Tissue hypoxia is an important element in tumor
progression and therapeutic resistance as tumor cells can activate
a range of adaptive molecular mechanisms that facilitate
glioblastoma multiforme (GBM) development (1). Hypoxia inducible factor-1 (HIF-1) is a
basic helix-loop-helix transcription factor that is expressed in
most cells in response to hypoxia. It regulates the cellular
response to oxygen deficit (hypoxia) in order to minimize tissue
damage. At the gene level, HIF-1 is the primary oxygen-sensitive
transcriptional activator that helps cells to adapt to low oxygen
tension (2). HIF-1 is a
heterodimeric protein consisting of an α (HIF-1α) and a β (HIF-1β)
subunit (3). Under normoxic
conditions, HIF-1α has a short half-life where it is degraded
rapidly through the ubiquitin proteasome pathway (4–6). In
hypoxic conditions, it is stabilized and complexes with the β
subunit to form a functional transcription factor. The complete
HIF-1 molecule translocates to the nucleus and activates the
expression of downstream genes in response to hypoxia (7,8).
Although HIF-1α is primarily switched on during hypoxia, it is also
frequently activated in cancer cells even under normoxic conditions
through oncogene activation and/or tumor-suppressor gene inhibition
(9,10). Furthermore, HIF-1α also regulates a
number of genes involved in major events in carcinogenesis
including cell immortalization, angiogenesis, invasion and
metastasis.
The following review focuses on the relationship
between HIF-1α and its target genes/pathways associated with
glucose metabolic pathways, cancer proliferation, cell mobility and
chemoresistance. The roles of HIF-1α in the regulation of cell
proliferation, angiogenesis, apoptosis, migration and invasion are
explored. Methods of targeting HIF-1α by regulating signaling
pathways and subsequent modulation of the target genes involved in
glioblastoma development are also discussed.
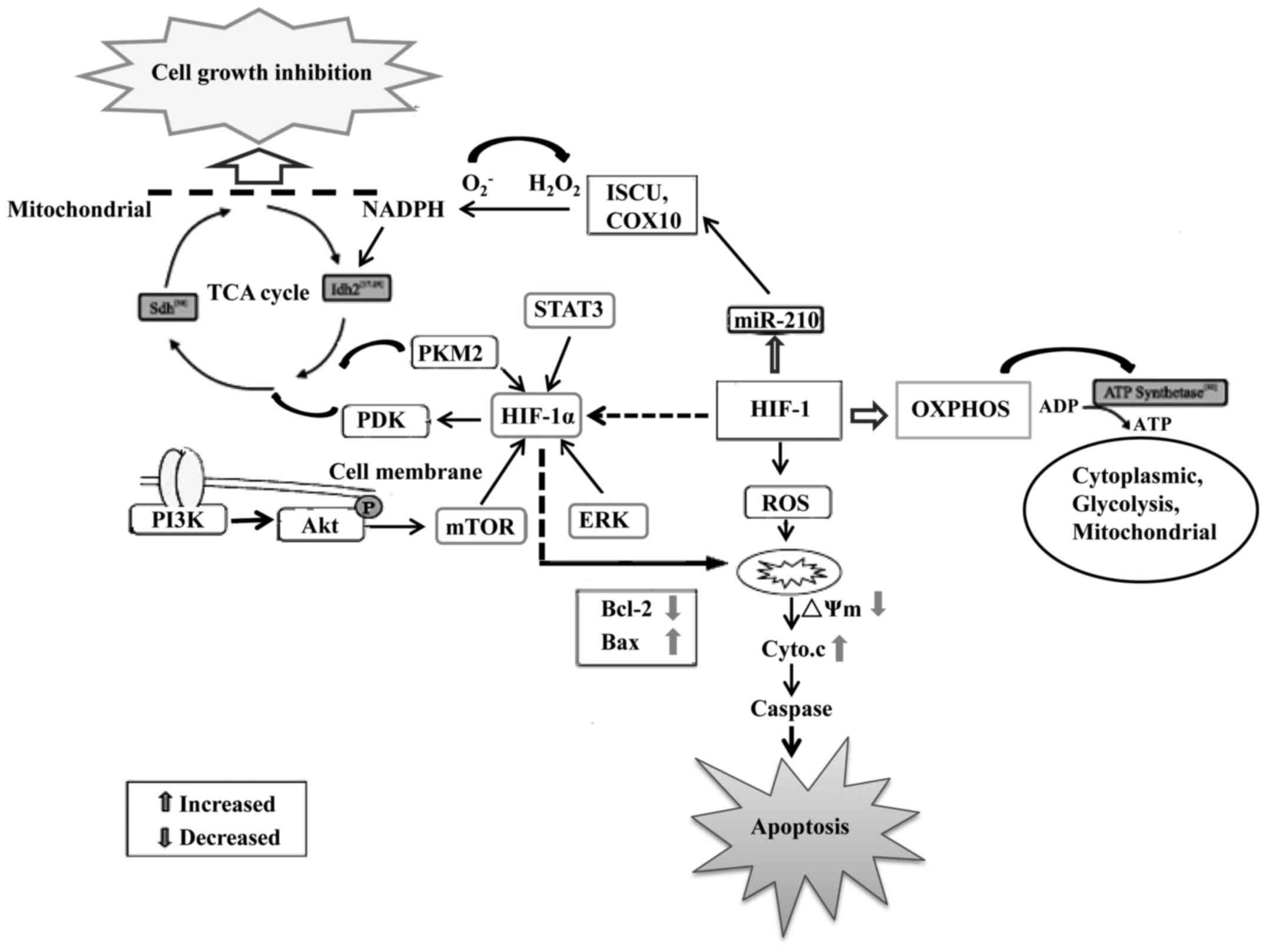
Numerous studies have shown that abnormal cellular
metabolism is a prominent feature in tumorigenesis (11,12).
Metabolic reprogramming is now recognized as a hallmark of tumors
and abnormal energy metabolism is found in GBM (13). Glioblastoma cells obtain their
energy primarily through glycolysis instead of mitochondrial
oxidative phosphorylation (OXPHOS) (14). The upregulation of glycolysis that
results in increased glucose consumption, first reported by Warburg
(15), appears to be a universal
feature in both primary and glioma stem cells (GSCs) (16).
It is generally recognized that ATP production from
OXPHOS in mitochondria is much more efficient than glycolysis in
the cytoplasm. To satisfy the energy needs of tumor cells, a large
amount of glucose must be made to fulfil the ATP needed to sustain
their growth and survival. During hypoxia, pyruvate dehydrogenase
kinase 1 (PDK1) is induced in cells to minimize the production of
reactive oxygen species (ROS) such as from glycolysis in
glioblastoma cells (17). HIF-1
also induces the transcription of miR-210, a microRNA that
partially rescues Myc antagonist (MNT) protein expression and
increases the apoptotic rate and caspase-3/−7 activity and
decreased invasive capacity, ROS and lactate production and
radioresistance in hypoxic GSCs (18,19).
Thus, HIF-1 is crucial during hypoxia to reduce mitochondrial
respiration, leakage of electrons from the electron transport
chain, and to prevent ROS production to ensure the survival of GBM
cells (20).
On the contrary, increased levels of HIF-1 may
induce glycolytic enzyme expression resulting in higher levels of
lactate being produced (21). This
biochemical shift from OXPHOS to anaerobic glycolysis is a major
hallmark of malignant glioma cells. The lactate produced causes the
acidification of the extracellular environment. Together with the
HIF-1α-induced expression of carbonic anhydrases, the pH ratio
between the intracellular and extracellular compartments is
significantly changed (22,23). As a consequence, the passive
absorption of many drugs may be decreased within the cell. Active
efflux of drugs is also taking place due to the HIF-1α-induced
transporter overexpression (24,25).
All of these actions are achieved by the hypoxia driven activation
of HIF-1 via stabilization of HIF-1α and its target genes (26,27).
Hypoxia activates the expression of HIF-1α which can
result in the switching from OXPHOS to anaerobic glycolysis,
angiogenesis, increased cell migration potential, and genetic
alterations that prevent hypoxia-induced apoptosis (28–30).
The activation of oncogenic signaling pathways, such as the
phosphatidylinositol-3 kinase/Akt (PI3K/Akt), mitogen-activated
protein kinase/extracellular signal-regulated kinase (MAPK/ERK),
signal transducer and activator of transcription 3 (STAT3)
signaling pathways, also promotes HIF-1α expression at
transcriptional levels and increases glucose utilization in GBM
even when oxygen is abundant (31–33).
Activation of HIF-1α can increase the expression of anti-apoptotic
proteins such as Bcl-2 to block glioblastoma cell apoptosis
(34–36). Aerobic glycolysis can also prevent
cells from undergoing apoptosis through the inhibition of
mitochondrial respiration, a mechanism involving the release of
cytochrome c and activation of the caspase cascade (37). Thus, HIF-1α plays a master
regulatory role in controlling aerobic glycolysis in glioblastoma
cells to meet their high-energy consumption and at the same time
preventing them from hypoxia-induced damage (38). The metabolic preference of
glioblastoma cells for glycolysis as an energy source is also a
potential therapeutic target. Thus, targeting HIF-1α, its regulated
pathways and metabolic enzymes may form a basis for the development
of effective glioblastoma therapies (Fig. 1). Further identification of more
detailed mechanisms on how HIF-1α regulates apoptosis and
pro-proliferative metabolic signaling pathways may be beneficial
for GBM therapy. As HIF-1α expression and aerobic glycolysis are
essential for glioblastoma growth, inhibiting both may enhance the
effects of antitumor agents. Furthermore, targeting HIF-1α and/or
glycolysis may be a potential option for selective glioblastoma
therapy.
Hypoxia, a common feature in cancers, is associated
with poor response to treatment. As discussed above, HIF-1 not only
facilitates glioblastoma cells to adapt to the hypoxic environment,
but also regulates a number of genes involved in major events in
carcinogenesis including cell immortalization, angiogenesis,
invasion and metastasis (39). The
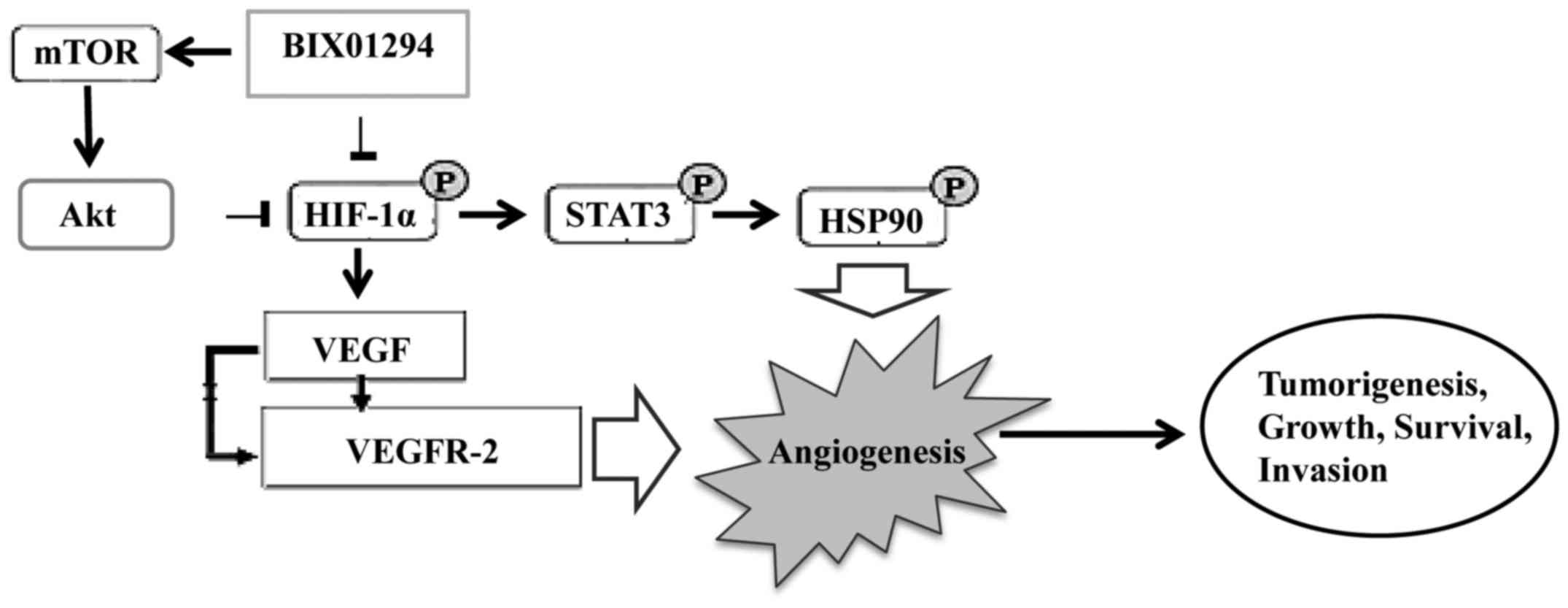
subunit HIF-1α is central to the response of mammalian cells to low
oxygen tension. It triggers the expression of vascular endothelial
growth factor (VEGF), which is central to angiogenesis by
stimulating endothelial cell growth, migration and invasion into
the extracellular matrix forming new blood vessels to support tumor
development (40). Thus, HIF-1α
appears to be a promising therapeutic target in
angiogenesis-related GBM.
BIX01294 (BIX), a G9a histone
methyltransferase-specific inhibitor, has been shown to promote
apoptosis and suppress glioma cell proliferation, migration and
invasion. BIX01294 induced an Akt-dependent increase in HIF-1α
expression and activity. Furthermore, Akt-HIF-1α axis driven
PKM2-YAP1 crosstalk activates autophagic responses in glioma cells
by G9a inhibition (41). Similarly,
ER-400583-00 targets HIF-1α signal transduction causing it to
become less stable and effectively decreased the expression of VEGF
in U251 cells. As a result, it achieved sustained HIF-1α
suppression in xenograft gliomas in animal studies. Altogether,
ER-400583-00 exhibited enhanced cytotoxicity against hypoxic glioma
cells and enhanced antitumor activity in combination with radiation
therapy (42). These findings
indicate that inhibition of HIF-1α could provide new insights into
the discovery of drugs for cancer treatment.
Hypoxia-inducible factors, particularly HIF-1α and
STAT3 have important roles in angiogenesis and are associated with
VEGF. Inhibitors of STAT3 and HIF-1α downmodulated the
hypoxia-induced immunosuppressive effects of GSCs. Thus, hypoxia
further enhances GBM-mediated immunosuppression. Thus, immune
therapeutic approaches can be used successfully in the vast
majority of GBM patients by demonstrating the importance of the
tumor hypoxic environment (43).
These findings suggest that GBM-mediated immunosuppression is
mediated through HIF-1α and STAT-3, and the inhibition of STAT3 and
HIF-1α is a promising anti-angiogenic strategy in GBM (Fig. 2).
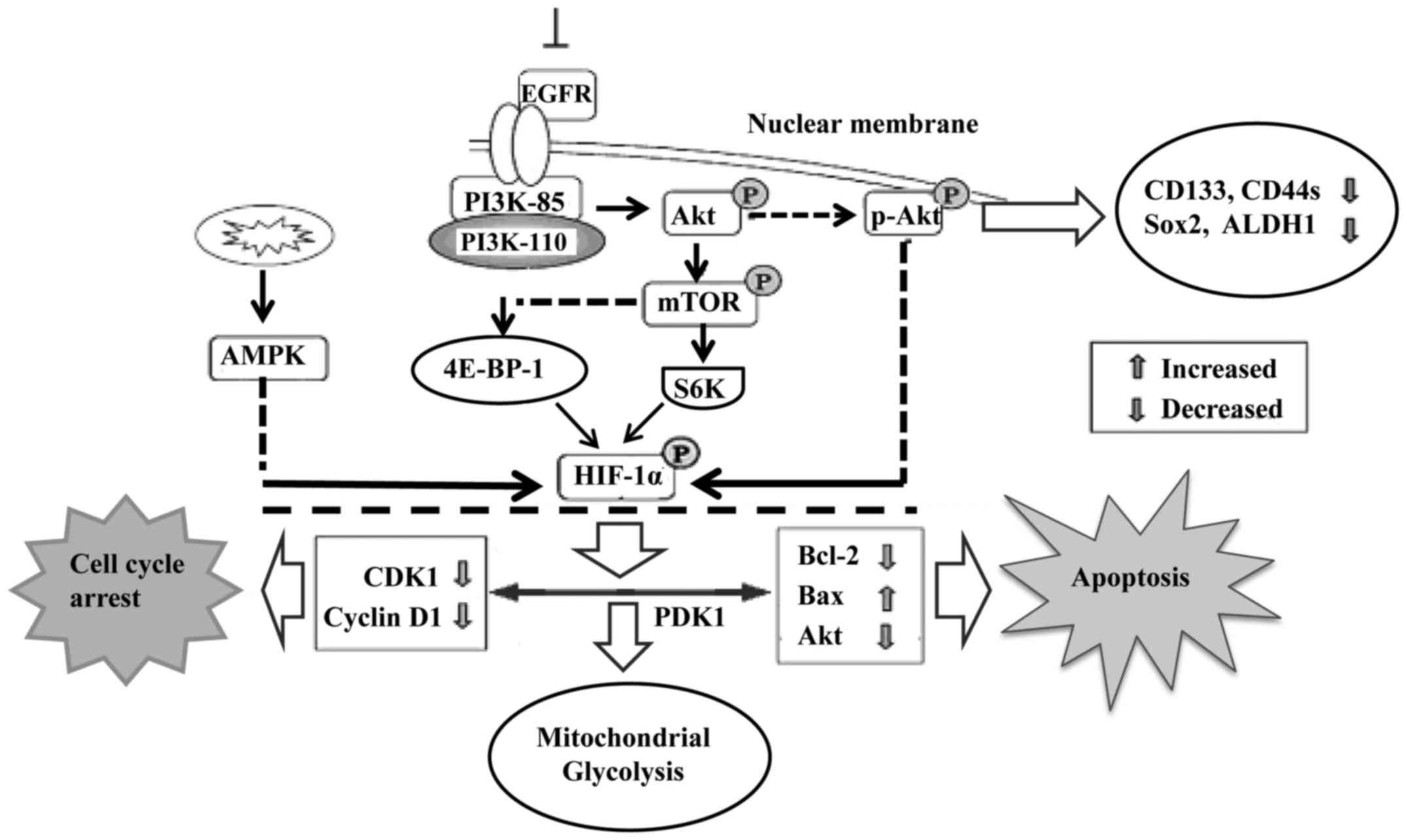
The PI3K/Akt signaling pathway is involved in cell
proliferation, apoptosis, angiogenesis, transformation and tumor
growth. Increased expression of the PI3K/Akt/HIF-1α pathway is
therefore closely associated with tumor differentiation (44,45).
Moreover, endothelial cells were programmed by GBM cell-derived
hypoxic exosomes to secrete several potent growth factors and
cytokines and to stimulate pericyte PI3K/AKT signaling activation
and migration. Active hypoxia-related molecules including HIF-1α,
Glut-1, p27 (Kip1), and p-Akt were found to be significantly
increased in glioma stem cells, particularly under hypoxic
conditions. Inhibition of endogenous Akt by LY294002 resulted in
decreased expression of several CSC-related markers (46), including CD133, Sox2, CD44s and
aldehyde dehydrogenase 1 (ALDH1) (Fig.
3).
Mitochondrial dysfunction is one of the hallmarks of
cancer progression. It represses HIF-1α expression through
phosphorylation of p70S6K and 4E-BP-1 (48). Mitochondrial dysfunction was also
found to decrease intracellular ATP levels and elevate AMPK
phosphorylation. Reducing AMPK activity by inhibitors or
gene-knockdown was found to partially rescue the mitochondrial
dysfunction-repressed HIF-1α expression (Fig. 3). Interfering with mitochondrial
function and reducing HIF-1α activity may be a novel approach to
treat hypoxic tumors that are resistant to both chemotherapy and
radiotherapy. Furthermore, Akt accumulates in the mitochondria
during hypoxia and phosphorylates PDK1 to inactivate the pyruvate
dehydrogenase complex, which switches tumor metabolism toward
glycolysis, antagonizes apoptosis and autophagy, hampers oxidative
stress and maintains tumor cell proliferation in the face of severe
hypoxia. Mitochondrial Akt-PDK1 signaling may provide an
‘actionable’ therapeutic target for GBM (49).
An adaption of glioma cells to low-oxygen
environment is the induction of HIFs. In many types of cancer
cells, HIF-1 is primarily responsible for the expression of the
apoptosis inhibitor apoptosis repressor with a CARD domain (ARC).
It acts through direct binding to a region, known as the hypoxia
response element (HRE), 190 bp upstream of the transcription site
(50). ARC is not expressed in the
normal brian but is expressed in GBM and HIFs have been implicated
in GBM pathogenesis even under normoxic conditions. In GBM, HIF-1α
activity-dependent protein carbonic anhydrase IX (CAIX) expression
was identified as a translatable non-invasive biomarker with
potential clinical significance. This suggests that increased HIF
mediates ARC expression and along with CAIX promote GBM cell
survival (51).
In high grade glioma (HGG), HIF-1α and FAT atypical
cadherin (FAT1) are two key tumor-promoting factors in the hypoxic
microenvironment. FAT1 with HIF1α and its target genes such as CA9,
GLUT1, VEGFA, MCT4, HK2, BNIP3 and REDD1 in GBM specimens, reveals
the significance of the FAT1-HIF1α axis in controlling the
invasiveness of glioblastoma. Furthermore, FAT1
depletion-associated reduction in the level of HIF1α was due to a
compromised EGFR-Akt signaling as well as increased VHL-dependent
proteasomal degradation of HIF1α. These results indicate that FAT1
represents a novel potential therapeutic target for glioblastomas
(52). Similarly, inhibition of
inositol requiring enzyme 1 (IRE1) modified the effect of hypoxia
on the expression of genes. IRE1 eliminated the sensitivity to
hypoxia of IGFBP7 and IGFBP9/NOV genes, suppressed the effect of
hypoxia on IGFBP6, IGFBP10/CYR61 and WISP2 genes, and slightly
enhanced hypoxic regulation of WISP1 gene expression in glioma
cells (53). Moreover, inhibition
of IRE1, which correlates with suppression of cell proliferation
and glioma growth, downregulated expression of pro-proliferative
IGFBP genes. Taken together, HIF-1α plays a crucial role in
mediating the interactions between oncogenes and GBM.
The hypoxic tumor microenvironment serves as a niche
for maintaining glioma-initiating cells (GICs) that are critical
for GBM occurrence and recurrence. Hypoxia-induced miR-215 is vital
for reprogramming GICs to fit the hypoxic microenvironment via
suppressing the expression of epigenetic regulator KDM1B and
modulating activities of multiple pathways (54). Notably, biogenesis of miR-215 and
several miRNAs is post-transcriptionally accelerated by
hypoxia-inducible factors (HIFs) through HIF-Drosha interaction.
Moreover, miR-215 expression is inversely correlated with KDM1B
while is positively correlated with HIF1α and GBM progression in
patients. It appears that the HIF regulates miRNA biogenesis and
consequently activates the miR-215-KDM1B-mediated signaling
required for GIC adaptation to hypoxia (55). Additionally, miR-497 is
overexpressed in glioma and hypoxia can induce the expression of
miR-497 at the transcriptional level by binding with the hypoxia
response element in the promoter (56).
Recent research also indicates that microRNA-584-3p
(miR-584-3p) is upregulated in hypoxic glioma cells and in
high-grade human glioma tumors (WHO grades III–IV). High-grade
glioma patients with high miR-584-3p expression had significantly
prolonged postoperative survival time. Mechanically, miR-584-3p
suppressed the migration and invasion of glioma cells by disrupting
hypoxia-induced stress fiber formation. Altogether, miR-584-3p may
function as a potent tumor suppressor and as a prognostic biomarker
for malignant glioma (57).
Furthermore, miR-584-3p is a potential therapeutic target for
malignant glioma, particularly for patients with WHO III–IV
GBMs.
Most researchers agree that the highly aggressive
GBM subtype with its necrotic tissues, are affected similarly by
hypoxia. The extent of the influence of hypoxia on these processes
makes it an attractive therapeutic strategy for glioma (58). Considering that miRNA research has
advanced from the identification of an initial association with
glioma to the commercial development of miRNA-based therapeutics in
less than a decade, the anticipation of signifcant developments in
this field with the ultimate improvement of patient outcomes is
reasonable (59).
Hypoxia is an essential condition in tumor
development and glioblastoma cells can utilize a range of adaptive
molecular mechanisms leading to their subsequent therapeutic
resistance (60). These mechanisms
include switching from OXPHOS to anaerobic glycolysis,
angiogenesis, increased cell migration potential and genetic
alterations that help avoid hypoxia-induced apoptosis. These
hypoxic areas can either promote cell death or provoke an adaptive
response leading to death resistance (61–63).
Once GBM cells become adaptive to hypoxia, they are more resistant
to apoptosis and less responsive to targeted therapy. Tumor hypoxia
is thought to play a crucial role in pathologic characteristics of
GBM, including invasiveness, necrosis and microvascular
hyperplasia. It also contributes to resistance to chemotherapy,
immunotherapy and radiotherapy due to the possibility of
dysregulation of apoptotic pathway or other mechanisms (64). Using in vitro models of
glioblastoma, rhabdomyosarcoma and Ewing's sarcoma, it has been
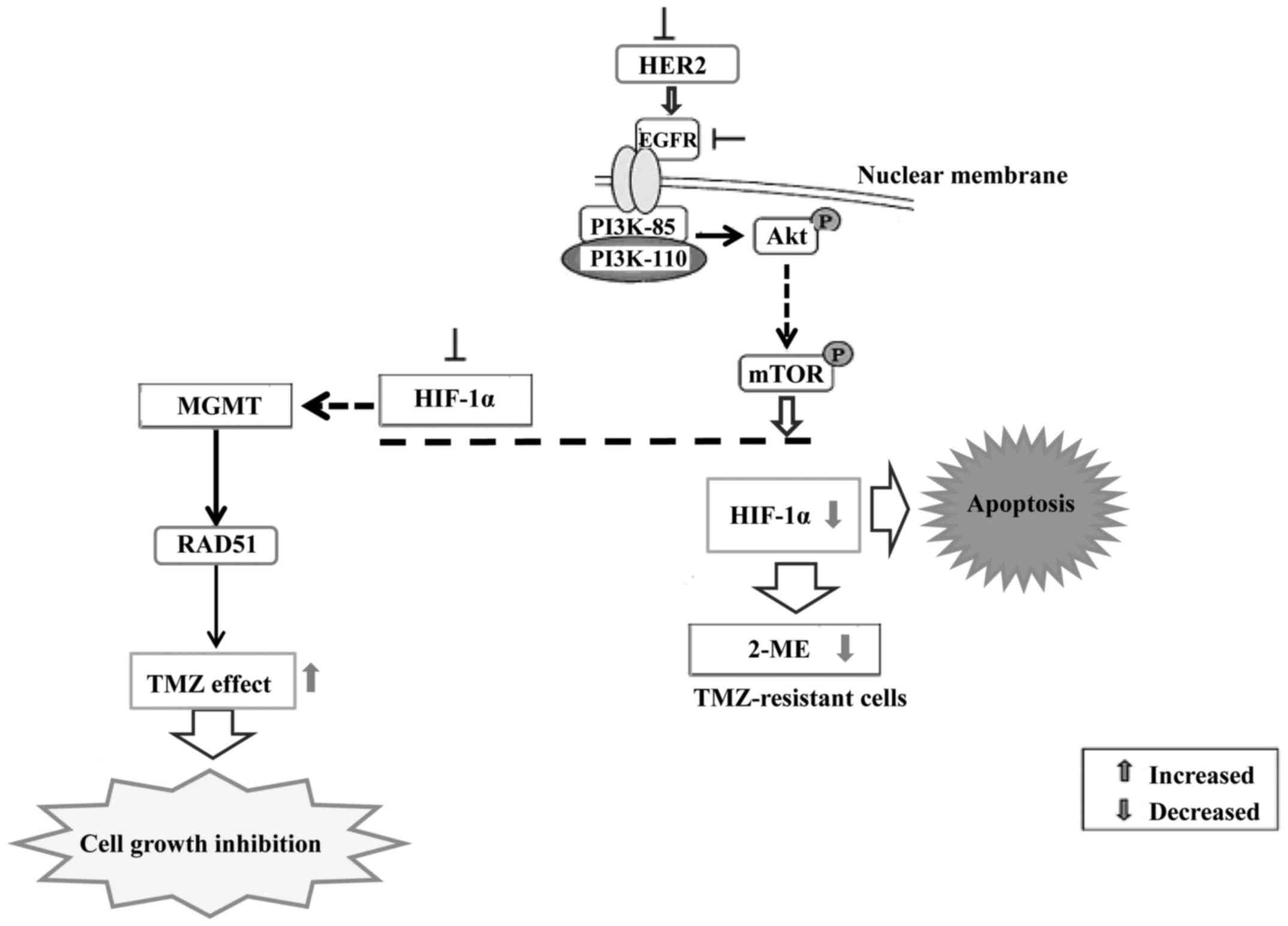
shown that the resistance to chemotherapy is dependent on HIF-1.
Inhibition of HIF-1α sensitizes glioma cells to temozolomide (TMZ)
through the downregulation of the DNA repair enzyme
O6-methylguanine-DNA methyltransferase (MGMT). HIF-1α
downregulation sensitized U251 cells to TMZ treatment and enhanced
the proliferation-inhibiting, invasion/migration-suppressing,
apoptosis-inducing and differentiation-promoting effects exerted by
TMZ. HIF-1α downregulation sensitized U251 glioma cells to
temozolomide treatment via inhibiting MGMT expression and Notch1
pathway activation (65).
Suppression of HIF-1α expression by gene knockdown
or by an inhibitor of 2-methoxyestradiol (2ME) increased the
efficacy of TMZ on human pituitary adenoma cells (66). Furthermore, downmodulation of MGMT
decreased DNA repair through a decrease in RAD51 protein
expression. Thus, 2ME may be a useful adjuvant to enhance the
efficacy of TMZ in the treatment of gliomas. HER2 or kinase
inhibitors suppress the expression of HIF-1α in cancer cells,
suggesting that the HER2-driven PI3K/Akt/mTOR pathway is involved
(Fig. 4).
Overall, hypoxia-induced resistance is implicated in
treatment resistance not only to radiotherapy, but also to
chemotherapy (67). Hypoxia induces
resistance to several anticancer agents in neurons (68), but also in glioma cells (69). Moreover, stabilization of HIF-1α in
normoxia by cobalt choride or suppression of HIF-1α in hypoxia by
various means (shRNAi, siRNA, dominant-negative HIF, small-molecule
NSC-134754) did not induce drug resistance. Taken together, further
evaluation of the regulation of HIF-1α needs to be clarified in
radiotherapy/temozolomide in clinical trials for patients with
glioblastoma.
The importance of HIFs in cancer progression
particularly in reduced oxygen conditions has led to the
development of HIF-1α and HIF-2α inhibitors (70). Concomitant administration of
inhibitors of HIF-1α and its target genes and pathways may be novel
strategy for treating GBM. However, a major difficulty in targeting
HIF-1α is the lack of specificity of the available inhibitors and
most of them exhibit a number of off-target effects (71,72).
Nevertheless, priority may be given to an inhibitor that could
effectively reduce HIF-1α levels and the expression of its target
genes (VEGF, IGF2 and PDK1) rather than its cytotoxic effect.
During the early stages in carcinogenesis, inhibition of the HIF-1
system may be beneficial particularly in reducing the development
of resistance to cytotoxic and targeted drugs (Table I).
Since aerobic glycolysis provides the major source
of energy for cancer cell growth and proliferation, strategies
targeting this process could be a promising therapeutic option. A
synthetic Toll-like receptor (TLR) 7/8 ligand imiquimod (IMQ) was
found to enhance aerobic glycolysis by upregulating HIF-1α
expression through ROS-mediated STAT3- and Akt-dependent pathways
rather than through TLR7/8 signaling (73). Silencing of HIF-1α repressed
IMQ-induced aerobic glycolysis and sensitized cells to apoptosis
due to a faster depletion of ATP and Mcl-1. Co-treatment with a
glucose analog (2-DG) and an Hsp90 inhibitor (17-AAG) enhanced the
effect of IMQ in inducing cancer cell apoptosis in vitro and
inhibiting tumor growth in vivo. These observations support
the use of inhibitors of HIF-1α and glycolysis to enhance the
antitumor effects of IMQ. Additionally, IMQ modulates the activity
of the glioma-associated oncogene (GLI) transcription factors by
engaging signaling components of other pathways. GLI1 is a direct
transcriptional target of GLI2 and GLI3, serves as a robust and
sensitive functional read-out for the Hedgehog (HH) pathway
activity (74,75).
The anticancer action of sodium dichloroacetate
(DCA) caused ambiguous effects varying from tumor growth
stimulation to significant anticancer activity. Under hypoxic
conditions, the anticancer efficacy of DCA against glioma С6 cells
was significantly enhanced (76).
DCA was found to inhibit the glycolytic pathway through glycolytic
inhibition which in turn diminished acid production. Moreover, DCA
treatment also led to an alteration in the multidrug resistance
(MDR) phenotype of GBM cells. Thus, altering glucose metabolism in
GBM cells with hypoxia-induced resistance could be enhanced by
DCA.
Resveratrol, a natural compound in food, has
anticancer effects, antioxidant properties and an impact on glucose
metabolism. It markedly suppressed glioma U87 and U251 cell
migration and invasion under a hypoxia condition and higher doses
led to stronger blockage (77). It
has been proven that hypoxia promotes aggressiveness, angiogenesis
and resistance in glioma. Mechanically, hypoxia-induced
upregulation of phosphorylated STAT3 was blocked by resveratrol.
Notably, miR-34a was downregulated under hypoxia, but upregulated
by resveratrol which consequently inhibited hypoxia-induced
migration and invasion possibly via the p-STAT3/miR-34a axis and
this effect was both time- and dose-dependent.
The close relationship between HIF-activated gene
products and tumor progression/metabolism pinpoints HIF-1α as an
attractive therapeutic target. Several studies have already
established that inhibition of the HIF-1 pathway can inhibit
malignant characteristics such as glucose metabolism in gliomas
(78,79). Indeed, a number of small-molecule
inhibitors of HIF-1α signaling are already undergoing clinical
trials. The search for new HIF-1α inhibitors continues. Echinomycin
and ‘programmable’ polyamides inhibited HIF-1α, and blocked
hypoxia-induced expression of dynamin-related protein 1 (Drp1).
Notably, Drp1 inhibitor Mdivi-1 efficiently attenuated
hypoxia-induced mitochondrial fission and migration of U251 cells
(80). One of the lead inhibitors
is KCN1
[3,4-dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzene-sulfonamide],
which is a new class of arylsulfonamide inhibitors of the HIF-1
pathway, inhibited HIF-1α transcriptional activity through the
disruption of the interaction between the HIF-1α subunit and
transcriptional co-activator p300/CBP (70,81).
The latter acts as a bridge between transcription factors to the
transcriptional machinery. p300/CBP has a critical role in HIF
function since blocking HIF-1α-p300/CBP interaction markedly
attenuates HIF activity (71). A
recent study also found that KCN1 impaired the recruitment of these
co-factors to preassembled HRE-HIF complexes on the chromatin, and
prevented hypoxia-induced transcription in malignant glioma tumor
xenografts. Mechanistically, KCN1 on the CH1 domains of p300 and
CBP are predicted to block the interaction with HIF-1α (82).
HIF-1α inhibitor OKN-007 reduced HIF-1α expression
even under hypoxia, acted on GLUT-1 and MIB-1 to decrease cell
proliferation, and increased apoptosis through cleavage of
caspase-3 (83). OKN-007,
previously known as NXY-059, is a very effective compound against
in vivo adult glioma models (84–86),
and it is currently undergoing clinical trial assessment as a new
investigational drug for recurrent adult GBMs. OKN-007 is a small
molecule that can traverse the blood-brain barrier and also has
anti-inflammatory, antioxidant, pro-apoptotic (87,88)
and anti-angiogenic properties (85,86).
It was also previously demonstrated that OKN-007 is an effective
anti-angiogenic compound in vivo, by directly decreasing
microvessel density (MVD) (CD-31) and HIF-1α levels in both F98 and
U87 glioma models (85), and ex
vivo by directly decreasing the levels of VEGFR-2 in C6 rat
gliomas (86). OKN-007 was also
able to decrease the levels of VEGFR-2 in a preclinical GL261 mouse
glioma model (89). OKN-007
treatment substantially decreased VEGFR-2 levels in a GL261 glioma
model, and is considered as an anti-angiogenic therapy in human
gliomas. Furthermore, OKN-007 was able to significantly decrease
SULF2 and platelet-derived growth factor receptor-α (PDGFR-α)
immunoexpression, and significantly increased decorin expression in
responsive mice. This study indicates that OKN-007 may be an
effective anticancer agent for some patients with GBMs by
inhibiting cell proliferation and angiogenesis, possibly via the
PDGFRα pathway, and could be considered as an additional therapy
for pediatric brain tumor patients (90).
CpG oligodeoxynucleotides (CpG ODNs) are specific
inhibitors to knock down gene expression. CpG ODN107 in combination
with radiotherapy significantly decreased MVD, the VEGF level and
HIF-1α expression in orthotopic implantation glioma. In conclusion,
CpG ODN107 significantly increased the radiosensitivity of U87
human glioma cells in vitro and in vivo. The
radiosensitizing effect of CpG ODN107 was tightly related to its
anti-angiogenic activity via suppression of the HIF-1α/VEGF pathway
(91). The mRNAs of some target
genes were also downregulated. Thus, CpG ODN may be effective
therapeutics in the future.
Histone deacetylases are enzymes that can regulate
protein functions by removing their acetyl groups. Histones are
typically deacetylated resulting in stronger binding to DNA. These
enzymes can also regulate transcription factors such as HIF-1. The
latter has been reported to upregulate histone demethylases
(92).
The importance of HIF-1 in GBM has sparked a search
for their inhibitors. They include small molecule inhibitors
(93–95) and HDACIs (96). The latter not only suppressed HIF-1α
activity, but also the expression of HIF-regulated genes (97,98).
Among the HDACIs developed, vorinostat, romidepsin and
suberanilohydroxamic acid (SAHA) are most promising and are now
approved for treating GBM (99).
Vorinostat is an orally active, potent inhibitor of HDAC activity
that crosses the blood-brain barrier. Among the pleiotropic effects
of HDAC inhibitors is the ability to attenuate inflammation, an
action seen at concentrations lower than those required to slow
cancer cell growth (100,101). The combination of vorinostat and
tranylcypromine reduced GSC viability and displayed efficacy in a
U87 xenograft model (102).
Inhibition of histone deacetylases not only causes
hyperacetylation of histones, but other proteins as well such as
the chaperon Hsp90 (103,104). Hsp90 regulates the expression and
stability of HIF-1α that in turn regulates VEGF that promotes
glioma proliferation and metastasis (105,106). The translation of HIF-1α is
mediated, at least in part, by the PI3K/Akt pathway since
inhibition of this pathway is sufficient to reduce normoxic HIF-1α
protein levels (107). HDACIs
suppress constitutive Akt activation resulting in decrease HIF-1α
protein levels (108,109). The inhibition of histone
deacetylase may vary with time. The pan-HDACI PCI-24781 accelerates
cell apoptosis by downregulating the expression of AKT, mTOR, p70
ribosomal protein S6 kinase (p70s6k), glycogen synthase kinase 3A
and B (GSK3a/b) and eukaryotic initiation factor 4E binding protein
1 (4E-BP1), and enhances the accumulation of HIF-1α (110).
Another broad spectrum HDACI valproic acid (VPA)
exhibited significant anticancer activity in gliomas (111). It inhibited HIF-1α expression and
cell migration and knockdown of histone deacetylase 2 (HDAC2) could
mimic these effects (112). In
glioma tumors, HDACIs mainly induced cytostasis and apoptosis
(113,114). A number of mechanisms have been
proposed to explain the HDACI-induced apoptosis but how these
inhibitors work is still not fully understood. Unraveling the
molecular actions of HDACIs on HIF-1α may not only increase our
understanding of the HIF signaling pathways but also allow the
development of novel and more specific treatment options for
GBM.
A number of compounds derived from natural sources
have anticancer properties that are linked to HIF-1α. For example,
a dietary chalcone-type flavonoid called isoliquiritigenin (ISL)
was found to suppress sprout formation in VEGF-treated aortic
rings. It also inhibited VEGF expression in breast cancer cells by
fostering HIF-1α proteasome degradation, and blocking VEGFR-2
kinase activity by binding to it directly. ISL inhibited breast
cancer growth in vivo, suppressed VEGF/VEGFR-2 signaling,
elevated apoptosis ratio but with minimal toxic effects (115). It has been reported that ISL had a
reversible inhibitory effect on DNA topoisomerase I (TOP I)
activity, reduced the rate of single-strand DNA unwinding in tumor
cells, and upregulated p21/WAF1 and p27 in inducing the apoptosis
of U87 glioma cells (116,117).
However, the hypoxic microenvironment in glioma is
reductive in nature, and the effects of natural compounds an HIF-1α
protein are unclear in GBM. This is being exploited to selectively
activate drugs such as ISL or celastrol targeting HIF-1α in GBM is
still a promising option since it regulates key cellular processes
such as angiogenesis.
It is important to ascertain how HIF-1 plays such a
significant role in GBM since silencing HIF-2α alone impairs tumor
growth in vivo (121,122). Conflicting views are expressed in
the scientific community regarding the roles of HIF-1α and HIF-2α.
Some report that HIF-1α is a tumor-suppressor gene while others
report that HIF-2α is an oncogene. Functional studies have shown
that overexpression of HIF-1α can suppress tumor growth while
suppressing it enhances tumor growth (123). A recent study also indicated a
novel signaling mechanism mediated by HIF-2α in regulating
invasiveness and stemness characteristics. It suggested that under
hypoxic conditions, U87MG and A172 glioma cells acquire more
migratory potential by increased Pan Mena and Mena INV expression
as a consequence of this HIF-2α mediated increase in Oct-4 and
Sox-2 (124). These properties may
help glioma cells to form a new nidus after local invasion or
metastasis.
Metabolic reprogramming is now an established fact
in GBM biology. In this process, HIF-1 plays a crucial role in
switching energy metabolism from OXPHOS to glycolysis particularly
under hypoxic conditions to provide GBM cells a survival advantage.
In many types of cancers, poor prognosis is associated with
abnormal levels and activity of HIF-1. Therefore, HIF-1 and its
mediated metabolic pathways may be promising targets for treating
GBM (125). Drugs such as DCA, and
IMQ are effective in inhibiting HIF-1 expression and its activity
and thus block tumor growth (73,76).
Drugs targeting metabolic enzymes downstream of HIF-1 are also
effective inhibitors of GBM progression. Therefore, combination
therapy using both groups of drugs may provide an even more
effective treatment regimen.
As HIFs are a group of transcription factors that
regulate a large number of target genes, it is possible that they
can act in an opposite fashion. How they work in a particular
context depends on the shift in their balance between
tumor-suppressive and oncogenic properties. At early stages of GBM
development, HIF-1-mediated anti-apoptosis may be important. As
time progresses, more mutations accumulate leading to more
signaling pathways being re-programmed eventually leading to
evasion of apoptosis. The anti-apoptotic function of HIF-1 becomes
non-essential at later stages of GBM development and the balance
may shift towards more tumor-suppression leading to a selective
pressure on eliminating HIF-1. Nevertheless, targeting HIF-1α is
still a promising option since it regulates key cellular processes
such as angiogenesis and epithelial to mesenchymal transition which
are important for metastasis (126,127). HIF-1α can further enhance the
already activated signaling pathways in GSCs supporting their
enrichment in GBM (35,128). Targeting HIF-1α directly,
indirectly or eliminating the hypoxic regions in gliomas may be
workable for treating aggressive GBM (129,130).
A growing body of evidence supports the facilitating
role of HIF-1α in GBM progression/metabolism. Targeting HIF-1α is a
potent strategy for GBM, particularly as HIF-1α is the key
transcription factor responsible for the transactivation of a wide
array of genes, many of which enhance the survival and metabolism
of GBM cells. For example, Akt/mTOR is one of the major oncogenic
pathways that shift the balance of HIF-1α accumulation (131,132). A number of drugs are now in
clinical trials but they only show low to moderate activity against
gliomas (117,119).
Owing to the notable improvements in blocking the
HIF-1 function, it may be expected to interfere with multiple
attributes of tumor cells and eventually lead to tumor regression.
Not surprisingly, significant efforts and resources have been
invested into identifying small molecules that may potently and
specifically inhibit HIF-1α. Despite this, new inhibitors of the
HIF-1 pathway, preferably with a defined mechanism of action, need
to be identified, and we have yet to determine which agents may
have the best antitumor efficacy and safety profile. Furthermore,
drugs targeting these regulators of the HIF-1 system, which
eventually degrade them in GBM cells, may be the future for
developing novel treatment strategies.
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81673827) and
also by a grant from the Shanghai Municipal Commission of Health
and Family Planning Research Project of Traditional Chinese
Medicine (no. 2016JP008). The present review is distributed under
the terms of noncommercial use, distribution and reproduction in
any medium, provided the original author(s) and source are
credited. The present review is part of a Special Issue entitled
‘Targeting HIF-1α and regulate its downstream pathways in GBM cells
for future glioblastoma therapies’.
|
1
|
Søndergaard KL, Hilton DA, Penney M,
Ollerenshaw M and Demaine AG: Expression of hypoxia-inducible
factor 1α in tumours of patients with glioblastoma. Neuropathol
Appl Neurobiol. 28:210–217. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Solaini G, Baracca A, Lenaz G and Sgarbi
G: Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys
Acta. 1797:1171–1177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jewell UR, Kvietikova I, Scheid A, Bauer
C, Wenger RH and Gassmann M: Induction of HIF-1alpha in response to
hypoxia is instantaneous. FASEB J. 15:1312–1314. 2001.PubMed/NCBI
|
|
5
|
Huang LE, Gu J, Schau M and Bunn HF:
Regulation of hypoxia-inducible factor 1alpha is mediated by an
O2-dependent degradation domain via the
ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 95:7987–7992.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salceda S and Caro J: Hypoxia-inducible
factor 1alpha (HIF-1alpha) protein is rapidly degraded by the
ubiquitin-proteasome system under normoxic conditions. Its
stabilization by hypoxia depends on redox-induced changes. J Biol
Chem. 272:22642–22647. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Majmundar AJ, Wong WJ and Simon MC:
Hypoxia-inducible factors and the response to hypoxic stress. Mol
Cell. 40:294–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papandreou I, Cairns RA, Fontana L, Lim AL
and Denko NC: HIF-1 mediates adaptation to hypoxia by actively
downregulating mitochondrial oxygen consumption. Cell Metab.
3:187–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rankin EB and Giaccia AJ: The role of
hypoxia-inducible factors in tumorigenesis. Cell Death Differ.
15:678–685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zundel W, Schindler C, Haas-Kogan D, Koong
A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson
AB, et al: Loss of PTEN facilitates HIF-1-mediated gene expression.
Genes Dev. 14:391–396. 2000.PubMed/NCBI
|
|
11
|
Wang G, Wang J, Zhao H, Wang J and To SS
Tony: The role of Myc and let-7a in glioblastoma, glucose
metabolism and response to therapy. Arch Biochem Biophys.
580:84–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Zhu S, Tong J, Hao H, Yang J, Liu Z
and Wang Y: Suppression of lactate dehydrogenase A compromises
tumor progression by downregulation of the Warburg effect in
glioblastoma. Neuroreport. 27:110–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Özcan E and Çakır T: Reconstructed
metabolic network models predict flux-level metabolic reprogramming
in glioblastoma. Front Neurosci. 10:1562016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu J, Liu Y, Cho K, Dong X, Teng L, Han D,
Liu H, Chen X, Chen X, Hou X, et al: Downregulation of TRAP1
sensitizes glioblastoma cells to temozolomide chemotherapy through
regulating metabolic reprogramming. Neuroreport. 27:136–144. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuen CA, Asuthkar S, Guda MR, Tsung AJ and
Velpula KK: Cancer stem cell molecular reprogramming of the Warburg
effect in glioblastomas: A new target gleaned from an old concept.
CNS Oncol. 5:101–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang W, Finniss S, Cazacu S, Xiang C,
Brodie Z, Mikkelsen T, Poisson L, Shackelford DB and Brodie C:
Repurposing phenformin for the targeting of glioma stem cells and
the treatment of glioblastoma. Oncotarget. Jul 29–2016.(Epub ahead
of print). doi: 10.18632/oncotarget.10919.
|
|
17
|
Shen H, Hau E, Joshi S, Dilda PJ and
McDonald KL: Sensitization of glioblastoma cells to irradiation by
modulating the glucose metabolism. Mol Cancer Ther. 14:1794–1804.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang W, Wei J, Guo T, Shen Y and Liu F:
Knockdown of miR-210 decreases hypoxic glioma stem cells stemness
and radioresistance. Exp Cell Res. 326:22–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agrawal R, Pandey P, Jha P, Dwivedi V,
Sarkar C and Kulshreshtha R: Hypoxic signature of microRNAs in
glioblastoma: Insights from small RNA deep sequencing. BMC
Genomics. 15:6862014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Velpula KK, Bhasin A, Asuthkar S and Tsung
AJ: Combined targeting of PDK1 and EGFR triggers regression of
glioblastoma by reversing the Warburg effect. Cancer Res.
73:7277–7289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nie Q, Guo P, Guo L, Lan J, Lin Y, Guo F,
Zhou S, Ge J, Mao Q, Li X, et al: Overexpression of isocitrate
dehydrogenase-1R123H enhances the proliferation of A172
glioma cells via aerobic glycolysis. Mol Med Rep. 11:3715–3721.
2015.PubMed/NCBI
|
|
22
|
Inukai M, Hara A, Yasui Y, Kumabe T,
Matsumoto T and Saegusa M: Hypoxia-mediated cancer stem cells in
pseudopalisades with activation of hypoxia-inducible factor-1α/Akt
axis in glioblastoma. Hum Pathol. 46:1496–1505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu G, Wang M, Xie W and Bai X:
Hypoxia-inducible factor-1 alpha C1772T gene polymorphism and
glioma risk: A hospital-based case-control study from China. Genet
Test Mol Biomarkers. 15:461–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adams DJ, Waud WR, Wani MC, Manikumar G,
Flowers JL, Driscoll TA and Morgan LR: BACPTDP: A water-soluble
camptothecin pro-drug with enhanced activity in hypoxic/acidic
tumors. Cancer Chemother Pharmacol. 67:855–865. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Florczyk SJ, Wang K, Jana S, Wood DL,
Sytsma SK, Sham JG, Kievit FM and Zhang M: Porous
chitosan-hyaluronic acid scaffolds as a mimic of glioblastoma
microenvironment ECM. Biomaterials. 34:10143–10150. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maroni P, Matteucci E, Drago L, Banfi G,
Bendinelli P and Desiderio MA: Hypoxia induced E-cadherin involving
regulators of Hippo pathway due to HIF-1α stabilization/nuclear
translocation in bone metastasis from breast carcinoma. Exp Cell
Res. 330:287–299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lv L, Yuan J, Huang T, Zhang C, Zhu Z,
Wang L, Jiang G and Zeng F: Stabilization of Snail by HIF-1α and
TNF-α is required for hypoxia-induced invasion in prostate cancer
PC3 cells. Mol Biol Rep. 41:4573–4582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mashiko R, Takano S, Ishikawa E, Yamamoto
T, Nakai K and Matsumura A: Hypoxia-inducible factor 1α expression
is a prognostic biomarker in patients with astrocytic tumors
associated with necrosis on MR image. J Neurooncol. 102:43–50.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Birner P, Piribauer M, Fischer I,
Gatterbauer B, Marosi C, Ambros PF, Ambros IM, Bredel M, Oberhuber
G, Rössler K, et al: Vascular patterns in glioblastoma influence
clinical outcome and associate with variable expression of
angiogenic proteins: Evidence for distinct angiogenic subtypes.
Brain Pathol. 13:133–143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Flynn JR, Wang L, Gillespie DL, Stoddard
GJ, Reid JK, Owens J, Ellsworth GB, Salzman KL, Kinney AY and
Jensen RL: Hypoxia-regulated protein expression, patient
characteristics, and preoperative imaging as predictors of survival
in adults with glioblastoma multiforme. Cancer. 113:1032–1042.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan G, Yan SF, Xue H, Zhang P, Sun JT and
Li G: Cucurbitacin I induces protective autophagy in glioblastoma
in vitro and in vivo. J Biol Chem. 289:10607–10619. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang YT, Ju TC and Yang DI: Induction of
hypoxia inducible factor-1 attenuates metabolic insults induced by
3-nitropropionic acid in rat C6 glioma cells. J Neurochem.
93:513–525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qiang L, Wu T, Zhang HW, Lu N, Hu R, Wang
YJ, Zhao L, Chen FH, Wang XT, You QD, et al: HIF-1α is critical for
hypoxia-mediated maintenance of glioblastoma stem cells by
activating Notch signaling pathway. Cell Death Differ. 19:284–294.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gammoh N, Fraser J, Puente C, Syred HM,
Kang H, Ozawa T, Lam D, Acosta JC, Finch AJ, Holland E, et al:
Suppression of autophagy impedes glioblastoma development and
induces senescence. Autophagy. 12:1431–1439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maugeri G, D'Amico A Grazia, Reitano R,
Magro G, Cavallaro S, Salomone S and D'Agata V: PACAP and VIP
inhibit the invasiveness of glioblastoma cells exposed to hypoxia
through the regulation of HIFs and EGFR expression. Front
Pharmacol. 7:1392016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li J, Ke Y, Huang M, Huang S and Liang Y:
Inhibitory effects of B-cell lymphoma 2 on the vasculogenic mimicry
of hypoxic human glioma cells. Exp Ther Med. 9:977–981.
2015.PubMed/NCBI
|
|
37
|
Lin H, Patel S, Affleck VS, Wilson I,
Turnbull DM, Joshi AR, Maxwell R and Stoll EA: Fatty acid oxidation
is required for the respiration and proliferation of malignant
glioma cells. Neuro Oncol. Jun 29–2016.(Epub ahead of print). pii:
now128.
|
|
38
|
Han D, Wei W, Chen X, Zhang Y, Wang Y,
Zhang J, Wang X, Yu T, Hu Q, Liu N, et al: NF-κB/RelA-PKM2 mediates
inhibition of glycolysis by fenofibrate in glioblastoma cells.
Oncotarget. 6:26119–26128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Womeldorff M, Gillespie D and Jensen RL:
Hypoxia-inducible factor-1 and associated upstream and downstream
proteins in the pathophysiology and management of glioblastoma.
Neurosurg Focus. 37:E82014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie Q, Bao X, Chen ZH, Xu Y, Keep RF,
Muraszko KM, Xi G and Hua Y: Role of protease-activated receptor-1
in glioma growth. Acta Neurochir. (Suppl 121). S355–S360. 2016.
|
|
41
|
Ahmad F, Dixit D, Joshi SD and Sen E: G9a
inhibition induced PKM2 regulates autophagic responses. Int J
Biochem Cell Biol. 78:87–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Okamoto K, Ito D, Miyazaki K, Watanabe S,
Tohyama O, Yokoi A, Ozawa Y, Asano M, Kawamura T, Yamane Y, et al:
Microregional antitumor activity of a small-molecule
hypoxia-inducible factor 1 inhibitor. Int J Mol Med. 29:541–549.
2012.PubMed/NCBI
|
|
43
|
Wei J, Wu A, Kong LY, Wang Y, Fuller G,
Fokt I, Melillo G, Priebe W and Heimberger AB: Hypoxia potentiates
glioma-mediated immunosuppression. PLoS One. 6:e161952011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kucharzewska P, Christianson HC, Welch JE,
Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain
E, Bengzon J and Belting M: Exosomes reflect the hypoxic status of
glioma cells and mediate hypoxia-dependent activation of vascular
cells during tumor development. Proc Natl Acad Sci USA.
110:7312–7317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pore N, Jiang Z, Shu HK, Bernhard E, Kao
GD and Maity A: Akt1 activation can augment hypoxia-inducible
factor-1alpha expression by increasing protein translation through
a mammalian target of rapamycin-independent pathway. Mol Cancer
Res. 4:471–479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Joshi S, Singh AR and Durden DL: MDM2
regulates hypoxic hypoxia-inducible factor 1α stability in an E3
ligase, proteasome, and PTEN-phosphatidylinositol
3-kinase-AKT-dependent manner. J Biol Chem. 289:22785–22797. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Muh CR, Joshi S, Singh AR, Kesari S,
Durden DL and Makale MT: PTEN status mediates 2ME2 anti-tumor
efficacy in preclinical glioblastoma models: Role of HIF1α
suppression. J Neurooncol. 116:89–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hsu CC, Wang CH, Wu LC, Hsia CY, Chi CW,
Yin PH, Chang CJ, Sung MT, Wei YH, Lu SH, et al: Mitochondrial
dysfunction represses HIF-1α protein synthesis through AMPK
activation in human hepatoma HepG2 cells. Biochim Biophys Acta.
1830:4743–4751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chae YC, Vaira V, Caino MC, Tang HY, Seo
JH, Kossenkov AV, Ottobrini L, Martelli C, Lucignani G, Bertolini
I, et al: Mitochondrial Akt regulation of hypoxic tumor
reprogramming. Cancer Cell. 30:257–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Razorenova OV, Castellini L, Colavitti R,
Edgington LE, Nicolau M, Huang X, Bedogni B, Mills EM, Bogyo M and
Giaccia AJ: The apoptosis repressor with a CARD domain (ARC) gene
is a direct hypoxia-inducible factor 1 target gene and promotes
survival and proliferation of VHL-deficient renal cancer cells. Mol
Cell Biol. 34:739–751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lo Dico A, Martelli C, Valtorta S,
Raccagni I, Diceglie C, Belloli S, Gianelli U, Vaira V, Politi LS,
Bosari S, et al: Identification of imaging biomarkers for the
assessment of tumour response to different treatments in a
preclinical glioma model. Eur J Nucl Med Mol Imaging. 42:1093–1105.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Madan E, Dikshit B, Gowda SH, Srivastava
C, Sarkar C, Chattopadhyay P, Sinha S and Chosdol K: FAT1 is a
novel upstream regulator of HIF1α and invasion of high grade
glioma. Int J Cancer. 139:2570–2582. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Minchenko OH, Kharkova AP, Minchenko DO
and Karbovskyi LL: Effect of hypoxia on the expression of genes
that encode some IGFBP and CCN proteins in U87 glioma cells depends
on IRE1 signaling. Ukr Biochem J. 87:52–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hu J and Wang XF: HIF-miR-215-KDM1B
promotes glioma-initiating cell adaptation to hypoxia. Cell Cycle.
15:1939–1940. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hu J, Sun T, Wang H, Chen Z, Wang S, Yuan
L, Liu T, Li HR, Wang P, Feng Y, et al: MiR-215 is induced
post-transcriptionally via HIF-Drosha complex and mediates
glioma-initiating cell adaptation to hypoxia by targeting KDM1B.
Cancer Cell. 29:49–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lan J, Xue Y, Chen H, Zhao S, Wu Z, Fang
J, Han C and Lou M: Hypoxia-induced miR-497 decreases glioma cell
sensitivity to TMZ by inhibiting apoptosis. FEBS Lett.
588:3333–3339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xue H, Guo X, Han X, Yan S, Zhang J, Xu S,
Li T, Guo X, Zhang P, Gao X, et al: MicroRNA-584-3p, a novel tumor
suppressor and prognostic marker, reduces the migration and
invasion of human glioma cells by targeting hypoxia-induced ROCK1.
Oncotarget. 7:4785–4805. 2016.PubMed/NCBI
|
|
58
|
Jensen RL: Brain tumor hypoxia:
Tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a
therapeutic target. J Neurooncol. 92:317–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tivnan A and McDonald KL: Current progress
for the use of miRNAs in glioblastoma treatment. Mol Neurobiol.
48:757–768. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kalkan R: Hypoxia is the driving force
behind GBM and could be a new tool in GBM treatment. Crit Rev
Eukaryot Gene Expr. 25:363–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ruan K, Song G and Ouyang G: Role of
hypoxia in the hallmarks of human cancer. J Cell Biochem.
107:1053–1062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Oliver L, Olivier C, Marhuenda FB, Campone
M and Vallette FM: Hypoxia and the malignant glioma
microenvironment: Regulation and implications for therapy. Curr Mol
Pharmacol. 2:263–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tang JH, Ma ZX, Huang GH, Xu QF, Xiang Y,
Li N, Sidlauskas K, Zhang EE and Lv SQ: Downregulation of HIF-1a
sensitizes U251 glioma cells to the temozolomide (TMZ) treatment.
Exp Cell Res. 343:148–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chen W, Xiao Z, Zhao Y, Huang L and Du G:
HIF-1α inhibition sensitizes pituitary adenoma cells to
temozolomide by regulating MGMT expression. Oncol Rep.
30:2495–2501. 2013.PubMed/NCBI
|
|
67
|
Winkler F, Kozin SV, Tong RT, Chae SS,
Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E,
et al: Kinetics of vascular normalization by VEGFR2 blockade
governs brain tumor response to radiation: Role of oxygenation,
angiopoietin-1, and matrix metalloproteinases. Cancer Cell.
6:553–563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wick A, Wick W, Waltenberger J, Weller M,
Dichgans J and Schulz JB: Hypoxic neuroprotection requires
sequential activation of vascular endothelial growth factor
receptor and Akt. J Neurosci. 22:6401–6407. 2002.PubMed/NCBI
|
|
69
|
Henze AT, Riedel J, Diem T, Wenner J,
Flamme I, Pouyseggur J, Plate KH and Acker T: Prolyl hydroxylases 2
and 3 act in gliomas as protective negative feedback regulators of
hypoxia-inducible factors. Cancer Res. 70:357–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mancini M, Gariboldi MB, Taiana E, Bonzi
MC, Craparotta I, Pagin M and Monti E: Co-targeting the IGF system
and HIF-1 inhibits migration and invasion by (triple-negative)
breast cancer cells. Br J Cancer. 110:2865–2873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Onnis B, Rapisarda A and Melillo G:
Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med.
13:2780–2786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Xia Y, Choi HK and Lee K: Recent advances
in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem.
49:24–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Huang SW, Kao JK, Wu CY, Wang ST, Lee HC,
Liang SM, Chen YJ and Shieh JJ: Targeting aerobic glycolysis and
HIF-1alpha expression enhance imiquimod-induced apoptosis in cancer
cells. Oncotarget. 5:1363–1381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Dai P, Akimaru H, Tanaka Y, Maekawa T,
Nakafuku M and Ishii S: Sonic Hedgehog-induced activation of the
Gli1 promoter is mediated by GLI3. J Biol Chem. 274:8143–8152.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Regl G, Neill GW, Eichberger T, Kasper M,
Ikram MS, Koller J, Hintner H, Quinn AG, Frischauf AM and Aberger
F: Human GLI2 and GLI1 are part of a positive feedback mechanism in
Basal Cell Carcinoma. Oncogene. 21:5529–5539. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Fedorchuk AG, Pyaskovskaya ON, Gorbik GV,
Prokhorova IV, Kolesnik DL and Solyanik GI: Effectiveness of sodium
dichloroacetate against glioma C6 depends on administration
schedule and dosage. Exp Oncol. 38:80–83. 2016.PubMed/NCBI
|
|
77
|
Wang H, Feng H and Zhang Y: Resveratrol
inhibits hypoxia-induced glioma cell migration and invasion by the
p-STAT3/miR-34a axis. Neoplasma. 63:532–539. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li S, Wang J, Wei Y, Liu Y, Ding X, Dong
B, Xu Y and Wang Y: Crucial role of TRPC6 in maintaining the
stability of HIF-1α in glioma cells under hypoxia. J Cell Sci.
128:3317–3329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Luan W, Wang Y, Chen X, Shi Y, Wang J,
Zhang J, Qian J, Li R, Tao T, Wei W, et al: PKM2 promotes glucose
metabolism and cell growth in gliomas through a mechanism involving
a let-7a/c-Myc/hnRNPA1 feedback loop. Oncotarget. 6:13006–13018.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wan YY, Zhang JF, Yang ZJ, Jiang LP, Wei
YF, Lai QN, Wang JB, Xin HB and Han XJ: Involvement of Drp1 in
hypoxia-induced migration of human glioblastoma U251 cells. Oncol.
32:619–626. 2014.
|
|
81
|
Adamski J, Price A, Dive C and Makin G:
Hypoxia-induced cytotoxic drug resistance in osteosarcoma is
independent of HIF-1Alpha. PLoS One. 8:e653042013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cook KM, Hilton ST, Mecinovic J,
Motherwell WB, Figg WD and Schofield CJ: Epidithiodiketopiperazines
block the interaction between hypoxia-inducible factor-1alpha
(HIF-1alpha) and p300 by a zinc ejection mechanism. J Biol Chem.
284:26831–26838. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Maples KR, Green AR and Floyd RA:
Nitrone-related therapeutics: Potential of NXY-059 for the
treatment of acute ischaemic stroke. CNS Drugs. 18:1071–1084. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gillespie DL, Whang K, Ragel BT, Flynn JR,
Kelly DA and Jensen RL: Silencing of hypoxia inducible
factor-1alpha by RNA interference attenuates human glioma cell
growth in vivo. Clin Cancer Res. 13:2441–2448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sydserff SG, Borelli AR, Green AR and
Cross AJ: Effect of NXY-059 on infarct volume after transient or
permanent middle cerebral artery occlusion in the rat; studies on
dose, plasma concentration and therapeutic time window. Br J
Pharmacol. 135:103–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hashizume R, Ozawa T, Dinca EB, Banerjee
A, Prados MD, James CD and Gupta N: A human brainstem glioma
xenograft model enabled for bioluminescence imaging. J Neurooncol.
96:151–159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
de Souza PC, Smith N, Pody R, He T, Njoku
C, Silasi-Mansat R, Lupu F, Meek B, Chen H, Dong Y, et al: OKN-007
decreases VEGFR-2 levels in a preclinical GL261 mouse glioma model.
Am J Nucl Med Mol Imaging. 5:363–378. 2015.PubMed/NCBI
|
|
88
|
Bourne DW: BOOMER, a simulation and
modeling program for pharmacokinetic and pharmacodynamic data
analysis. Comput Methods Programs Biomed. 29:191–195. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yamaoka K, Nakagawa T and Uno T:
Application of Akaike's information criterion (AIC) in the
evaluation of linear pharmacokinetic equations. J Pharmacokinet
Biopharm. 6:165–175. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
de Souza P Coutinho, Mallory S, Smith N,
Saunders D, Li XN, McNall-Knapp RY, Fung KM and Towner RA:
Inhibition of pediatric glioblastoma tumor growth by the
anti-cancer agent OKN-007 in orthotopic mouse xenografts. PLoS One.
10:e01342762015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Liu D, Cao G, Cen Y, Liu T, Peng W, Sun J,
Li X and Zhou H: The radiosensitizing effect of CpG ODN107 on human
glioma cells is tightly related to its antiangiogenic activity via
suppression of HIF-1α/VEGF pathway. Int Immunopharmacol.
17:237–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wellmann S, Bettkober M, Zelmer A, Seeger
K, Faigle M, Eltzschig HK and Bührer C: Hypoxia upregulates the
histone demethylase JMJD1A via HIF-1. Biochem Biophys Res Commun.
372:892–897. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Belozerov VE and Van Meir EG: Hypoxia
inducible factor-1: A novel target for cancer therapy. Anticancer
Drugs. 16:901–909. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Melillo G: Inhibiting hypoxia-inducible
factor 1 for cancer therapy. Mol Cancer Res. 4:601–605. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Semenza GL: Evaluation of HIF-1 inhibitors
as anticancer agents. Drug Discov Today. 12:853–859. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lee P, Murphy B, Miller R, Menon V, Banik
NL, Giglio P, Lindhorst SM, Varma AK, Vandergrift WA III, Patel SJ,
et al: Mechanisms and clinical significance of histone deacetylase
inhibitors: Epigenetic glioblastoma therapy. Anticancer Res.
35:615–625. 2015.PubMed/NCBI
|
|
97
|
Qian DZ, Wang X, Kachhap SK, Kato Y, Wei
Y, Zhang L, Atadja P and Pili R: The histone deacetylase inhibitor
NVP-LAQ824 inhibits angiogenesis and has a greater antitumor effect
in combination with the vascular endothelial growth factor receptor
tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res.
64:6626–6634. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kerbel RS: Tumor angiogenesis. N Engl J
Med. 358:2039–2049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Moeini A, Cornellà H and Villanueva A:
Emerging signaling pathways in hepatocellular carcinoma. Liver
Cancer. 1:83–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
von Burstin J, Eser S, Paul MC, Seidler B,
Brandl M, Messer M, von Werder A, Schmidt A, Mages J, Pagel P, et
al: E-cadherin regulates metastasis of pancreatic cancer in vivo
and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex.
Gastroenterology. 137:361–371, 371.e1–371.e5. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Falkenberg KJ and Johnstone RW: Histone
deacetylases and their inhibitors in cancer, neurological diseases
and immune disorders. Nat Rev Drug Discov. 13:673–691. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Singh MM, Johnson B, Venkatarayan A,
Flores ER, Zhang J, Su X, Barton M, Lang F and Chandra J:
Preclinical activity of combined HDAC and KDM1A inhibition in
glioblastoma. Neuro Oncol. 17:1463–1473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Scroggins BT, Robzyk K, Wang D, Marcu MG,
Tsutsumi S, Beebe K, Cotter RJ, Felts S, Toft D and Karnitz L: An
acetylation site in the middle domain of Hsp90 regulates chaperone
function. Mol Cell. 25:151–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Siegel D, Hussein M, Belani C, Robert F,
Galanis E, Richon VM, Garcia-Vargas J, Sanz-Rodriguez C and Rizvi
S: Vorinostat in solid and hematologic malignancies. J Hematol
Oncol. 2:312009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Miyar A, Habibi I, Ebrahimi A, Mansourpour
D, Mokarizadeh A, Rajabi A, Farshgar R, Eshaghzadeh M,
Zamani-Ahmadmahmudi M and Nodushan SM: Predictive and prognostic
value of TLR9 and NFKBIA gene expression as potential biomarkers
for human glioma diagnosis. J Neurol Sci. 368:314–317. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zagzag D, Nomura M, Friedlander DR, Blanco
CY, Gagner JP, Nomura N and Newcomb EW: Geldanamycin inhibits
migration of glioma cells in vitro: A potential role for
hypoxia-inducible factor (HIF-1alpha) in glioma cell invasion. J
Cell Physiol. 196:394–402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Simioni C, Cani A, Martelli AM, Zauli G,
Alameen AA, Ultimo S, Tabellini G, McCubrey JA, Capitani S and Neri
LM: The novel dual PI3K/mTOR inhibitor NVP-BGT226 displays
cytotoxic activity in both normoxic and hypoxic hepatocarcinoma
cells. Oncotarget. 6:17147–17160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Huang WJ, Liang YC, Chuang SE, Chi LL, Lee
CY, Lin CW, Chen AL, Huang JS, Chiu CJ, Lee CF, Huang CY and Chen
CN: NBM-HD-1: A novel histone deacetylase inhibitor with anticancer
activity. Evid Based Complement Alternat Med. 2012:7814172012.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Huang WJ, Lin CW, Lee CY, Chi LL, Chao YC,
Wang HN, Chiou BL, Chen TJ, Huang CY and Chen CN: NBM-HD-3, a novel
histone deacetylase inhibitor with anticancer activity through
modulation of PTEN and AKT in brain cancer cells. J Ethnopharmacol.
136:156–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang W, Lv S, Liu J, Zang Z, Yin J, An N,
Yang H and Song Y: PCI-24781 down-regulates EZH2 expression and
then promotes glioma apoptosis by suppressing the PIK3K/Akt/mTOR
pathway. Genet Mol Biol. 37:716–724. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Redjal N, Reinshagen C, Le A, Walcott BP,
McDonnell E, Dietrich J and Nahed BV: Valproic acid, compared to
other antiepileptic drugs, is associated with improved overall and
progression-free survival in glioblastoma but worse outcome in
grade II/III gliomas treated with temozolomide. J Neurooncol.
127:505–514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hoja S, Schulze M, Rehli M, Proescholdt M,
Herold-Mende C, Hau P and Riemenschneider MJ: Molecular dissection
of the valproic acid effects on glioma cells. Oncotarget. Aug
18–2016.(Epub ahead of print). doi: 10.18632/oncotarget.11379.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Pont LM Berghauser, Kleijn A, Kloezeman
JJ, van den Bossche W, Kaufmann JK, de Vrij J, Leenstra S, Dirven
CM and Lamfers ML: The HDAC inhibitors scriptaid and LBH589
combined with the oncolytic virus Delta24-RGD exert enhanced
anti-tumor efficacy in patient-derived glioblastoma cells. PLoS
One. 10:e01270582015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Vasilatos SN, Katz TA, Oesterreich S, Wan
Y, Davidson NE and Huang Y: Crosstalk between lysine-specific
demethylase 1 (LSD1) and histone deacetylases mediates
antineoplastic efficacy of HDAC inhibitors in human breast cancer
cells. Carcinogenesis. 34:1196–1207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wang Z, Wang N, Han S, Wang D, Mo S, Yu L,
Huang H, Tsui K, Shen J and Chen J: Dietary compound
isoliquiritigenin inhibits breast cancer neoangiogenesis via
VEGF/VEGFR-2 signaling pathway. PLoS One. 8:e685662013. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhao S, Chang H, Ma P, Gao G, Jin C, Zhao
X, Zhou W and Jin B: Inhibitory effect of DNA topoisomerase
inhibitor isoliquiritigenin on the growth of glioma cells. Int J
Clin Exp Pathol. 8:12577–12582. 2015.PubMed/NCBI
|
|
117
|
Zhou GS, Song LJ and Yang B:
Isoliquiritigenin inhibits proliferation and induces apoptosis of
U87 human glioma cells in vitro. Mol Med Rep. 7:531–536.
2013.PubMed/NCBI
|
|
118
|
Ma J, Han LZ, Liang H, Mi C, Shi H, Lee JJ
and Jin X: Celastrol inhibits the HIF-1α pathway by inhibition of
mTOR/p70S6K/eIF4E and ERK1/2 phosphorylation in human hepatoma
cells. Oncol Rep. 32:235–242. 2014.PubMed/NCBI
|
|
119
|
Zhou YX and Huang YL: Antiangiogenic
effect of celastrol on the growth of human glioma: An in vitro and
in vivo study. Chin Med J. 122:1666–1673. 2009.PubMed/NCBI
|
|
120
|
Huang Y, Zhou Y, Fan Y and Zhou D:
Celastrol inhibits the growth of human glioma xenografts in nude
mice through suppressing VEGFR expression. Cancer Lett.
264:101–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Bordji K, Grandval A, Cuhna-Alves L,
Lechapt-Zalcman E and Bernaudin M: Hypoxia-inducible factor-2α
(HIF-2α), but not HIF-1α, is essential for hypoxic induction of
class III β-tubulin expression in human glioblastoma cells. FEBS J.
281:5220–5236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Bache M, Rot S, Keßler J, Güttler A,
Wichmann H, Greither T, Wach S, Taubert H, Söling A, Bilkenroth U,
et al: mRNA expression levels of hypoxia-induced and stem
cell-associated genes in human glioblastoma. Oncol Rep.
33:3155–3161. 2015.PubMed/NCBI
|
|
123
|
Jonasch E, Futreal PA, Davis IJ, Bailey
ST, Kim WY, Brugarolas J, Giaccia AJ, Kurban G, Pause A, Frydman J,
et al: State of the science: An update on renal cell carcinoma. Mol
Cancer Res. 10:859–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Bhagat M, Palanichamy JK, Ramalingam P,
Mudassir M, Irshad K, Chosdol K, Sarkar C, Seth P, Goswami S, Sinha
S, et al: HIF-2α mediates a marked increase in migration and
stemness characteristics in a subset of glioma cells under hypoxia
by activating an Oct-4/Sox-2-Mena (INV) axis. Int J Biochem Cell
Biol. 74:60–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Miranda-Gonçalves V, Granja S, Martinho O,
Honavar M, Pojo M, Costa BM, Pires MM, Pinheiro C, Cordeiro M,
Bebiano G, et al: Hypoxia-mediated upregulation of MCT1 expression
supports the glycolytic phenotype of glioblastomas. Oncotarget. Jun
16–2016.(Epub ahead of print). doi: 10.18632/oncotarget.
|
|
126
|
Zhang J, Zhu L, Fang J, Ge Z and Li X:
LRG1 modulates epithelial-mesenchymal transition and angiogenesis
in colorectal cancer via HIF-1α activation. J Exp Clin Cancer Res.
35:292016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Li S, Zhang J, Yang H, Wu C, Dang X and
Liu Y: Copper depletion inhibits CoCl2-induced
aggressive phenotype of MCF-7 cells via downregulation of HIF-1 and
inhibition of Snail/Twist-mediated epithelial-mesenchymal
transition. Sci Rep. 5:124102015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ishii A, Kimura T, Sadahiro H, Kawano H,
Takubo K, Suzuki M and Ikeda E: Histological characterization of
the tumorigenic ‘peri-necrotic niche’ harboring quiescent stem-like
tumor cells in glioblastoma. PLoS One. 11:e01473662016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Xu H, Rahimpour S, Nesvick CL, Zhang X, Ma
J, Zhang M, Zhang G, Wang L, Yang C, Hong CS, et al: Activation of
hypoxia signaling induces phenotypic transformation of glioma
cells: Implications for bevacizumab antiangiogenic therapy.
Oncotarget. 6:11882–11893. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Clarke RH, Moosa S, Anzivino M, Wang Y,
Floyd DH, Purow BW and Lee KS: Sustained radiosensitization of
hypoxic glioma cells after oxygen pretreatment in an animal model
of glioblastoma and in vitro models of tumor hypoxia. PLoS One.
9:e1111992014. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Balamurugan K, Wang JM, Tsai HH, Sharan S,
Anver M, Leighty R and Sterneck E: The tumour suppressor C/EBPδ
inhibits FBXW7 expression and promotes mammary tumour metastasis.
EMBO J. 29:4106–4117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Sudhagar S, Sathya S and Lakshmi BS: Rapid
non-genomic signalling by 17β-oestradiol through c-Src involves
mTOR-dependent expression of HIF-1α in breast cancer cells. Br J
Cancer. 105:953–960. 2011. View Article : Google Scholar : PubMed/NCBI
|