Introduction
Lung cancer is one of the most prevalent and fatal
type of cancers in the world, accounting for ~20% of all
cancer-related death (1). Lung
cancer can be divided into two broad categories, small cell lung
cancer and non-small cell lung cancer (NSCLC). NSCLC accounting for
~80% of all lung cancer cases leads to the highest mortality with a
relative poor 5-year survival rate. Although surgical and
chemotherapeutic techniques have made great progress for the
treatment of lung cancer, the prognosis of NSCLC is very poor due
to toxicity, high incidence of recurrence, and other side effects,
with a 5-year survival rate as low as 15% (2). Therefore, it is urgent and necessary
for us to explore new strategies or drugs with fewer side effects
for the management of NSCLC.
Heat shock protein (Hsp) family is a group of
conserved molecular chaperons that facilitate proper protein
folding, modification, and transportation, and are known as
inhibitors of apoptosis (3). Hsp
expression usually increases in cells under stressful condition,
including increased temperature, hypoxia, and exposure to cytotoxic
agents, to protect cells from injury (4). Hsp70 is a member of Hsps, and Hsp70
overexpression has been reported to be associated with a wide range
of malignances (5,6). Increasing results have reported that
compared to healthy individuals, Hsp70 has been overexpressed in
serum and tissue samples from patients with NSCLC (7,8).
Furthermore, overexpression of Hsp70 is associated with adverse
prognosis and resistance to chemotherapy (5). Selective depletion of Hsp70 in lung
cancer cells results in apoptotic cell death, while not in normal
lung cells, suggesting that targeting Hsp70 may be a potential
approach for cancer therapeutics (9).
Previous results identified the small molecular
2-phenylethynesulfonamide (PES), also known as pifithrin-μ, as a
specific inhibitor of stress-inducible Hsp70, which induced tumor
cell death but markedly showed less toxic to non-transformed cells
(10). Several pivotal survival
pathways for cancer were impaired by PES through disruption of
HSP70/HSP90 chaperone system (11).
Data reported by Granato et al indicated that PES induced
immunogenic cell death via lysosomal cathepsin D release in primary
effusion lymphoma (12). In
addition, PES enhanced 17AAG, an Hsp90 inhibitor of antitumor
activities against acute leukemia and bladder cancer cells
(13,14). Furthermore, PES synergistically
enhanced antitumor activity of hyperthermia against prostate cancer
cells (15). However, little is
known about the effect of PES on human lung cancer cells, and no
study have been conducted to evaluate whether PES shows a
chemosensitizing effect on human lung cancer cells.
We investigated the ability of PES to inhibit
proliferation of NSCLC cell lines in vitro and in
vivo, and further explored the underlying molecular mechanisms.
In addition, we evaluated the antitumor effect of PES in
combination with tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL). Our results suggest that PES can effectively
inhibit cell proliferation and migration in human NSCLC cells.
Furthermore, PES can induce G0/G1 phase cell cycle arrest and cell
apoptosis via a caspase-dependent manner. Overexpression of Hsp70
in A549 cells attenuates the effect of PES on cell proliferation
inhibition. Knockdown of Hsp70 by siRNA in A549 cells plays a
potent synergistic effect on cell proliferation inhibition. In
addition to reduction of p-AKT and p-ERK, PES sensitized NSCLC cell
lines to TRAIL-induced cell proliferation inhibition and apoptosis
via upregulation of DR4 and DR5. Finally, our results show that
lung cancer xenografts in nude mice are efficiently inhibited by
PES treatment.
Materials and methods
Cell lines and reagents
The human non-small cell lung cancer (NSCLC) cells
(A549 and H460) purchased from the American Type Culture Collection
(Manassas, VA, USA) were maintained in DMEM supplemented with 10%
FBS (Gibco, Gaithersburg, MD, USA) at 37°C in a humidified
atmosphere with 5% CO2. The heat-shock protein 70
(Hsp70) inhibitor PES, (pifithrin-μ), was purchased from Calbiochem
(San Diego, CA, USA). Recombinant TRAIL was obtained from
Invitrogen (Carlsbad, CA, USA). PES and recombinant TRAIL were
dissolved in DMSO (Sigma, St. Louis, MO, USA) and PBS containing
0.1% (w/v) bovine serum albumin (BSA), respectively.
Plasmids and cell transfection
The plasmid pSG5-Hsp70 was kindly provided by
Professor X. Sun (School of Basic Medical Sciences, Wuhan
University, Wuhan, China). Hsp70 siRNA and control siRNA were
obtained from GenePharma (GenePharma, Shanghai, China). A549 cells
were transiently transfected with pSG5-Hsp70 using X-tremeGENE HP
DNA Transfection Reagent (Roche, Basel, Switzerland) according to
the manufacturer's instructions. A549 cells were transiently
transfected with Hsp70 siRNA or control siRNA using Oligofectamine
(Invitrogen) according to the manufacturer's instructions.
Cell viability analysis
The cell viability was determined by the Cell
Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan)
assay. Briefly, A549 and H460 cells were incubated in 96-well
plates at a density of 5×103 per 100 µl of culture
medium overnight. After treated with indicated concentration of PES
for 24 and 48 h, 10 µl of tetrazolium substrate were added to each
well of the plate. After incubation at 37°C for 1 h, the absorbance
was recorded at a wavelength of 450 nm using a microplate reader
(EXL800; BioTek, Winooski, VT, USA). Each experiment was determined
in triplicate and repeated at least three times.
Wound healing assay
A549 and H460 cells (5×105) were placed
and culture in 6-well plates overnight. A sterile 10-µl pipette tip
was used to create wounds in 6-well plates. The cells were washed
with PBS and cultured with fresh medium with or without PES. After
incubation for further 48 h, an inverted microscope was used to
determine the wounds.
Transwell migration assay
Transwell migration assay was conducted in a
Transwell chambers (Corning, New York, NY, USA) with a
polycarbonate membrane (8-µm polyester membrane filter pores).
Cells were starved for 24 h prior to the experiment, and then
1×105 cells were seeded into the upper chamber with 100
µl serum-free medium. The bottom chamber contained 500 µl of medium
containing 10% FBS to serve as a chemoattractant. Following
incubation at 37°C for 48 h, the cells adhering to the lower
surface of the membrane were fixed, stained, and captured.
Flow cytometry analysis
Cell cycle arrest and induction of apoptosis by PES
were assessed by flow cytometry (BD Biosciences). A549 and H460
cells (2×105/well) were seeded in 6-well plates and
treated with vehicle control or PES (20 µM) for 24 h, respectively.
For cell cycle analysis, above cells were collected and fixed with
500 µl of 70% ethanol at −20°C overnight. The fixed cells were
washed with cold PBS 3 times. Cell cycle distribution was
determined by using Cell cycle Detection kit (Multisciences,
Hangzhou, China) according to the manufacturer's instructions. For
apoptosis analysis, PES or vehicle control treatment of A549 and
H460 cells were collected. Annexin V-FITC/propidium iodide (PI)
Apoptosis Detection kit (Multisciences) was used to detect cell
apoptosis induced by PES according to the manufacturer's
instructions.
Caspase-3 activity assay
Caspase-3 activity assay kits from Beyotime
(Shanghai, China) were used to detect caspase-3 activity. In brief,
human NSCLC cells were treated with PES and TRAIL, either alone or
in combination for 24 h. The above cells were washed with cold PBS
3 times, followed by lysis buffer treatment on ice for 30 min.
After centrifuged at 12,000 g for 10 min, cell lysate supernatant
(10 µl), assay buffer (80 µl) and caspase-3 substrate (Ac-DEVD-pNA,
10 µl) were added to each well in a 96-well plate. The samples were
further incubated at 37°C for 12 h, and their optical density (OD)
was detected at a wavelength of 405 nm using a microplate reader
(BioTek, EXL800; BioTek). Each experiment was determined in
triplicate and repeated at least three times.
Western blotting
Cells were lysed in RIPA lysis buffer (Beyotime)
supplemented with 0.5% cocktail protease inhibitor (Roche). The
cell lysates were centrifuged at 12,000 g for 10 min at 4°C, and
the supernatants were collected. Protein concentrations in the
supernatants were measured according to the bicinchoninic acid
method using bovine serum albumin as a standard. Equal amounts of
proteins mixed with 5X loading buffer were subjected to 10%
SDS-PAGE gels and transferred to PVDF membranes (Bio-Rad, Hercules,
CA, USA). After blocking the membranes with 5% non-fat milk in TBST
for 1 h at room temperature, the membranes were incubated with
desired primary antibodies overnight at 4°C. After 3×5 min washes
in TBST, the membranes were incubated with corresponding
horseradish-peroxidase-conjugated secondary antibodies for 1 h at
room temperature. ECL systems (Bio-Rad) were used to detect
expression of antibody-bound proteins.
The primary antibodies used in this study were as
follows: GAPDH (10494-1-AP) from Proteintech (Peking, China),
Vimentin (5741), cleaved caspase-3 (9664), cleaved caspase-9
(7237), Hsp70 (4872), Akt (9272), p-Akt (4060), ERK (9102), and
p-ERK (4370) from Cell Signaling Technology (Cambridge, UK), p21
(sc-397), cyclin A (sc-751), CDK2 (sc-163), MMP9 (sc-21733),
E-cadherin (sc-8426) cleaved PARP (sc-56196), DR4 (sc-8411), and
DR5 (sc-166624) from Santa Cruz Biotechnology (Santa Cruz, CA,
USA). The secondary antibodies were
horseradish-peroxidase-conjugated secondary anti-mouse IgG (Kerui
Tech, Wuhan, China) or anti-rabbit IgG (Kerui Tech).
In vivo xenograft model
Athymic female nude mice (6 weeks of age) were
purchased from Beijing HFK Bioscience Co. Ltd. (Beijing, China) and
housed under pathogen-free conditions. Animal care and use were
approved by the Medical Ethics Committee of Wuhan University.
A549 cells (1×107) were suspended in
Matrigel (BD Biosciences) and inoculated subcutaneously into the
mice. Twelve mice bearing evident tumors were arbitrarily assigned
to PBS control group and PES treatment groups (six mice per group).
When tumors reached a size of ~5×5 mm2, mice were
treated with either a single of intraperitoneal injection of PES
(20 mg/kg) or PBS every two days. After 3-week treatment, mice were
euthanized with carbon dioxide. Tumor burdens were evaluated by
measuring body weight, tumor weight, and tumor volume. Tumor volume
was determined as 0.5 × length × width2. Tumor samples
were collected and fixed in 10% neutral buffered formalin.
Hematoxylin and eosin staining and immunohistochemistry for
histological analysis of tumor samples were performed as described
previously (16).
Statistical analysis
Data were expressed as the mean ± standard deviation
(mean ± SD). Statistical analysis was performed using SPSS software
(version 19.0, SPSS, Chicago, IL, USA). The significance of the
difference between two groups was determined using the Student's
t-test. Values of P<0.05 were considered statistically
significant.
Results
PES reduces cell viability of human
NSCLC cells
The chemical structure of PES is shown in Fig. 1A. To determine the effect of PES on
cell viability of human NSCLC cells, A549 and H460 cells were
exposed to vehicle control or a series of different PES
concentration, ranging from 2.5 to 40 µM, for 24 and 48 h,
respectively. CCK-8 assays were used to detect cell viability. As
shown in Fig. 1B, PES induced a
time- and dose-dependent loss of viability in A549 cells, with 50%
inhibitory concentration (IC50) of 44.9 and 25.7 µM by a
24- and 48-h PES treatment. Similar results were also found in H460
cells treated by a series of different PES concentration, and the
IC50 values were calculated to be 40.1 and 24.3 µM by a
24- and 48-h PES treatment (Fig.
1C). These results suggested that PES efficiently inhibited
cell proliferation of A549 and H460 cells.
PES suppresses the migration of human
NSCLC cells
Accumulating evidence has demonstrated that the
metastasis activity of cancer cells is a critical mechanism for
cancer mortality and invasion, which facilitated the development of
cancer. To this end, the wound healing assay and Transwell
migration assay were performed to study the effect of PES on
migration of human NSCLC cells. When compared to the control group,
PES suppressed the wound healing in A549 and H460 cells (Fig. 1D). To exclude the effect of cell
proliferation on cell migration, Transwell migration assay was
further used to evaluate effect of PES on cell migration.
Similarly, the inhibitory effect of PES on cell migration was
confirmed by the Transwell migration assay, in which the number of
migrated A549 and H460 cells was significantly reduced after
treatment with PES for 48 h (Fig.
1E). These results suggested that PES inhibited cell migration
of human NSCLC cells.
PES induces cell cycle arrest in human
NSCLC cells
To determine the mechanism by which PES inhibits
human NSCLC cell proliferation, flow cytometry was used to analyze
cell cycle contribution of A549 and H460 cells after PES (20 µM)
treatment for 24 h. As shown in Fig.
2A, PES treatment markedly inhibited cell cycle progression in
A549 and H460 cells, which was observed by a significant decrease
in the percentage of cells in the S phase and concomitant increases
in cells in the G0/G1 phase. When compared to the control,
treatment of PES increased the fraction of G0/G1 from 32.8 to
54.1%, decreased the fraction of S phase from 55.4 to 32.8% in A549
cells (Fig. 2B). Similarly, when
compared to the control, PES increased the fraction of G0/G1 from
34.2 to 48.4%, decreased the fraction of S phase from 44.6 to 33.5%
in H460 cells (Fig. 2C). Notably, a
marked change in percentage of cells in G2/M phase was not found in
A549 and H460 cells (Fig. 2B and
C).
Consistent with the observation by flow cytometry,
western blotting results indicated that PES inhibited expression of
cyclin A and CDK2, which are cell cycle progression markers
(Fig. 2D). In contrast, expression
of CDK inhibitor p21, a negative regulator of cell cycle
progression, was increased by PES treatment (Fig. 2D). Since inhibition of cell
migration may be partly caused by inhibition of cell proliferation,
western blotting was used to further detect the expression of genes
associated with cell invasion. Consistent with the results of wound
healing assay, PES increased expression of the epithelial marker
E-cadherin and reduced expression of vimentin and MMP9, suggesting
that PES inhibited human NSCLC cell migration and invasion via
regulation of epithelial to mesenchymal transition (EMT) (Fig. 2D).
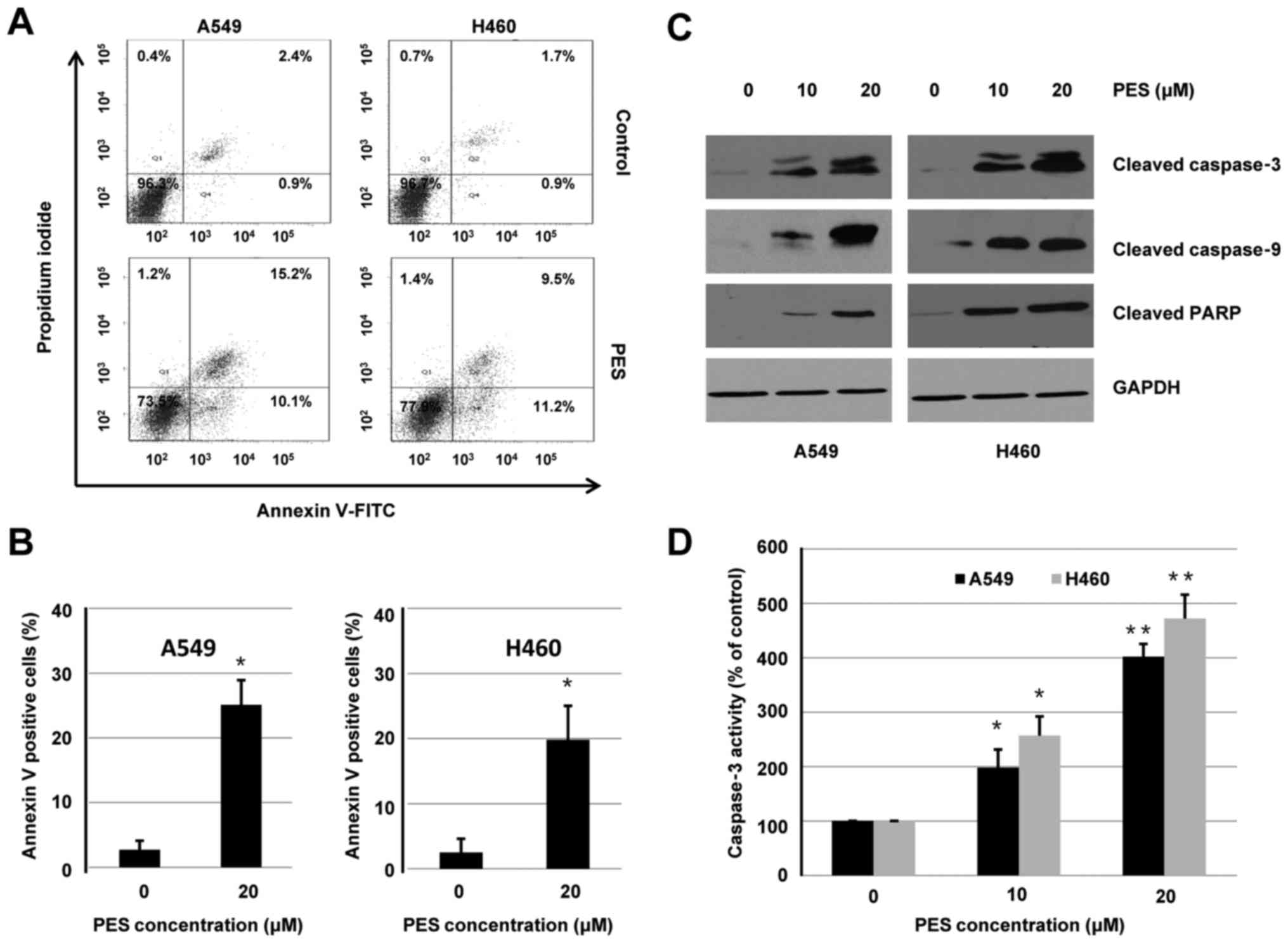
PES induces cell apoptosis in human
NSCLC cells
To determine whether the inhibitory effect of PES on
cell viability was associated with the induction of cell apoptosis,
A549 and H460 cells were treated with or without PES (20 µM) for 24
h. Flow cytometry was used to analyze apoptosis induced by PES. As
shown in Fig. 3A, the induction of
apoptosis by PES was validated by increased percentage of Annexin
V-positive cells. Quantitative analysis of Annexin V-positive cells
induced by PES treatment is shown in Fig. 3B.
In addition to Annexin V-FITC assay, western blot
analysis was used to evaluate the effect of PES on induction of
apoptosis. A549 and H460 cells were treated with or without PES (10
or 20 µM) for 48 h. Whole-cell extracts were harvested and
subjected to western blotting. As shown in Fig. 3C, PES treatment increased expression
of cleaved caspase-3, cleaved caspase-9, and cleaved PARP in both
A549 and H460 cells. Furthermore, activities of caspase-3 were
dose-dependently increased by PES treatment in A549 and H460 cells
(Fig. 3D). These results suggested
that PES induced apoptosis, at least in part, through a
caspase-dependent intrinsic mitochondrial pathway in human NSCLC
cells.
PES inhibits activation of Akt and ERK
pathway in human NSCLC cells
AKT and MAPK signaling pathway is essential to the
action of chemotherapeutic drugs in the regulation of cell cycle
progression and apoptosis (17,18).
Previous reports have indicated that AKT and ERK are key modulators
of cell proliferation, and activation of AKT and ERK by
phosphorylation is associated with tumor aggressiveness and
prognosis in NSCLC (19,20). To determine the effect of PES on AKT
and ERK signaling pathway, A549 and H460 cells were treated with
vehicle control or PES (10 or 20 µM) for 48 h. Results shown in
Fig. 4A indicate that PES
dose-dependently decreased phosphorylated AKT and ERK in A549 and
H460 cells. In addition, DR4 and DR5 were increased following PES
treatment.
 | Figure 4.PES inhibits activities of AKT, ERK,
and Hsp70 in human NSCLC cells. Effect of PES on activities of AKT
and ERK. (A) A549 and H460 cells were treated with vehicle control
or PES (10 or 20 µM) for 48 h. Whole cell extracts were collected
and immunoblotted with Hsp70, DR4, DR5, AKT, p-AKT, ERK, and p-ERK.
GAPDH was used for loading control. (B and C) A549 cells
transfected with or without pSG5-Hsp70 for 4 h were treated with or
without PES for further 44 h. Whole cells were collected and
subjected to western blotting or CCK-8 assay as in Materials and
methods above, respectively. (D and E) A549 cells transfected with
or without siHsp70 for 4 h were treated with or without PES for
further 44 h. Whole cells were collected and subjected to western
blotting or CCK-8 assay as in Materials and methods,
respectively. |
Previous results have indicated that Hsp70 is
essential to NSCLC growth regulation, and compared to normal
counterpart cell lines, Hsp70 was overexpressed in NSCLC cells
(5). Since PES is an Hsp70
inhibitor, the effect of PES on Hsp70 was evaluated in human NSCLC
cells. As shown in Fig. 4A, PES did
not reduce expression of Hsp70 in A549 and H460 cells, suggesting
that PES might inhibit activity of Hsp70 via specifically
interacting with the ATPase binding domain.
PES-induced cell proliferation
inhibition is involved in the regulation of Hsp70 in human NSCLC
cells
To further validate if cell viability decrease by
PES treatment is caused by acting on Hsp70, pSG5-Hsp70 or vehicle
control (pSG5) were transiently transfected into A549 cells. At 4 h
after transfection, cells were treated with or without PES (20 µM)
for further 44 h. CCK-8 assay was used to detect cell viability
after overexpression of Hsp70. As shown in Fig. 4B, Hsp70 was successfully
overexpressed in A549 cells. Though overexpression of Hsp70 itself
did not increase cell viability, it can efficiently attenuate the
inhibitory efficiency of PES (Fig.
4C). To further characterize the role of Hsp70 in cell
proliferation, Hsp70 was knocked down in A549 cells by siRNA.
Results shown in Fig. 4D confirmed
reduction of Hsp70 expression following by transfection with
siHsp70. Moreover, Hsp70 knockdown by siHsp70 alone decreased cell
viability by 58.8%. Most importantly, A549 cells transfected with
siHsp70 were more sensitive to PES-induced cell proliferation
inhibition (Fig. 4E). These results
suggested that PES inhibited cell proliferation of human NSCLS
cells through Hsp70-dependent mechanism.
PES sensitizes NSCLC cell lines to
TRAIL-induced cell proliferation inhibition and apoptosis
TRAIL belongs to a member of the tumor necrosis
factor family that can trigger apoptosis in a broad spectrum of
tumor cells while not in most normal cells (21). TRAIL can selectively trigger
apoptosis of tumor cells through recognizing and binding to DR4 and
DR5 on cell surfaces (22). Based
on the upregulation of DR4 and DR5 in response to PES, we sought to
investigate whether PES combined with TRAIL synergistically
inhibits cell proliferation and enhance induction of apoptosis in
NSCLC cells. A549 and H460 cells were treated with or without PES
(20 µM) in the absence or presence of TRAIL (40 ng/ml) for 24 h,
cell were harvested and subjected to cell viability, apoptosis, and
caspase-3 activation analysis, respectively.
As shown in Fig. 5A,
PES alone and TRAIL alone reduced cell viability by 67.2 and 84.9%,
while co-treatment resulted in cell viability inhibition by 28.5%
in A549 cells. Similarly, PES alone and TRAIL alone reduced cell
viability by 59.9 and 66.1%, while co-treatment resulted in cell
viability inhibition by 19.7% in H460 cells (Fig. 5B). Flow cytometry results suggested
that co-treatment with PES and TRAIL enhances cell apoptosis to a
greater degree compared to PES treatment alone. Interestingly,
TRAIL alone did not result in a marked induction of apoptosis in
A549 and H460 cells. Quantitative analysis is shown in Fig. 5C and D. As expected, co-treatment of
A549 and H460 cells with PES and TRAIL led to a marked increase in
caspase-3 activation, when compared to PES or TRAIL treatment
alone. Results indicated in Fig. 5E
demonstrate that treatment of cells with TRAIL alone did not induce
the cleavage of PARP protein, while co-treatment of cells with PES
and TRAIL enhanced expression of cleaved PARP to a greater degree
compared to PES treatment alone. Taken together, these results
suggested that PES sensitized A549 and H460 cells to TRAIL-induced
cell proliferation inhibition and apoptosis.
In vivo effects of PES on A549 lung
tumor xenografts
To evaluate the antitumor activity of PES in a
xenograft model, A549 cells (1×107) suspended in
Matrigel were injected subcutaneously into flanks of female mice.
After 7 days, mice bearing visible tumors (~5×5 mm2)
were treated with PES (10 mg/kg) or PBS via intraperitoneal
injection every two days. Three weeks later, the mice were
euthanized, and tumors were measured and weighed. As shown in
Fig. 6A and B, compared to PBS
treatment, tumor volume and weight were reduced by PES treatment.
Moreover, no significant mouse body weight loss was demonstrated in
PES treatment groups (Fig. 5C).
Representative images of mice treated with either
PBS or PES alone are shown in Fig.
6D. To further determine PES-associated toxicity in mice, major
organs and tumors were dissected and subjected to hematoxylin and
eosin staining and immunohistochemistry analysis. Results shown as
Fig. 6E indicated that no notable
differences between PES and PBS treatment group were observed among
the organs involved, the liver, kidney, spleen, and lung. In
addition, immunohistochemistry analysis results demonstrated that
tumor tissues from the mice treated with PES also exhibited growth
inhibition and increased apoptosis, as assessed by the staining for
cleaved caspase-3, Ki-67, and TUNEL (Fig. 6F). Taken collectively, the above
data suggested that PES exerts a potent in vivo inhibitory
effect on lung carcinoma xenograft growth by inducing tumor cell
apoptosis in mice. Moreover, PES used in this study made no marked
signs of systemic toxicity, indicating that the dosage of PES in
vivo in this study was safe.
Discussion
Over the past decades, lung cancer is one of the
most commonly diagnosed cancers, and its morbidity and mortality
have markedly increased in the world (23). Therefore, the effective agents and
strategies for the treatment of lung cancer are urgently necessary.
Cancer is known as a complex disease accompanying with multiple
abnormally upregulated oncogenic proteins, which involve in
activation of prosurvival signaling pathway (24). The chaperon function of Hsp family
play an important role in the stability of most oncoproteins.
Accumulating results have indicated that molecular chaperone
function of Hsp family members is an attractive therapeutic target
for the treatment of cancer (6,25,26).
In this study, the biologic effects of a small
molecular Hsp70 inhibitor, PES, on human NSCLC cells were
determined in vitro and in vivo. First of all, PES
were found to time- and dose-dependently inhibit cell proliferation
in human NSCLC cells. Wound healing assay results indicated that
compared to the vehicle control, wound width was significant wider
after treatment by PES, suggesting that PES can efficiently inhibit
cell migration of human NSCLC cells. Though inhibition of cell
migration may be partly caused by inhibition of cell proliferation,
Transwell migration assay results demonstrated that the number of
migrated A549 and H460 cells was significantly reduced after
treatment with PES for 48 h, which confirmed the inhibition of cell
migration by PES treatment. EMT is identified as a critical
cellular phenomenon regulating tumor progression, and inhibition of
EMT is considered a potent target for cancer therapy (27). The expression of E-cadherin, MMP-9,
and Vimentin are related to cell invasion. Our results by western
blotting demonstrated that PES reduced expression of MMP-9 and
Vimentin, while increased expression of epithelial marker,
E-cadherin. Therefore, PES inhibited human NSCLC cell migration at
least in part via regulation of EMT.
In addition to inhibitory effect of growth, PES can
induce cell cycle arrest and apoptosis. Flow cytometry results
suggested that PES disrupted cell cycle progression, which was
shown as a markedly increased percentage of NSCLC cells in G0/G1
phase. Furthermore, cell cycle progression markers, CDK2 and Cyclin
A, were decreased, while CDK inhibitor, p21 was increased by PES
treatments. The answer for the cell death through apoptotic or
non-apoptotic pathway induced by PES is controversial. PES resulted
in cell death in primary effusion lymphoma through induction of
lysosomal cathepsin D release, not through canonical apoptotic
processes (12). However, PES
induced apoptotic cell death in pancreatic cell lines via both
caspase-dependent and -independent processes (28). Our results demonstrated that PES
induced cell apoptosis in human NSCLC cells. Caspase activation is
the key event of apoptosis. Activated caspase-9 activates
downstream caspase-3 and induces PARP cleavage (29). As expected, following treatment of
human NSCLC cells by PES, expression of cleaved caspase-9 was
increased, accompanied by increased cleaved caspase-3 and cleaved
PARP. Furthermore, caspase-3 activation assay results also
confirmed the findings above, indicating that PES induced cell
apoptosis, at least in part, in a caspase-dependent manner.
Multiple signaling pathways, such as ERK and AKT
signal transduction pathways, play an important role in cell
survival and regulation of apoptosis (30). Our results demonstrated that PES
decreased the level of phosphorylated AKT and ERK in human NSCLC
cells. These results concur with the reports in lung cancer cells
treated with VER-155008 (5). In
addition to protect cells from repeat exposure of harmful stimuli,
Hsp70 can assist folding of nascent polypeptides and protein,
indicating the importance of Hsp70 in growth and survival of tumor
cells (31). Previous reports
indicated that expression of Hsp70 was inhibited by triptolide,
which resulted in pancreatic cancer cell death (6). Quercetin and gemcitabine
synergistically enhanced apoptosis in lung cancer cells via
inhibition of Hsp70 (32). Since
PES is identified as an specific Hsp70 inhibitor, the regulation of
Hsp70 by PES has been evaluated in human NSCLC cells. Though
expression of Hsp70 was undetectably reduced by PES, overexpression
of Hsp70 significantly attenuated the inhibitory effect of PES on
cell proliferation in A549 cells, suggesting that PES targeted the
ATPase binding domain of Hsp70, and then inhibited its protein
chaperoning activity. As expected, knockdown of Hsp70 by siRNA in
A549 cells enhanced sensitivity of PES to cell growth
inhibition.
TRAIL, also referred as Apo-2L, is an attractive
antitumor therapeutic, which can selectively induce tumor cell
apoptosis through binding to DR4 and DR5 in the cell surface
(22). Since increased DR4 and DR5
by PES treatment were shown as the results above, the effects of
combination treatment of PES and TRAIL on human NSCLC cells were
determined. Our results indicated that combination treatment with
PES and TRAIL synergistically inhibited cell proliferation and
increased the percentage of Annexin V-positive cells in human NSCLC
cells. Interestingly, TRAIL treatment alone in human NSCLC cells
did not induce significant apoptosis, suggesting that human NSCLC
cells presented resistance to TRAIL. The mechanisms of TRAIL
resistance may contribute to the regulation of DRs, for increased
DR4 and DR5 by PES treatment sensitized NSCLC cell lines to the
effect of TRAIL. Even so, the specific mechanisms of TRAIL
resistance to human NSCLC cells require further study.
In addition to the effect of PES on human NSCLC
cells in vitro, this study also investigated the therapeutic
effect of PES against NSCLC in vivo using a mouse xenograft
model. Our results demonstrated that PES significantly repressed
growth of tumor xenografts, which could be confirmed by decreased
volume and weight of solid tumors. Moreover, that no notable
differences were determined among mouse weight and major organs,
suggesting that few side effects were observed by PES treatment in
mice.
In conclusion, these results indicated the abilities
of PES in vitro and in vivo to inhibit cell viability
in human NSCLC cells. In addition, inhibition of cell viability by
PES is related to the regulation of Hsp70 in human NSCLC cells.
Though human NSCLC cells are resistant to TRAIL treatment, TRAIL
showed a potent synergistic effects on cell proliferation
inhibition via combination with PES. Taken together, PES may be a
promising compound for the management of human NSCLC, and
synergistic anticancer action of PES and TRAIL require further
study.
Acknowledgements
This study was supported by the intramural funding
from Jingzhou First People's Hospital of Hubei Province (to L.C.)
and the research grant provided by JingZhou Hospital Affiliated to
Huazhong University of Science and Technology.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Erridge SC, Møller H, Price A and Brewster
D: International comparisons of survival from lung cancer: Pitfalls
and warnings. Nat Clin Pract Oncol. 4:570–577. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jego G, Hazoumé A, Seigneuric R and
Garrido C: Targeting heat shock proteins in cancer. Cancer Lett.
332:275–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ritossa F: Discovery of the heat shock
response. Cell Stress Chaperones. 1:97–98. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wen W, Liu W, Shao Y and Chen L:
VER-155008, a small molecule inhibitor of HSP70 with potent
anti-cancer activity on lung cancer cell lines. Exp Biol Med
(Maywood). 239:638–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Phillips PA, Dudeja V, McCarroll JA,
Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM and Saluja AK:
Triptolide induces pancreatic cancer cell death via inhibition of
heat shock protein 70. Cancer Res. 67:9407–9416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki K, Ito Y, Wakai K, Kawado M,
Hashimoto S, Seki N, Ando M, Nishino Y, Kondo T, Watanabe Y, et al:
Serum heat shock protein 70 levels and lung cancer risk: A
case-control study nested in a large cohort study. Cancer Epidemiol
Biomarkers Prev. 15:1733–1737. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang Q, Zu Y, Fu X and Wu T: Expression
of heat shock protein 70 and 27 in non-small cell lung cancer and
its clinical significance. J Huazhong Univ Sci Technolog Med Sci.
25:693–695. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhuang H, Jiang W, Zhang X, Qiu F, Gan Z,
Cheng W, Zhang J, Guan S, Tang B, Huang Q, et al: Suppression of
HSP70 expression sensitizes NSCLC cell lines to TRAIL-induced
apoptosis by upregulating DR4 and DR5 and downregulating c-FLIP-L
expressions. J Mol Med (Berl). 91:219–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leu JI, Pimkina J, Frank A, Murphy ME and
George DL: A small molecule inhibitor of inducible heat shock
protein 70. Mol Cell. 36:15–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leu JI, Pimkina J, Pandey P, Murphy ME and
George DL: HSP70 inhibition by the small-molecule
2-phenylethynesulfonamide impairs protein clearance pathways in
tumor cells. Mol Cancer Res. 9:936–947. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Granato M, Lacconi V, Peddis M, Lotti LV,
Di Renzo L, Gonnella R, Santarelli R, Trivedi P, Frati L, D'Orazi
G, et al: HSP70 inhibition by 2-phenylethynesulfonamide induces
lysosomal cathepsin D release and immunogenic cell death in primary
effusion lymphoma. Cell Death Dis. 4:e7302013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma L, Sato F, Sato R, Matsubara T, Hirai
K, Yamasaki M, Shin T, Shimada T, Nomura T, Mori K, et al: Dual
targeting of heat shock proteins 90 and 70 promotes cell death and
enhances the anticancer effect of chemotherapeutic agents in
bladder cancer. Oncol Rep. 31:2482–2492. 2014.PubMed/NCBI
|
|
14
|
Kaiser M, Kühnl A, Reins J, Fischer S,
Ortiz-Tanchez J, Schlee C, Mochmann LH, Heesch S, Benlasfer O,
Hofmann WK, et al: Antileukemic activity of the HSP70 inhibitor
pifithrin-μ in acute leukemia. Blood Cancer J. 1:e282011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sekihara K, Harashima N, Tongu M, Tamaki
Y, Uchida N, Inomata T and Harada M: Pifithrin-μ, an inhibitor of
heat-shock protein 70, can increase the antitumor effects of
hyperthermia against human prostate cancer cells. PLoS One.
8:e787722013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi ES, Nam JS, Jung JY, Cho NP and Cho
SD: Modulation of specificity protein 1 by mithramycin A as a novel
therapeutic strategy for cervical cancer. Sci Rep. 4:71622014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hers I, Vincent EE and Tavaré JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang JM, He QY, Guo RX and Chang XJ:
Phosphorylated Akt overexpression and loss of PTEN expression in
non-small cell lung cancer confers poor prognosis. Lung Cancer.
51:181–191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sprick MR, Weigand MA, Rieser E, Rauch CT,
Juo P, Blenis J, Krammer PH and Walczak H: FADD/MORT1 and caspase-8
are recruited to TRAIL receptors 1 and 2 and are essential for
apoptosis mediated by TRAIL receptor 2. Immunity. 12:599–609. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan KC, Ting CM, Chan PS, Lo MC, Lo KW,
Curry JE, Smyth T, Lee AW, Ng WT, Tsao GS, et al: A novel Hsp90
inhibitor AT13387 induces senescence in EBV-positive nasopharyngeal
carcinoma cells and suppresses tumor formation. Mol Cancer.
12:1282013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen W, Sin SH, Wen KW, Damania B and
Dittmer DP: Hsp90 inhibitors are efficacious against Kaposi sarcoma
by enhancing the degradation of the essential viral gene LANA, of
the viral co-receptor EphA2 as well as other client proteins. PLoS
Pathog. 8:e10030482012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Li Z, Lin Z, Zhao B, Wang Y, Peng
R, Wang M, Lu C, Shi G and Shen Y: 17-DMCHAG, a new geldanamycin
derivative, inhibits prostate cancer cells through Hsp90 inhibition
and survivin downregulation. Cancer Lett. 362:83–96. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baek SH, Ko JH, Lee JH, Kim C, Lee H, Nam
D, Lee J, Lee SG, Yang WM, Um JY, et al: Ginkgolic acid inhibits
invasion and migration and TGF-β-induced EMT of lung cancer cells
through PI3K/Akt/mTOR inactivation. J Cell Physiol. 232:346–354.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Monma H, Harashima N, Inao T, Okano S,
Tajima Y and Harada M: The HSP70 and autophagy inhibitor
pifithrin-μ enhances the antitumor effects of TRAIL on human
pancreatic cancer. Mol Cancer Ther. 12:341–351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding X, Zhang B, Pei Q, Pan J, Huang S,
Yang Y, Zhu Z, Lv Y and Zou X: Triptolide induces apoptotic cell
death of human cholangiocarcinoma cells through inhibition of
myeloid cell leukemia-1. BMC Cancer. 14:2712014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meng G, Wang W, Chai K, Yang S, Li F and
Jiang K: Combination treatment with triptolide and
hydroxycamptothecin synergistically enhances apoptosis in A549 lung
adenocarcinoma cells through PP2A-regulated ERK, p38 MAPKs and Akt
signaling pathways. Int J Oncol. 46:1007–1017. 2015.PubMed/NCBI
|
|
31
|
Zorzi E and Bonvini P: Inducible hsp70 in
the regulation of cancer cell survival: Analysis of chaperone
induction, expression and activity. Cancers (Basel). 3:3921–3956.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee SH, Lee EJ, Min KH, Hur GY, Lee SH,
Lee SY, Kim JH, Shin C, Shim JJ, In KH, et al: Quercetin enhances
chemosensitivity to gemcitabine in lung cancer cells by inhibiting
heat shock protein 70 expression. Clin Lung Cancer. 16:e235–e243.
2015. View Article : Google Scholar : PubMed/NCBI
|