated the sensitivity of HCC cells to sorafenib.
The results showed that sorafenib induced the apoptosis of HCC
cells in a dose-dependent manner (Fig.
5A). Then we transfected HepG2 cells with PCBP2 siRNAs#3 or
control siRNA, and exposed the cells to sorafenib (50 µM) or DMSO.
The flow cytometric analysis showed that depletion of PCBP2
significantly increased the percentage of apoptotic cells in the
absence of sorafenib treatment (33.56 vs. 55.64%). Following
sorafenib treatment, PCBP2-depleted cells exhibited a markedly
increased level of apoptosis (59.01 vs. 78.38%), compared with the
mock-transfected group (Fig. 5B).
The bar chart shows the ratio of apoptotic cells by densitometry
(Fig. 5C). These results revealed
that interference of PCBP2 could potentiate HCC cells to
sorafenib-induced apoptosis. Furthermore, western blot analysis
showed that transfection with PCBP2 siRNA#3 significantly increased
the level of active caspase-3, Bax and inhibited the level of
Bcl-2, especially after sorafenib treatment (Fig. 5D). The bar chart shows the ratio of
PCBP2, active caspase-3, Bax and Bcl-2 expression to GAPDH
(Fig. 5E). These results indicate
that high expression of PCBP2 may contribute to sorafenib
resistance by regulating Bcl-2 proteins in HCC cells.
 | Figure 5.High expression of poly(C)-binding
protein 2 (PCBP2) contributes to sorafenib resistance in
hepatocellular carcinoma (HCC) cells. (A) Annexin V/PI staining
analysis of the apoptosis in HepG2 cells following different doses
(0, 10, 20, 30, 40 and 50 µM) of sorafenib exposure for 24 h. Data
are represented as the mean ± SEM from three independent
experiments (*P<0.05). (B) FITC-Annexin V/PI apoptotic analysis
of PCBP2-deleted HepG2 cells with or without sorafenib (50 µM).
Upper left quadrants (UL), debris and damaged cells; upper right
quadrants (UR), late apoptotic and necrotic cells; lower left
quadrants (LL), negative control cells; lower right quadrants (LR),
early apoptotic cells. We consider UR and LR as apoptotic cells.
(C) The bar chart indicates the ratio of apoptotic cells by
densitometry. Data are represented as the mean ± SEM from three
independent experiments (*p<0.05, *#p<0.05). (D)
HepG2 cells were transfected with control siRNA or PCBP2 siRNA#3,
and then incubated with 50 µM sorafenib or vehicle (DMSO) for 24 h.
The cell lysates were subjected to western blot analysis using the
indicated antibodies. (E) The bar chart shows the ratio of PCBP2,
active caspase-3, Bax and Bcl-2 expression to GAPDH. Data are
represented as the mean ± SEM from three independent experiments
(*, #, %, ^p<0.05). |
Discussion
HCC represents one of the most common cancer types
worldwide, with a low survival rate (30). Despite that significant improvements
have been made to better understanding the molecular mechanism
underlying HCC initiation and progression, the diagnosis of HCC
remains delayed and the prognosis of HCC patients remains poor
(2,31). Thus, the development of novel
molecular targets and more effective anti-neoplastic therapies are
urgent for us to improve the survival rate of this deadly
disease.
PCBP2, as a member of the PCBP family, has been
reported to be overexpressed in pancreatic cancer, glioma, gastric
carcinoma and prostate cancer. Consistently, we found that PCBP2
was also highly expressed in HCC. Moreover, our study showed that
PCBP2 expression was associated with HBsAg expression. Since HCC is
closely related with HBV infection in many countries, we speculated
that this result may indicate a potential involvement of PCBP2 in
HBV-associated HCC development. At the same time, the Cox
regression analysis suggested that PCBP2 represented a novel
independent indicator of HCC prognosis. In particular, we found
that PCBP2 is associated with HCC cell growth and sorafenib
resistance. Taken together, the above data suggest that PCBP2 may
be a novel prognostic indicator and therapeutic target of HCC.
The molecular mechanism underlying the oncogenic
property of PCBP2 remains largely elusive. There have been several
studies indicating that PCBP2 functions via regulation of miRNA
pathways, such as miR-328, miR-34a and miR-16, to promote the
growth of tumors (16,17,32).
Koganti et al found that STAT3 exploited cellular PCBP2 to
regulate the refractory state in EBV-positive Burkitt lymphoma
(33). It has been found that PCBP2
depletion inhibits tumor cell growth through inhibition of
cell-cycle progression and induction of apoptosis (15,17).
In our study, we discovered that PCBP2 promoted HCC cell growth
involving dysregulated expression of cyclin-dependent kinase
inhibitor p27, which plays a pivotal roles in cell cycle
regulation. Then, we speculated that PCBP2 aberrantly regulated
cell cycle progression and eventually transformed into HCC, which
was confirmed by flow cytometry analysis (Fig. 4E). More importantly, we first found
that depletion of PCBP2 enhanced sorafenib cytotoxicity in HCC
cells, which was accompanied by altered expression of Bax and
Bcl-2. Rencently, the genomic amplification of oncogenes has been
reported to play a key role in hepatocarcinogenesis and drug
resistance (34,35). According to our study, we believe
that the genomic amplification of PCBP2 may occur in human HCC,
which may be associated with HCC development and sorafenib
resistance. However, the specific mechanism remains obscure and
needs further study.
In conclusion, our study first revealed that PCBP2
is overexpressed in HCC and is correlated with poor prognosis and
shorter survival rate in HCC patients. Moreover, the expression of
PCBP2 promotes cell cycle progression in HCC cells. In addition, we
found that high expression of PCBP2 may contribute to aberrant
proliferation and drug resistance in HCC. We expect that these
findings will accelerate our understanding of HCC development and
PCBP2 may serve a candidate drug target for improving the survival
of HCC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (nos. 81502072, 81402024 and 81272708),
the Nantong University on Key Basic Research Project (14Z023) and
the Nantong Science and Technology Project (MS12015054) and a
project funded by the Priority Academic Program Development of
Jiangsu Higher Education Institutions (PAPD).
References
|
1
|
Tsochatzis EA, Meyer T and Burroughs AK:
Hepatocellular carcinoma. N Engl J Med. 366:92–93; author reply
92–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malek NP, Schmidt S, Huber P, Manns MP and
Greten TF: The diagnosis and treatment of hepatocellular carcinoma.
Dtsch Arztebl Int. 111:101–106. 2014.PubMed/NCBI
|
|
3
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finn RS: Development of molecularly
targeted therapies in hepatocellular carcinoma: Where do we go now?
Clin Cancer Res. 16:390–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andino R, Böddeker N, Silvera D and
Gamarnik AV: Intracellular determinants of picornavirus
replication. Trends Microbiol. 7:76–82. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blyn LB, Towner JS, Semler BL and
Ehrenfeld E: Requirement of poly(rC) binding protein 2 for
translation of poliovirus RNA. J Virol. 71:6243–6246.
1997.PubMed/NCBI
|
|
7
|
Collier B, Goobar-Larsson L, Sokolowski M
and Schwartz S: Translational inhibition in vitro of human
papillomavirus type 16 L2 mRNA mediated through interaction with
heterogenous ribonucleoprotein K and poly(rC)-binding proteins 1
and 2. J Biol Chem. 273:22648–22656. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang FM, Li WM, Chen Y, Wang DM and Han J:
Expression of hnRNP K in lung adenocarcinoma cells. Sichuan Da Xue
Xue Bao Yi Xue Ban. 39:823–826. 2008.(In Chinese). PubMed/NCBI
|
|
9
|
He Y, Brown MA, Rothnagel JA, Saunders NA
and Smith R: Roles of heterogeneous nuclear ribonucleoproteins A
and B in cell proliferation. J Cell Sci. 118:3173–3183. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou ZJ, Dai Z, Zhou SL, Fu XT, Zhao YM,
Shi YH, Zhou J and Fan J: Overexpression of HnRNP A1 promotes tumor
invasion through regulating CD44v6 and indicates poor prognosis for
hepatocellular carcinoma. Int J Cancer. 132:1080–1089. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Gai L, Liu J, Cui Y, Zhang Y and
Feng J: Expression of poly(C)-binding protein 1 (PCBP1) in NSCLC as
a negative regulator of EMT and its clinical value. Int J Clin Exp
Pathol. 8:7165–7172. 2015.PubMed/NCBI
|
|
12
|
Sean P, Nguyen JH and Semler BL: The
linker domain of poly(rC) binding protein 2 is a major determinant
in poliovirus cap-independent translation. Virology. 378:243–253.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sean P, Nguyen JH and Semler BL: Altered
interactions between stem-loop IV within the 5′ noncoding region of
coxsackievirus RNA and poly(rC) binding protein 2: Effects on
IRES-mediated translation and viral infectivity. Virology.
389:45–58. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Molinaro RJ, Jha BK, Malathi K, Varambally
S, Chinnaiyan AM and Silverman RH: Selection and cloning of
poly(rC)-binding protein 2 and Raf kinase inhibitor protein RNA
activators of 2′,5′-oligoadenylate synthetase from prostate cancer
cells. Nucleic Acids Res. 34:6684–6695. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han W, Xin Z, Zhao Z, Bao W, Lin X, Yin B,
Zhao J, Yuan J, Qiang B and Peng X: RNA-binding protein PCBP2
modulates glioma growth by regulating FHL3. J Clin Invest.
123:2103–2118. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin X, Yang B, Liu W, Tan X, Wu F, Hu P,
Jiang T, Bao Z, Yuan J, Qiang B, et al: Interplay between PCBP2 and
miRNA modulates ARHGDIA expression and function in glioma migration
and invasion. Oncotarget. 7:19483–19498. 2016.PubMed/NCBI
|
|
17
|
Hu CE, Liu YC, Zhang HD and Huang GJ: The
RNA-binding protein PCBP2 facilitates gastric carcinoma growth by
targeting miR-34a. Biochem Biophys Res Commun. 448:437–442. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan C, Gong C, Zhang H, Hua L, Li X, Chen
X, Chen Y, Ding X, He S, Cao W, et al: β2-adrenergic receptor
signaling promotes pancreatic ductal adenocarcinoma (PDAC)
progression through facilitating PCBP2-dependent c-myc expression.
Cancer Lett. 373:67–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fukushi S, Okada M, Kageyama T, Hoshino
FB, Nagai K and Katayama K: Interaction of poly(rC)-binding protein
2 with the 5′-terminal stem loop of the hepatitis C-virus genome.
Virus Res. 73:67–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tingting P, Caiyun F, Zhigang Y, Pengyuan
Y and Zhenghong Y: Subproteomic analysis of the cellular proteins
associated with the 3′ untranslated region of the hepatitis C virus
genome in human liver cells. Biochem Biophys Res Commun.
347:683–691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Jeng KS and Lai MM:
Poly(C)-binding protein 2 interacts with sequences required for
viral replication in the hepatitis C virus (HCV) 5′ untranslated
region and directs HCV RNA replication through circularizing the
viral genome. J Virol. 85:7954–7964. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shukla RS, Qin B, Wan YJ and Cheng K:
PCBP2 siRNA reverses the alcohol-induced pro-fibrogenic effects in
hepatic stellate cells. Pharm Res. 28:3058–3068. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leidgens S, Bullough KZ, Shi H, Li F,
Shakoury-Elizeh M, Yabe T, Subramanian P, Hsu E, Natarajan N,
Nandal A, et al: Each member of the poly-r(C)-binding protein 1
(PCBP) family exhibits iron chaperone activity toward ferritin. J
Biol Chem. 288:17791–17802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Wang Y, Xiang J, Ji F, Deng Y,
Tang C, Yang S, Xi Q, Liu R and Di W: Knockdown of CRM1 inhibits
the nuclear export of p27(Kip1) phosphorylated at serine 10 and
plays a role in the pathogenesis of epithelial ovarian cancer.
Cancer Lett. 343:6–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Liu F, Mao F, Hang Q, Huang X, He
S, Wang Y, Cheng C, Wang H, Xu G, et al: Interaction with cyclin
H/cyclin-dependent kinase 7 (CCNH/CDK7) stabilizes C-terminal
binding protein 2 (CtBP2) and promotes cancer cell migration. J
Biol Chem. 288:9028–9034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akiba N, Hayakawa I, Keh ES and Watanabe
A: Antifungal effects of a tissue conditioner coating agent with
TiO2 photocatalyst. J Med Dent Sci. 52:223–227.
2005.PubMed/NCBI
|
|
27
|
Cragg MS and Glennie MJ: Antibody
specificity controls in vivo effector mechanisms of anti-CD20
reagents. Blood. 103:2738–2743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okuda K, Ohtsuki T, Obata H, Tomimatsu M,
Okazaki N, Hasegawa H, Nakajima Y and Ohnishi K: Natural history of
hepatocellular carcinoma and prognosis in relation to treatment.
Study of 850 patients. Cancer. 56:918–928. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rahbari NN, Mehrabi A, Mollberg NM, Müller
SA, Koch M, Büchler MW and Weitz J: Hepatocellular carcinoma:
Current management and perspectives for the future. Ann Surg.
253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eiring AM, Harb JG, Neviani P, Garton C,
Oaks JJ, Spizzo R, Liu S, Schwind S, Santhanam R, Hickey CJ, et al:
miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation
of mRNA translation in leukemic blasts. Cell. 140:652–665. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koganti S, Clark C, Zhi J, Li X, Chen EI,
Chakrabortty S, Hill ER and Bhaduri-McIntosh S: Cellular STAT3
functions via PCBP2 to restrain Epstein-Barr Virus lytic activation
in B lymphocytes. J Virol. 89:5002–5011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Midorikawa Y, Yamamoto S, Ishikawa S,
Kamimura N, Igarashi H, Sugimura H, Makuuchi M and Aburatani H:
Molecular karyotyping of human hepatocellular carcinoma using
single-nucleotide polymorphism arrays. Oncogene. 25:5581–5590.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo X and Feng GS: VEGFA genomic
amplification tailors treatment of HCCs with sorafenib. Cancer
Discov. 4:640–641. 2014. View Article : Google Scholar : PubMed/NCBI
|