Introduction
Osteosarcoma is one of the most common malignant
bone tumors. It originates in stromal cell lines and is
characterized by rapid growth (1).
Nearly 80% of osteosarcoma cases occur within the age range of
10–20 years, causing extensive damage in the children and
adolescents who suffer from the disease (2). Osteosarcoma cells tend toward division
and proliferation because of an unregulated cell cycle. Moreover,
osteosarcoma usually metastasizes at an early stage.
Epidemiological surveys have reported that approximately 40% of
osteosarcoma patients die from early metastasis, especially
pulmonary metastasis (3). The
prognosis is far from optimistic, with an overall five-year
survival rate of 20% in cases with metastases (4). With improvements in limb salvage
operations combined with pre- or post-operative neoadjuvant
chemotherapy, the five-year survival rate has increased to
approximately 60% (5). However, the
high doses of chemotherapeutics cause toxicity in normal tissues
and damage the liver and kidneys. Cellular chemoresistance presents
another challenge, limiting the long-term curative effect of
chemotherapy (6,7). Thus, safe and effective therapy
options for osteosarcoma are urgently needed.
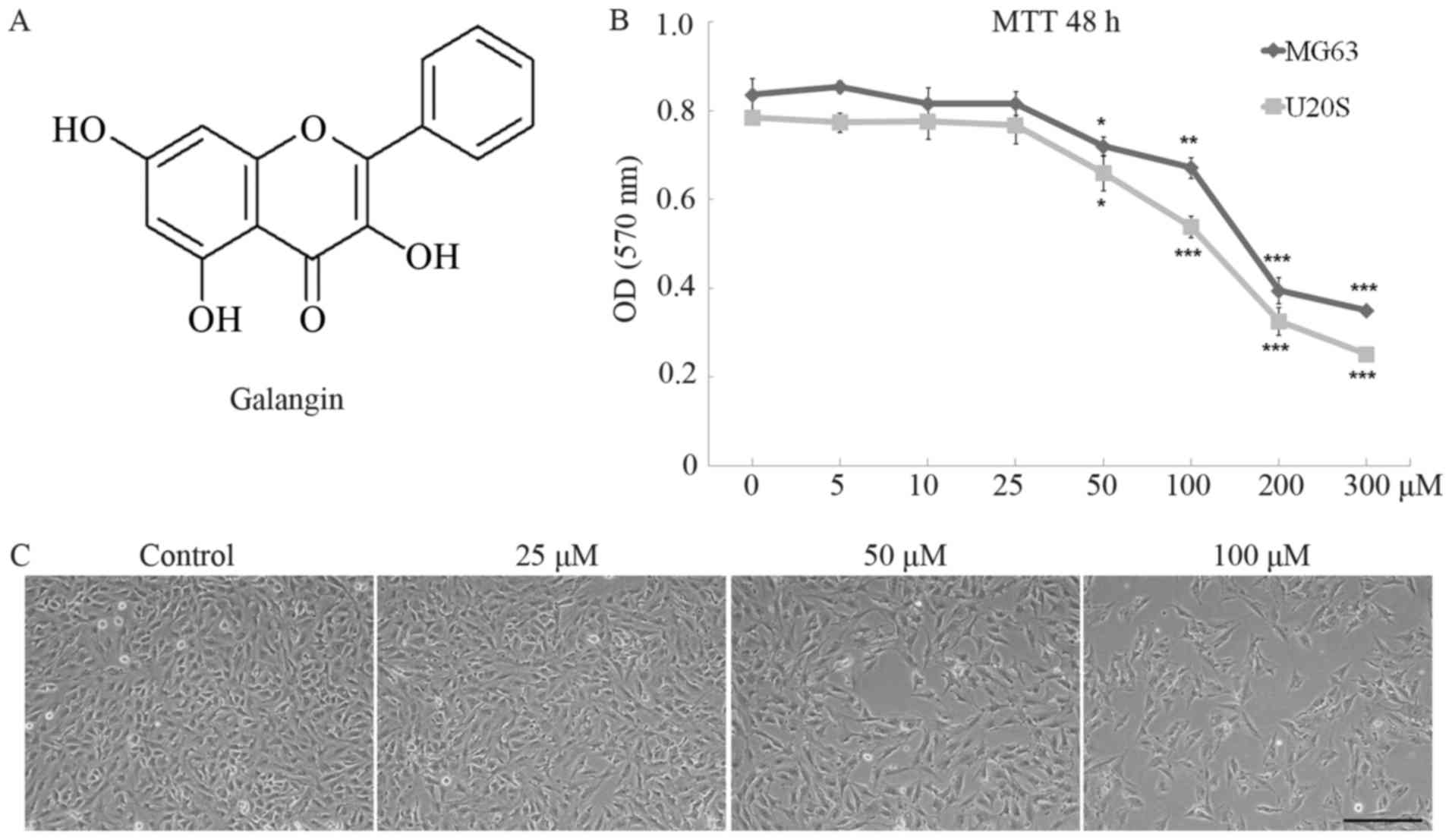
Galangin (3,5,7-trihydroxyflavone, Fig. 1A) is a natural bioflavonoid
primarily extracted from the rhizome of Alpinia officinarum,
which has been used as an herbal medicine in Asia for decades.
Previous studies have showed that galangin has anti-inflammatory
(8,9), antibacterial (10,11),
and antiviral (12) activities
in vitro. Currently, it is in focus for its antitumor
property. Studies have shown that galangin suppresses the
proliferation and functions of various tumor cells, including renal
carcinoma Caki cells (13),
hepatocellular carcinoma (14),
fibrosarcoma (15), and squamous
carcinoma (16). However, the
effects of galangin on osteosarcoma are still not clear. The aims
of this study were to evaluate the effects of galangin on the
proliferation, apoptosis, and invasion of osteosarcoma cell lines
in vitro and to explore the underlying mechanism of
action.
Materials and methods
Cell culture
The human osteosarcoma cell lines MG63 and U20S
(Cell Bank of the Chinese Academy of Sciences, Shanghai, China)
were used in this study. Cells were cultured in Dulbecco's modified
Eagle's medium (DMEM), which was supplemented with 10% fetal bovine
serum and 1% streptomycin-penicillin. Cells were seeded into
96-well plates at a density of 8×103 cells/well or
6-well plates at a density of 1×105 cells/well for the
following procedures. The morphology of cultured cells was observed
with an inverted phase contrast microscope.
Cell proliferation assay
MG63 and U20S cells were treated with galangin at
concentrations of 0, 5, 10, 25, 50, 100, 200, and 300 µM. After
incubation for 48 h, 10 µl
3-(4,5-dimethylthiazol-2-y1)-2,5-di-phenyltetrazolium bromide (MTT)
solution (5 mg/ml in PBS) was added to each well. Cells were
incubated for another 4 h, and then the medium was replaced with
150 µl of dimethyl sulfoxide (DMSO) solution to solubilize the
crystals. The results were read at 570 nm. SPSS software version
19.0 was used for calculation of median lethal concentration
(LC50).
Hoechst 33258 staining
MG63 cells were treated with galangin at
concentrations of 0, 25, 50, and 100 µM. After incubation for 24 h,
the medium was removed and the cells were fixed with 4%
polyoxymethylene for 20 min and washed twice. Then, 10 µg/ml
Hoechst 33258 solution was added, and the cells were incubated in
the dark for 5 min and then washed three times. The cells were
observed under a fluorescence microscope, and those in brilliant
blue color were counted.
Flow cytometry
Cell apoptosis was evaluated using Annexin V
fluorescein isothiocyanate and propidium iodide (Annexin V-FITC/PI)
apoptosis detection kits (Life Technologies, Waltham, MA, USA).
MG63 cells were treated with galangin for 48 h at concentrations of
0, 25, 50, and 100 µM. Then they were harvested and stained with
Annexin V-FITC or PI and were analyzed on a FACScan flow cytometer
(Becton, Dickinson, and Company, Franklin Lakes, NJ, USA). Annexin
V(+)/PI(−) cells were considered early apoptotic cells, while
Annexin V(+)/PI(+) cells were considered late apoptotic cells.
Scratch-wound healing assay
MG63 cells were seeded in 6-well plates and cultured
to confluence, followed by serum starvation overnight prior to
wounding, and treated with galangin at concentrations of 0, 25, 50,
and 100 µM for 24 and 48 h. The wound area was observed under an
optical microscope.
Transwell assay
Transwell assays with matrigel were performed to
evaluate cell migration and invasion as previously described
(17). Briefly, MG63 cells were
seeded in the upper surface of Transwell chamber at density of
1×105, and treated with galangin for 48 h at
concentrations of 0, 25, 50, and 100 µM. Then cells on the upper
parts of chamber were removed, while the invaded cells were fixed,
stained and counted under a high-power microscope.
Western blotting
Radio-immunoprecipitation assay (RIPA) lysis buffer
was used to extract total cellular protein lysates, which were
subjected to electrophoretic separation and transferred to
nitrocellulose membranes via electroblotting. Next, the membranes
were blocked with 5% non-fat dry milk for 20 min, and the proteins
were probed with primary antibodies overnight at 4°C. Membranes
were washed three times in solution and incubated with horseradish
peroxidase-conjugated secondary antibodies (Sigma-Aldrich, St.
Louis, MO, USA) for another 2 h. LAS-4000 Science Imaging System
(Fujifilm, Tokyo, Japan) was used to observe the protein bands. The
following primary antibodies were used: phosphoinositide 3-kinase
(PI3K; 1:1,000, Cell Signaling Technology, Danvers, MA, USA),
Aktp-Thr308 (1:1,000, Abcam, Cambridge, UK), Akt
(1:1,000, Abcam), cyclin D1 (1:1,000, Proteintech, Rosemont, IL,
USA), p27Kip1 (1:1,000, Proteintech), caspase-3
(1:5,000, Abcam) or caspase-8 (1:5,000, Abcam), matrix
metalloproteinase 2 (MMP-2; 1:1,000, Abcam), matrix
metalloproteinase 9 (MMP-9; 1:1,000, Proteintech), and β-tubulin
(1:1,000, Bioworld Technology Inc., St. Louis Park, MN, USA).
Statistics
All data are presented as the mean ± SD. The
statistical significance was evaluated by one-way analysis of
variance (ANOVA) methodology for repeated measurement, followed by
Student-Newman-Keuls test. P<0.05, P<0.01 and P<0.001 were
considered to be statistically significant. SPSS software version
19.0 was used for the statistical analyses. All experiments were
conducted in triplicates.
Results
Galangin inhibits the proliferation
and cell cycle of osteosarcoma cells
Galangin significantly inhibited the proliferation
of MG63 and U20S cells in a dose-dependent manner. The optical
density (OD) value of MG63 cells remained stable in the 5, 10, and
25 µM galangin treatment groups and was equivalent to the value of
0.83±0.063 in the control group (Fig.
1B). However, the OD value dropped to 0.72±0.038 in the 50
µM-group (P=0.034) and markedly dropped to 0.67±0.040 in the 100 µM
group (P<0.001), 0.40±0.051 in the 200 µM group (P<0.001),
and 0.35±0.015 in the 300 µM group (P<0.001). Similar trends
were observed in U20S cells. The IC50 of galangin to
MG63 and U20S cells 48 h post-treatment were determined as 234.8
and 227.0 µM, respectively. MG63 cells were seeded at the same
density in 6-well plates and treated with galangin at
concentrations of 0, 25, 50, and 100 µM for 48 h. Images taken
using an inverted phase contrast microscope showed a slight
decrease in cultured cell numbers in the 25 µM galangin group
compared to those in the control group, and significantly reduced
cell numbers at the 50 and 100 µM galangin concentrations (Fig. 1C).
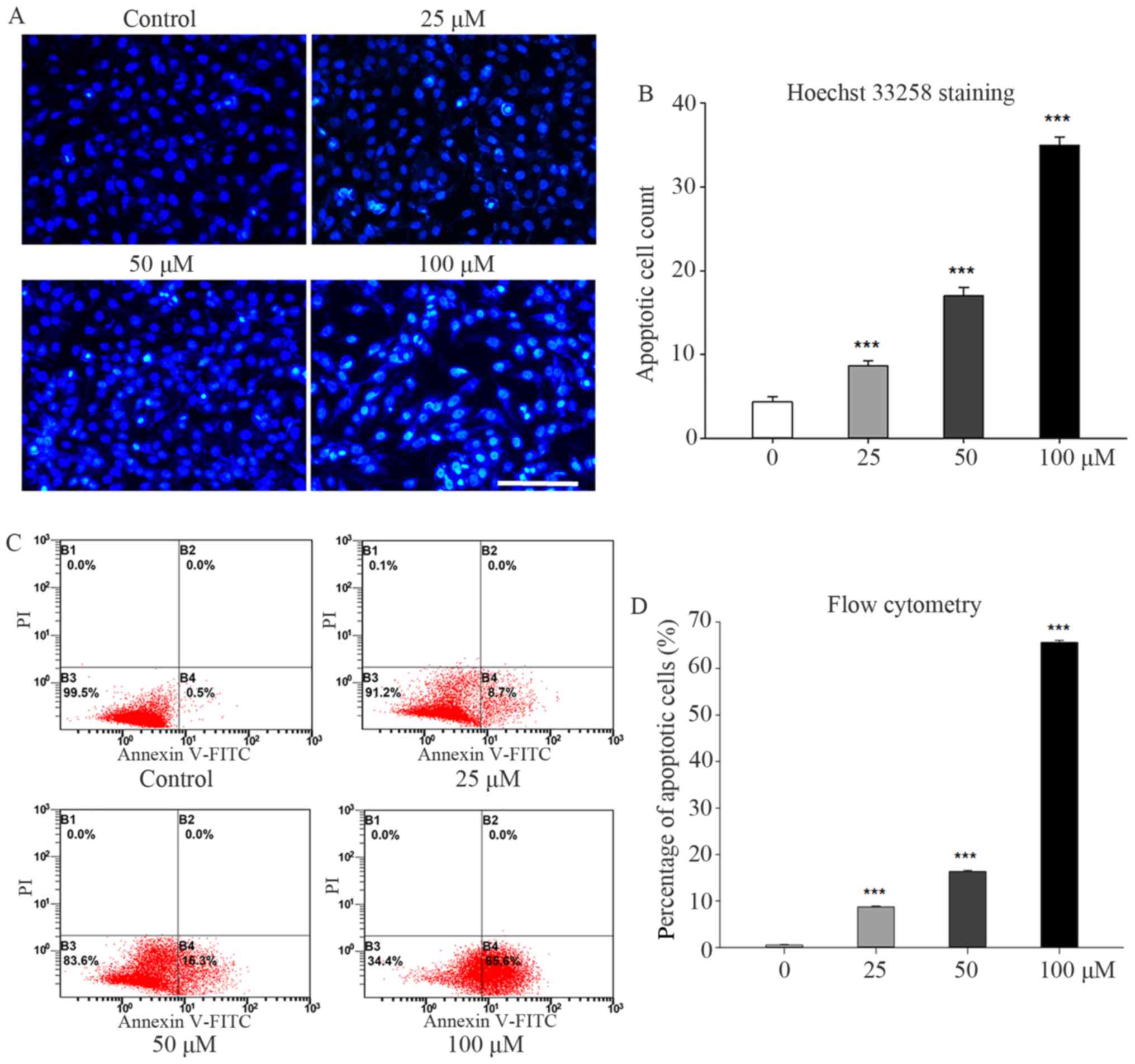
Galangin accelerated apoptosis in
osteosarcoma cells
Galangin treatment significantly enhanced apoptosis
in MG63 cells. The nucleus stained brilliant blue upon Hoechst
33258 staining, indicating condensed chromatin (Fig. 2A). The number of apoptotic MG63
cells observed under fluorescence microscopy increased to 8.6±0.57
(P<0.001), 17.0±1.00 (P<0.001), and 35.0±1.00 (P<0.001) in
the 25, 50, and 100 µM groups, respectively, compared to 4.3±0.57
in the control group (Fig. 2B).
Similar trends were observed in the flow cytometry results. The
percentages of early apoptotic cells increased to 8.7±0.20%
(P<0.001), 16.3±0.26% (P<0.001), and 65.6±0.44% (P<0.001)
in the 25, 50, and 100 µM groups, respectively, compared to
0.5±0.10% in the control group (Fig. 2C
and D).
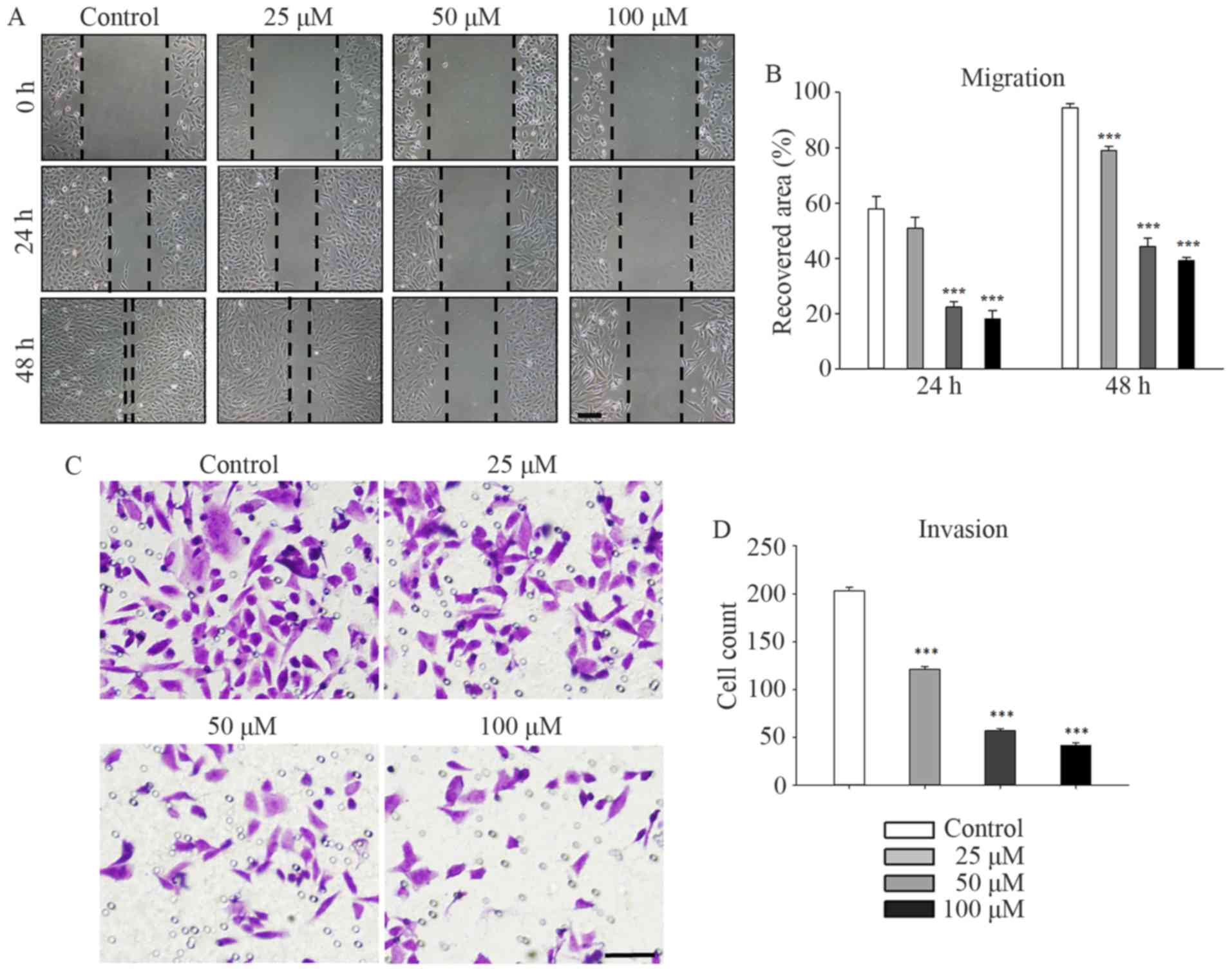
Galangin suppresses the migration and
invasion of osteosarcoma cells
Galangin markedly suppressed the migration and
invasion of MG63 cells in a concentration-dependent manner. MG63
cells were treated with galangin at concentrations of 0, 25, 50,
and 100 µM and subjected to a scratch-wound assay (Fig. 3A and B). After incubation for 48 h,
the recovered area decreased to 78.5±1.5% (P<0.001), 44.3±3.0%
(P<0.001), and 38.8±1.1% (P<0.001) of its original size in
the 25, 50, and 100 µM groups, respectively, compared to 93.9±1.5%
in the control group. Similar trends were observed at the 24-h time
point. Τranswell assays were performed to evaluate the inhibitory
effect of galangin on the invasion of MG63 cells. The numbers of
invading cells counted in the microscope images (Fig. 3C and D) were reduced to 121.3±2.5
(P<0.001), 57.0±2.0 (P<0.001), and 41.7±2.5 (P<0.001) in
the 25, 50, and 100 µM groups, respectively, compared to 203.3±3.5
invading cells in the control group.
Galangin regulated PI3K and its
downstream signaling pathway
Galangin markedly downregulated the protein levels
of PI3K and Aktp-Thr308 in a concentration-dependent
manner, while total Akt expression remained stable (Fig. 4A and B). We subsequently measured
the effects of galangin on the protein expression of cyclin D1 and
p27Kip1, which regulates G1 and S phase entry. The
results showed that galangin treatment decreased cyclin D1
expression and increased p27Kip1 expression in a
dose-dependent manner (Fig. 4C and
D). The expression levels of caspase-3 and caspase-8 were
significantly upregulated in galangin-treated MG63 cells (Fig. 4E and F). However, the expression
levels of MMP-2 and MMP-9 were significantly suppressed in
galangin-treated MG63 cells (Fig. 4G
and H).
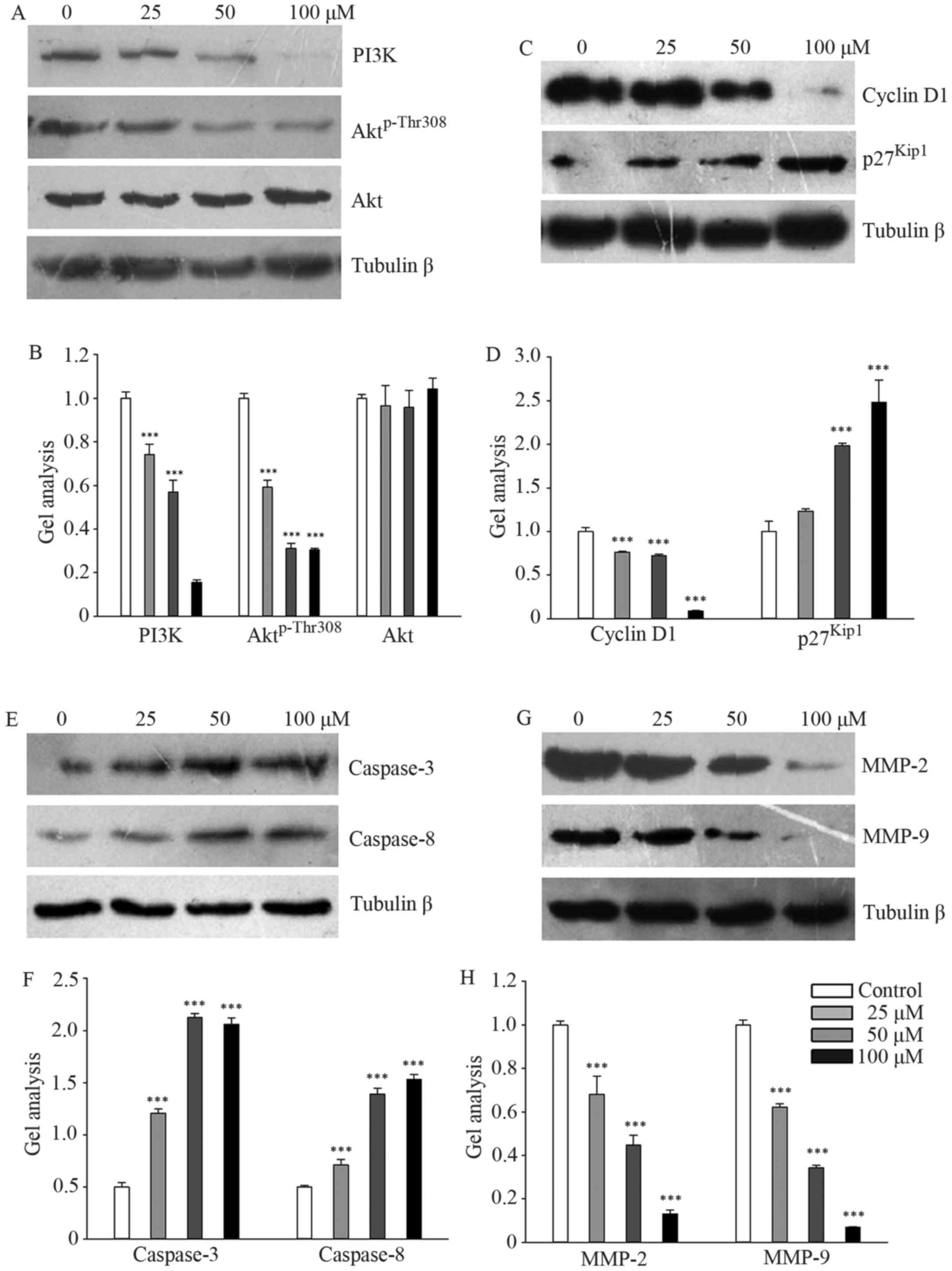 | Figure 4.PI3K and its downstream signaling
pathway targeted by galangin. (A, C, E and G) MG63 cells were
treated with galangin for 48 h at a concentration of 0, 25, 50, or
100 µM, and the levels of PI3K, Aktp-Thr308, Akt, cyclin
D1, p27Kip1, caspase-3, caspase-8, MMP-2, and MMP-9 were
analyzed by western blotting. β-tubulin was used as a loading
control. (B, D, F and H) Quantitative analyses of the western
blotting. Data are presented as the mean ± SD. ***P<0.001 versus
the control group. |
Discussion
Galangin, a compound extracted from herb medicine,
has been reported as an effective antitumor agent. It inhibits cell
proliferation and induces apoptosis in several cancer cell lines,
such a renal cell (18) and human
colon cancer (19). Our data showed
that the OD values of cultured osteosarcoma cells dropped
significantly after exposure to galangin, and the apoptotic rates
of cells significantly increased with galangin treatment. The
migratory and invasive abilities of cells also significantly
decreased after galangin treatment, which were accompanied by
reduced protein expression of PI3K, Aktp-Thr308, cyclin
D1, and MMP-2/9 and upregulation of p27Kip1, caspase-3,
and caspase-8. The results suggest that galangin suppressed the
proliferation and metastasis of osteosarcoma cells in a
concentration-dependent manner, and the underlying mechanism is
associated with inhibition of PI3K and its downstream signaling
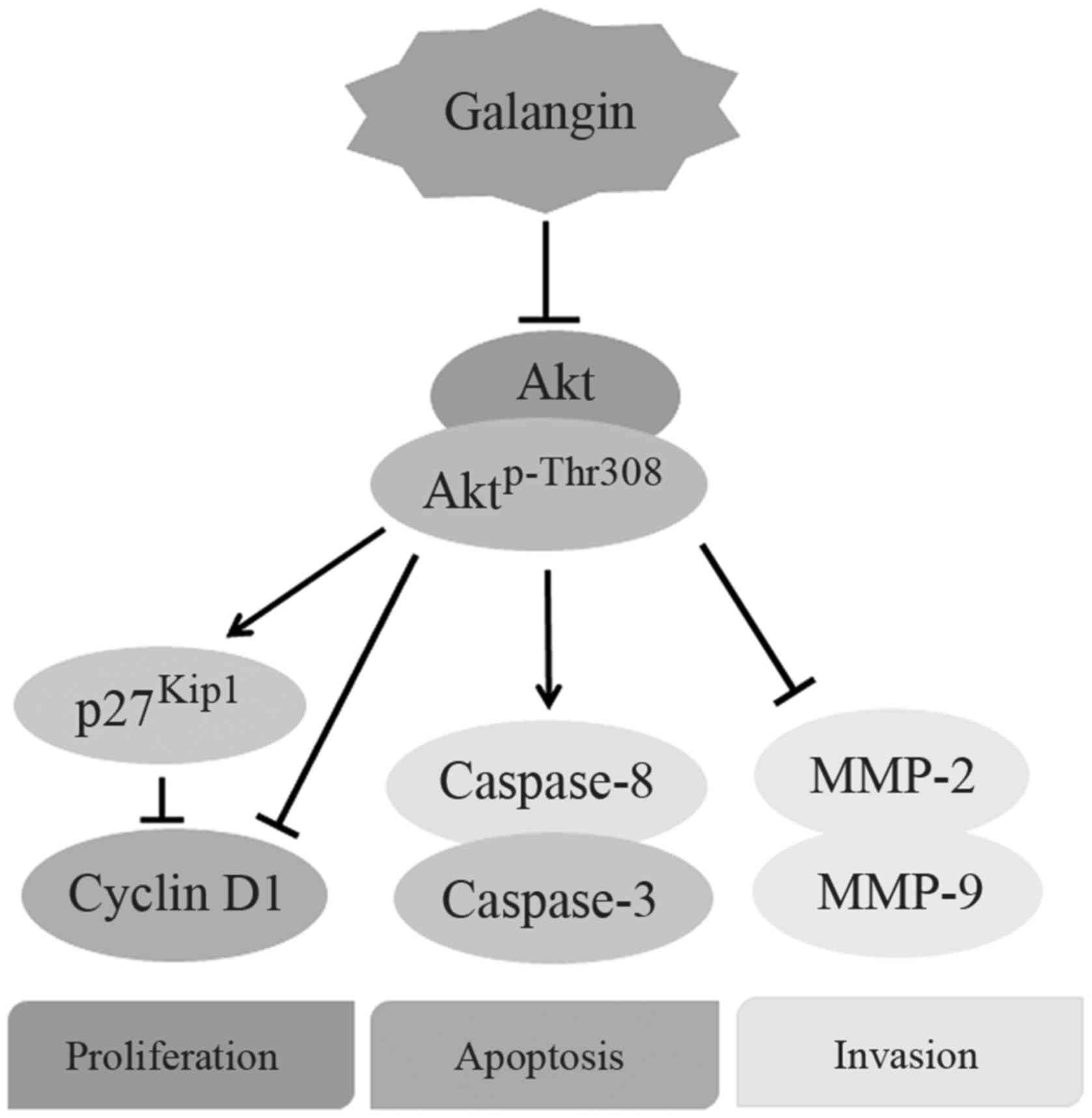
pathway (Fig. 5).
Galangin promotes apoptosis and inhibits
proliferation of osteosarcoma cells. Our data showed that galangin
inhibited the proliferation of osteosarcoma cells, rather than
MC3T3-E1 cells, in a dose-dependent manner, indicating that
galangin may be a tumor-targeted agent. The PI3K/Akt signaling
pathway plays a key role in the regulation of cellular functions,
and PI3K is activated largely in tumor cells than in non-malignant
cells (20). The protein expression
of PI3K in osteosarcoma cells was found to be significantly reduced
by galangin treatment in the present study. These findings indicate
that galangin may target osteosarcoma cells by reducing PI3K
expression and exhibit little toxicity toward non-malignant cells.
Both caspase-3 and caspase-8, proteases involved in extrinsic
apoptosis, were activated after neoadjuvant chemotherapy to elicit
cell death (21).
We also found that apoptosis in MG63 cells was
enhanced significantly after galangin treatment, which was
accompanied by increased protein expression levels of caspase-8 and
caspase-3. These results indicate that galangin may work as a
caspase agonist in tumor cells, and may represent an alternative
remedy for tumors. Moreover, we found that galangin markedly
reduced the protein expression of cyclin D1, a key regulator that
promotes the transition of cells from G1 phase to S phase, where
rapid DNA synthesis occur (22).
This may explain why galangin inhibits the proliferation of
osteosarcoma cells. Similarly, a previous study reported that
galangin induces significant cell cycle arrest of the human head
and neck squamous carcinoma cells at the G0/G1 phase, with
decreased expression of cyclin D1 (16), indicating that galangin mediates the
cell cycle through the PI3K/Akt/cyclin D1 signaling pathway.
In addition to the effect on cell proliferation,
galangin was further found to inhibit cell migration and invade
osteosarcoma cells. Efficient inhibition of tumor metastasis plays
a particularly crucial role in improving the prognosis of patients
with osteosarcoma because metastasis tends to occur at an early
stage of the disease. In the present study, we demonstrated using
Transwell assay that galangin treatment significantly reduces the
migration and invasion of MG63 cells, accompanied by downregulation
of protein expression levels of MMP-2 and MMP-9. Consistent with
these findings, a previous study showed that galangin
dose-dependently reduced the mRNA and protein expression levels of
MMP-2 and MMP-9 in liver cancer HepG2 cells (23). These findings suggested that
galangin could be used as an antimetastatic agent. Based on the
downregulation of both PI3K and Aktp-Thr308, we deduce
that galangin prevents the metastasis of osteosarcoma by inhibiting
the PI3K/Akt/MMP-2/MMP-9 signaling pathway.
Our study has several limitations. First, the
pharmacokinetic parameters of galangin remained to be unambiguously
elucidated. Previous studies showed that the oral bioavailability
of galangin was very low because it changed to glucuronidated forms
after hepatic metabolism (24,25).
The intravenous administration or molecular chemical modification
may improve its efficiency in vivo. Second, animal models to
evaluate the galangin antitumor effects in vivo of
osteosarcoma are required in the future. The effective
concentration of galangin in vivo remained undetected.
Therefore, physiologically relevant and attainable concentrations
of galangin in animal model need to be further explored.
In conclusion, our data established the properties
of galangin suppressing the proliferation, migration, and invasion
of osteosarcoma cells, while enhancing its apoptosis. These effects
occur at least through the inhibition of PI3K and its downstream
signaling pathway. The results presented in our study may broaden
the potential application of galangin, and may offer a promising
therapeutic strategy for antiosteosarcoma therapy.
Acknowledgements
This study was supported by the Natural Science
Foundation of Zhejiang Province (LQ16H160013 and LY15H060005) and
the National Natural Science Foundation of China (81572126).
References
|
1
|
Picci P: Osteosarcoma (osteogenic
sarcoma). Orphanet J Rare Dis. 2:62007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chou AJ, Geller DS and Gorlick R: Therapy
for osteosarcoma: Where do we go from here? Paediatr Drugs.
10:315–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu C and Wang W: Relationship between p15
gene mutation and formation and metastasis of malignant
osteosarcoma. Med Sci Monit. 22:656–661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allison DC, Carney SC, Ahlmann ER,
Hendifar A, Chawla S, Fedenko A, Angeles C and Menendez LR: A
meta-analysis of osteosarcoma outcomes in the modern medical era.
Sarcoma. 2012:7048722012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robl B, Pauli C, Botter SM,
Bode-Lesniewska B and Fuchs B: Prognostic value of tumor
suppressors in osteosarcoma before and after neoadjuvant
chemotherapy. BMC Cancer. 15:3792015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo W, Healey JH, Meyers PA, Ladanyi M,
Huvos AG, Bertino JR and Gorlick R: Mechanisms of methotrexate
resistance in osteosarcoma. Clin Cancer Res. 5:621–627.
1999.PubMed/NCBI
|
|
7
|
Zhang Z, Zhang Y, Lv J and Wang J: The
survivin suppressant YM155 reverses doxorubicin resistance in
osteosarcoma. Int J Clin Exp Med. 8:18032–18040. 2015.PubMed/NCBI
|
|
8
|
Jung YC, Kim ME, Yoon JH, Park PR, Youn
HY, Lee HW and Lee JS: Anti-inflammatory effects of galangin on
lipopolysaccharide-activated macrophages via ERK and NF-κB pathway
regulation. Immunopharmacol Immunotoxicol. 36:426–432. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zha WJ, Qian Y, Shen Y, Du Q, Chen FF, Wu
ZZ, Li X and Huang M: Galangin abrogates ovalbumin-induced airway
inflammation via negative regulation of NF-kappaB. Evid Based
Complement Alternat Med. 767689:20132013.
|
|
10
|
Cushnie TP and Lamb AJ: Assessment of the
antibacterial activity of galangin against 4-quinolone resistant
strains of Staphylococcus aureus. Phytomedicine. 13:187–191. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pepeljnjak S and Kosalec I: Galangin
expresses bactericidal activity against multiple-resistant
bacteria: MRSA, Enterococcus spp. and Pseudomonas aeruginosa. FEMS
Microbiol Lett. 240:111–116. 2004. View Article : Google Scholar
|
|
12
|
Meyer JJ, Afolayan AJ, Taylor MB and
Erasmus D: Antiviral activity of galangin isolated from the aerial
parts of Helichrysum aureonitens. J Ethnopharmacol. 56:165–169.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han MA, Lee DH, Woo SM, Seo BR, Min KJ,
Kim S, Park JW, Kim SH, Choi YH and Kwon TK: Galangin sensitizes
TRAIL-induced apoptosis through down-regulation of anti-apoptotic
proteins in renal carcinoma Caki cells. Sci Rep. 6:186422016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su L, Chen X, Wu J, Lin B, Zhang H, Lan L
and Luo H: Galangin inhibits proliferation of hepatocellular
carcinoma cells by inducing endoplasmic reticulum stress. Food Chem
Toxicol. 62:810–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi YJ, Lee YH and Lee ST: Galangin and
kaempferol suppress phorbol-12-myristate-13-acetate-induced matrix
metalloproteinase-9 expression in human fibrosarcoma HT-1080 cells.
Mol Cells. 38:151–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu L, Luo Q, Bi J, Ding J, Ge S and Chen
F: Galangin inhibits growth of human head and neck squamous
carcinoma cells in vitro and in vivo. Chem Biol Interact.
224:149–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Huang T, Jiang G, Gong W, Qian H and
Zou C: Synergistic apoptotic effect of crocin and cisplatin on
osteosarcoma cells via caspase induced apoptosis. Toxicol Lett.
221:197–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao J, Wang H, Chen F, Fang J, Xu A, Xi W,
Zhang S, Wu G and Wang Z: Galangin inhibits cell invasion by
suppressing the epithelial-mesenchymal transition and inducing
apoptosis in renal cell carcinoma. Mol Med Rep. 13:4238–4244.
2016.PubMed/NCBI
|
|
19
|
Ha TK, Kim ME, Yoon JH, Bae SJ, Yeom J and
Lee JS: Galangin induces human colon cancer cell death via the
mitochondrial dysfunction and caspase-dependent pathway. Exp Biol
Med (Maywood). 238:1047–1054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian F, Ding D and Li D: Fangchinoline
targets PI3K and suppresses PI3K/AKT signaling pathway in SGC7901
cells. Int J Oncol. 46:2355–2363. 2015.PubMed/NCBI
|
|
21
|
Chen ZH and Feng B: Effect of the change
of caspase-3 activity on the neoadjuvant chemotherapy-induced
apoptosis of large-intestinal carcinoma cells. Hunan Yi Ke Da Xue
Xue Bao. 28:117–120. 2003.(in Chinese). PubMed/NCBI
|
|
22
|
Resnitzky D and Reed SI: Different roles
for cyclins D1 and E in regulation of the G1-to-S transition. Mol
Cell Biol. 15:3463–3469. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chien ST, Shi MD, Lee YC, Te CC and Shih
YW: Galangin, a novel dietary flavonoid, attenuates metastatic
feature via PKC/ERK signaling pathway in TPA-treated liver cancer
HepG2 cells. Cancer Cell Int. 15:152015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen F, Tan YF, Li HL, Qin ZM, Cai HD, Lai
WY, Zhang XP, Li YH, Guan WW, Li YB, et al: Differential systemic
exposure to galangin after oral and intravenous administration to
rats. Chem Cent J. 9:142015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feng WH, Zhang HH, Zhang Y, Sun M and Niu
JL: Determination of galangin in rat plasma by UPLC and
pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life
Sci 998–999. 26–30. 2015. View Article : Google Scholar
|