Introduction
Osteosarcoma is the most prevalent aggressive
malignant bone tumor arising from primitive transformed cells of
mesenchymal origin in children and young adults (1). Osteosarcomas tend to occur at the
sites of bone growth, presumably due to the proliferation of
osteoblastic cells prone to acquire more osteosarcoma mutations
which could lead to carcinomatous change of cells (2). In the present study, the treatment
therapies of osteosarcoma was mainly performed by surgery combined
with chemotherapy due to its relatively resistance to radiotherapy.
However, the application of tumor chemotherapy drugs have many
adverse effects and tumor cells are prone to acquire drug
resistance (3,4). Thus, to find a low toxicity, high
efficiency anticancer drugs from natural compounds in the treatment
of osteosarcoma has important significance.
The tumor microenvironment is commonly considered as
an obligatory and significant component of almost all types of
cancer, and the cells infiltrating such microenvironment are a
source of inflammatory cytokines. Cytokines like IL-1β plays a key
role in regulating inflammation during the development and
progression of cancer (5). The
IL-1β have pleiotropic effects on various carcinoma cells in the
tumor microenvironment, particularly being capable of regulating
pro-oncogenic transcription factors STAT3 and NF-κB (6). Therefore, to reveal the IL-1β
involved, inflammation related mechanisms are vitally important for
the treatment of osteosarcoma.
Caspase-1 is a kind of cysteine protease that
proteolytically cleaves other proteins, such as the precursor forms
of the inflammatory cytokines IL-1β, into active mature peptides.
Consequently, IL-1β activate its downstream NF-κB signaling and
thus, enhance the release of inflammatory cytokines (7). Caspase-1 and IL-1β have been shown to
play important roles in inflammation, necrosis and pyroptosis, and
may function in various developmental stages (8–11). Our
preliminary experiments found that the expression of caspase-1 was
higher in osteosarcoma tissues than normal bone tissues. However,
to the best of our knowledge, the role of caspase-1 and its
down-strem target IL-1β in osteosarcoma have not been
clarified.
Berberine was derived from traditional Chinese
medicinal herbs which have drawn extensive attention towards its
antineoplastic effects. It has been reported to suppress growth of
a wide variety of tumor cells, including osteosarcoma, breast
cancer and gastric carcinoma (12–15).
Berberine potently inhibits osteosarcoma growth and metastasis as
reported in a previous extensive study and the underlying mechanism
is controversial and not that obscure (16,17).
Therefore, development of effective therapeutic strategies based on
a better understanding of the molecular mechanisms underlying the
anti-osteosarcoma property of berberine is urgently needed.
In the present research, we studied the effects of
berberine on osteosarcoma cells in vivo and in vitro.
Our data provided mechanistic insight into the role of berberine in
inhibition of osteosarcoma cell growth via downregulating
caspase-1/IL-1β inflammatory signaling pathway.
Materials and methods
Cell culture and treatment
Human Saos-2 and MG-63 cell lines were obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
Berberine was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cells were cultured in Dulbeccos modified Eagles medium (DMEM;
HyClone Laboratories, Inc., Logan, UT, USA) supplemented with 10%
(v/v) fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) in an
atmosphere of 95% humidified air and 5% CO2 at 37°C.
Cells were investigated within 8 h of harvest. To detect the
effects of berberine on osteosarcoma, cells were treated without or
with berberine (Sigma-Aldrich), respectively. To detect the effects
of caspase-1 on osteosarcoma, cells were treated without or with
selective caspase-1 inhibitor N-Ac-Tyr-Val-Ala-Asp-CMK
(Ac-YVAD-CMK) (Cayman Chemical, Ann Arbor, MI, USA),
respectively.
MTT assay of cell proliferation
Cell viability was determined by MTT assay according
to the manufacturer's instructions. Briefly, cells
(2×104 cells/well) were seeded in a 96-well plate and
treated differently based on the experimental purpose. Cells were
washed with phosphate-buffered saline (PBS), and then 20 µl of MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
solution (5 mg/ml) was added to each well. The plate was covered
and shaken at room temperature, after which the medium was
discarded. Next, dimethyl sulfoxide (DMSO) was added to each well
(200 µl), and the solution was vigorously mixed to dissolve the
purple tetrazolium crystals. The amount of produced purple formazan
is proportional to the percentage of cell viability. The absorbance
of each well was measured by automated microplate reader at a test
wavelength of 490 nm. All experiments were repeated at least three
times.
Western blot analysis
Western blotting was used to detect the expression
levels of the proteins of interest. Drugs were diluted and added to
cells for 24 h at 37°C before analysis by western blot. The cells
were washed using ice-cold phosphate-buffered saline, and total
protein was harvested with radioimmunoprecipitation assay buffer
(RIPA) containing 1% protease inhibitor (Sigma-Aldrich). Protein
(100 µg) per sample was separated using 12% SDS-PAGE, and then
transferred into nitrocellulose membranes. The membrane was blocked
with 5% non-fat milk (BD Biosciences, San Jose, CA, USA) and 0.1%
Tween-20 in Tris-buffered saline and immunoblotted overnight using
appropriate primary antibodies at 4°C with gentle shaking. After
that, fluorochrome labelled secondary antibody (Alexa Fluor 800;
LI-COR Biosciences, Lincoln, NE, USA) was used to identify the
appropriate primary antibody. Immunoreactivity was detected with
the Odyssey fluorescent scanning system (LI-COR Biosciences) and
analyzed by Image Studio software. β-actin was used as a loading
control.
cDNA synthesis and real-time PCR
analysis
Real-time PCR was used to measure caspase-1 and
IL-1β mRNA levels. Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) from tissues and cells.
First-strand cDNA was synthesized using a reverse transcriptase kit
(Applied Biosystems, Foster City, CA, USA) according to the
manufacturer's instructions. Real-time PCRs were carried out with a
SYBR-Green PCR Master Mix kit (Applied Biosystems) and performed on
7500 FAST Real-Time PCR system (Applied Biosystems, Carlsbad, CA,
USA). GAPDH was used as an internal control. The following primers
were used in the study. Caspase-1: forward,
5-ACACGTCTTGCCCTCATTATCT-3 and reverse, 5-ATAACCTTGGGCTTGTCTTTCA-3;
IL-1β: forward, 5-CCCTGCAGCTGGAGAGTGTGG-3 and reverse,
5-TGTGCTCTGCTTGAGAGGTGCT-3; GAPDH: forward, 5-ATCACTGCCACCCAGAAGAC3
and reverse, 5-TTTCTAGACGGCAGGTCAGG-3.
TUNEL assay
Cells were seeded on coverslips in 6-well culture
plates and grown overnight for adherence, then treated with
different drugs, respectively. Apoptosis was measured by an In
Situ Cell Death Detection kit according to the manufacturer's
instructions (Roche Applied Science). In brief, cells were fixed
with freshly prepared 4% paraformaldehyde for 60 min. The slides
were rinsed with PBS and incubated in 0.1% Triton X-100
permeabilization solution for 2 min. Then slides were incubated
with TUNEL reaction mixture for 60 min at 37°C in a humidified
chamber. the rinsed slides were counterstained with DAPI. Cells
were counted under a fluorescence microscopy and the green
fluorescence staining cells were calculated as positive-staining
cells. All experiments were repeated at least three times.
Establishment of a xenograft mouse
model
The experimental protocols were approved by the
Ethic Committee of Harbin Medical University (Harbin, China). The
use of animals followed the National Institutes of Health guide for
the care and use of laboratory animals published by the US National
Institutes of Health (NIH Publication no. 85–23, revised 1996).
BALB/c-nu/nu mice, male, 5–6-week old weighing 18–20 g were used.
The mice were housed with a regular 12-h light/12-h dark cycle and
ad libitum access to standard rodent chow diet and were kept
in a pathogen-free environment. For in vivo tracking, the
Saos-2 and MG-63 cells were stably transfected with firefly
luciferase. Saos-2 and MG-63 cells (1×107 cells were
suspended in 100 µl serum-free DMEM) were injected subcutaneously
into the back of mice. Eight days post-implantation, the mice were
randomly divided into three groups (n=6 for each group) and fed by
oral gavage with saline, berberine (20 mg/kg/day), or berberine and
intraperitoneal caspase-1 inhibitor Ac-YVAD-CHO (0.1 mg/kg/day).
Tumor growth was monitored by luciferase activity in Saos-2 and
MG-63 cells, and the emitted photons from the target site
penetrated through the mammalian tissue and could be externally
detected and quantified using a sensitive light imaging system.
Mice were euthanized 21 days after treatment and the tumors were
isolated for further detection.
Statistical analysis
Data are expressed as mean ± standard error of mean
(mean ± SEM) and analyzed with SPSS 13.0 software. Statistical
comparisons between the two groups were performed using the
Students t-test. Statistical comparisons among multiple groups were
performed using analysis of variance (ANOVA). A two-tailed
P<0.05 was taken to indicate a statistically significant
difference.
Results
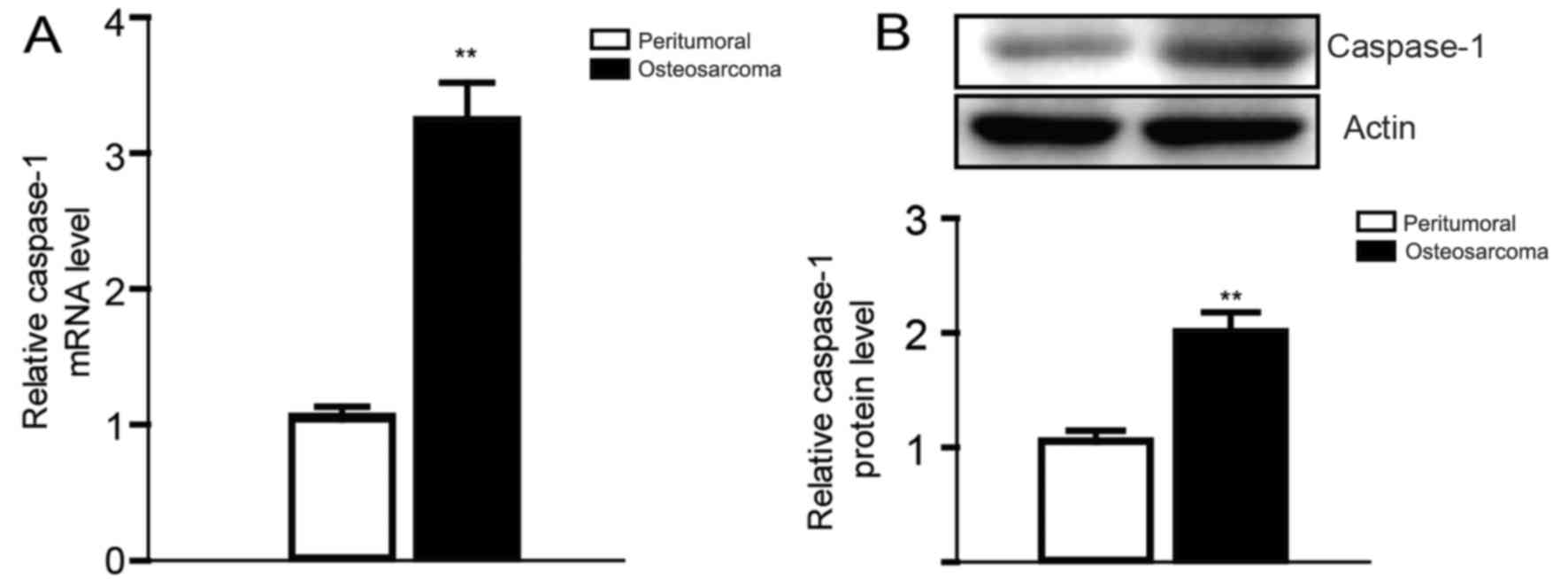
The expression of caspase-1 in
osteosarcoma tissues
Based on previous studies on the relationship
between osteosarcoma and inflammation, and the central role of
caspase-1 in the process of inflammation (18–20),
we first compared the expression level of caspase-1 in osteosarcoma
tissues from six pairs of clinical osteosarcoma cases with
peritumoral tissues. Then we found that the expression of caspase-1
was obviously elevated both in the mRNA and the protein level,
which is consistent to our expectation (Fig. 1).
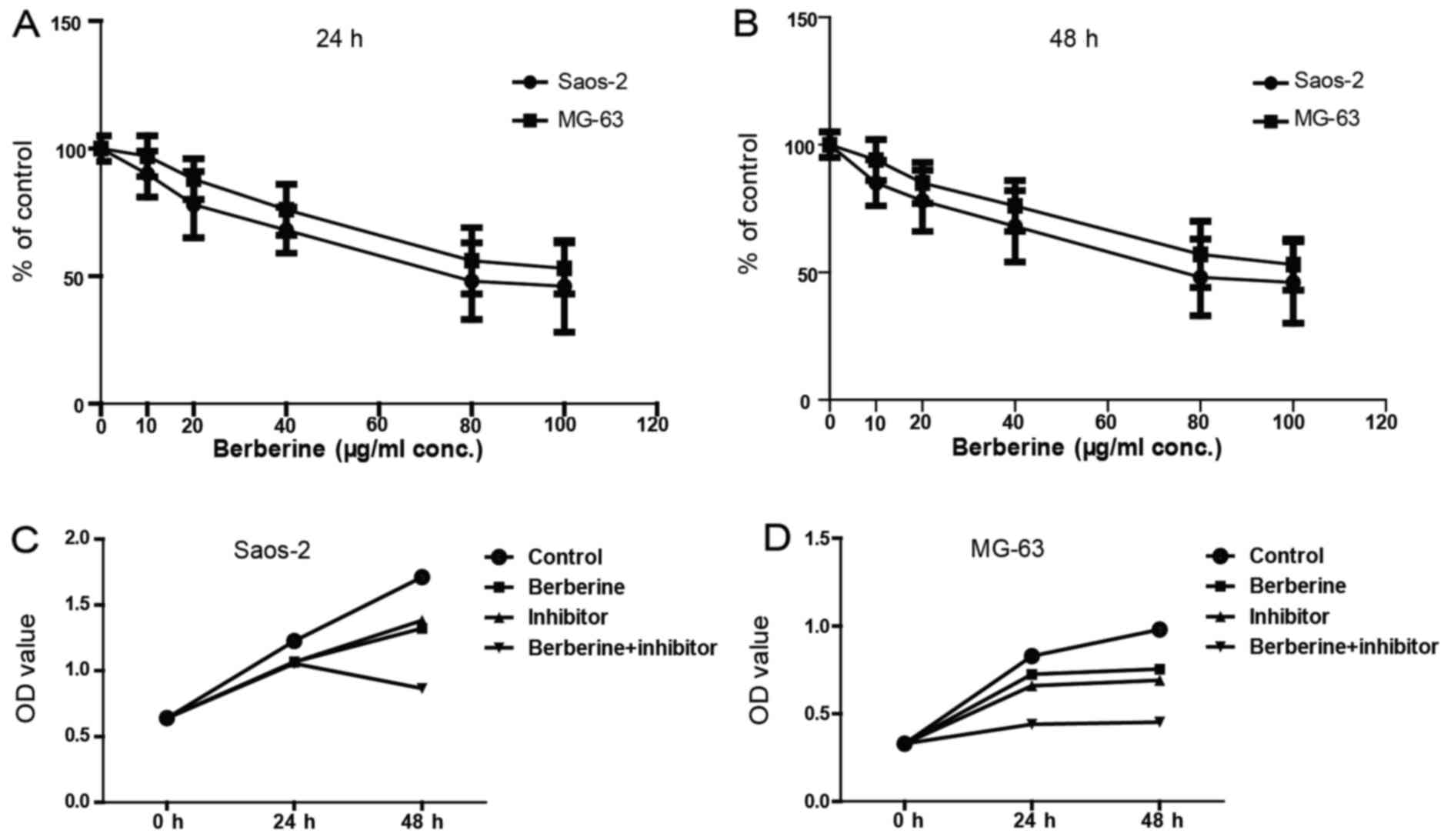
Berberine decreases the viability of
osteosarcoma cell
Next, we evaluated the effects of berberine on
Saos-2 and MG-63 cells by MTT assay. MTT assay results demonstrated
that berberine significantly inhibits the growth of Saos-2 and
MG-63 cells in a time- and dose-dependent manner. As shown in
Fig. 2A and B, the concentration of
berberine at 80 µM could inhibit the cell viability to the greatest
extent; and the viable cells at 48 h decreased more obviously than
24 h after treatment with 80 µM berberine. Thus, the following
administration of berberine were all at 48 h with 80 µM. Fig. 2C and D shows that berberine
significantly reduced osteosarcoma cell viability and caspase-1
inhibitor exerts similar effects, which suggest that caspase-1 may
be involved in the inhibition of osteosarcoma cell growth caused by
berberine.
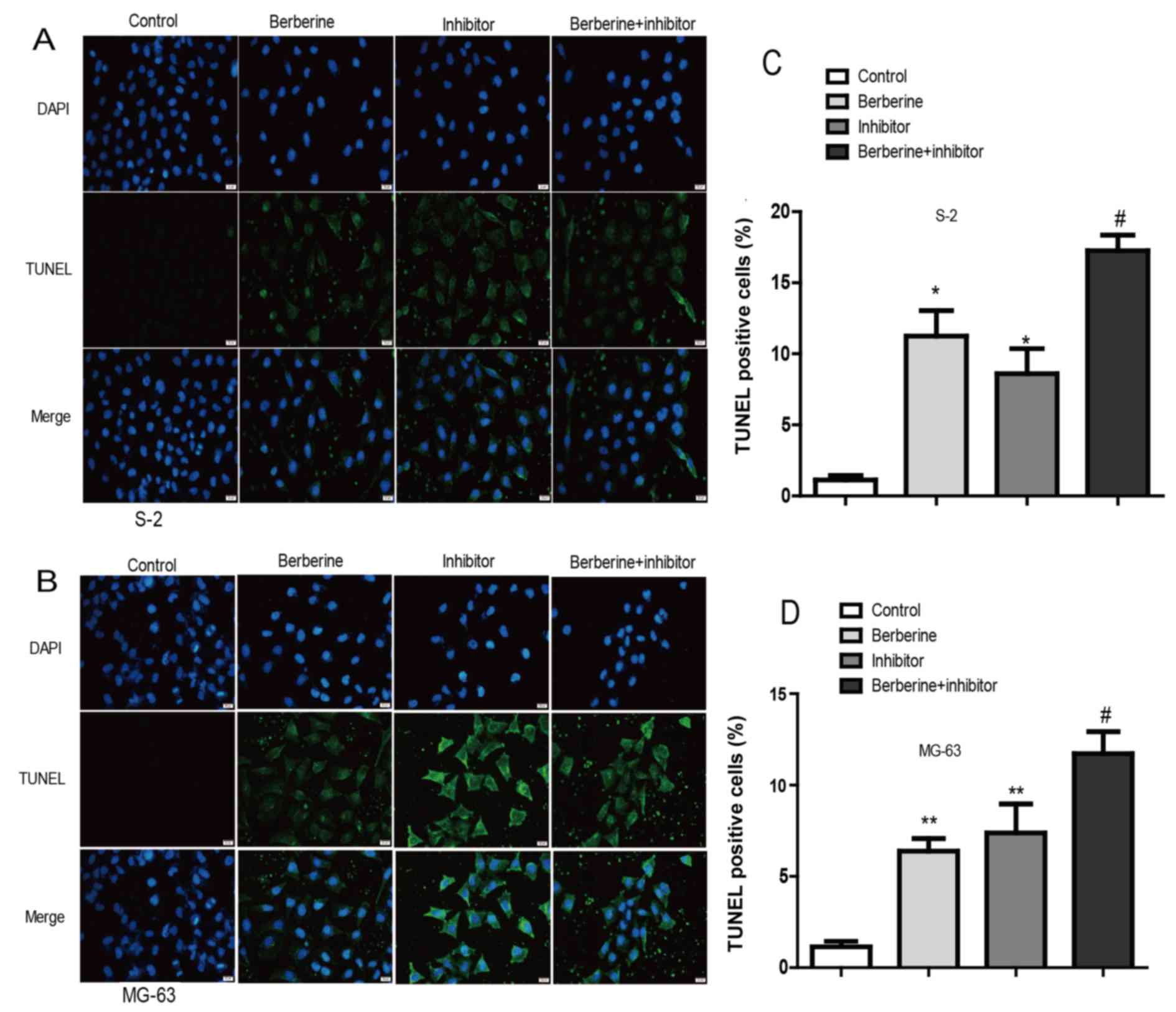
Berberine induces apoptosis of Saos-2
and MG-63 osteosarcoma cells
To study the effect of berberine administration on
osteosarcoma cell apoptosis, TUNEL assay was performed. Cells in
the images with green nuclei were considered apoptotic. In Fig. 3, we found few cells with nuclei
staining green in the control group. After being exposed to 80 µM
berberine for 48 h, ~13.12% of cells showed apoptotic hallmarks.
Moreover, caspase-1 inhibitor exert similar effect to berberine. In
addition, co-incubation berberine with caspase-1 inhibitor exerted
inhibitory effects to the greatest extent. Thus, these results
indicated that berberine could induce apoptosis of osteosarcoma
cells possibly by caspase-1 involved process.
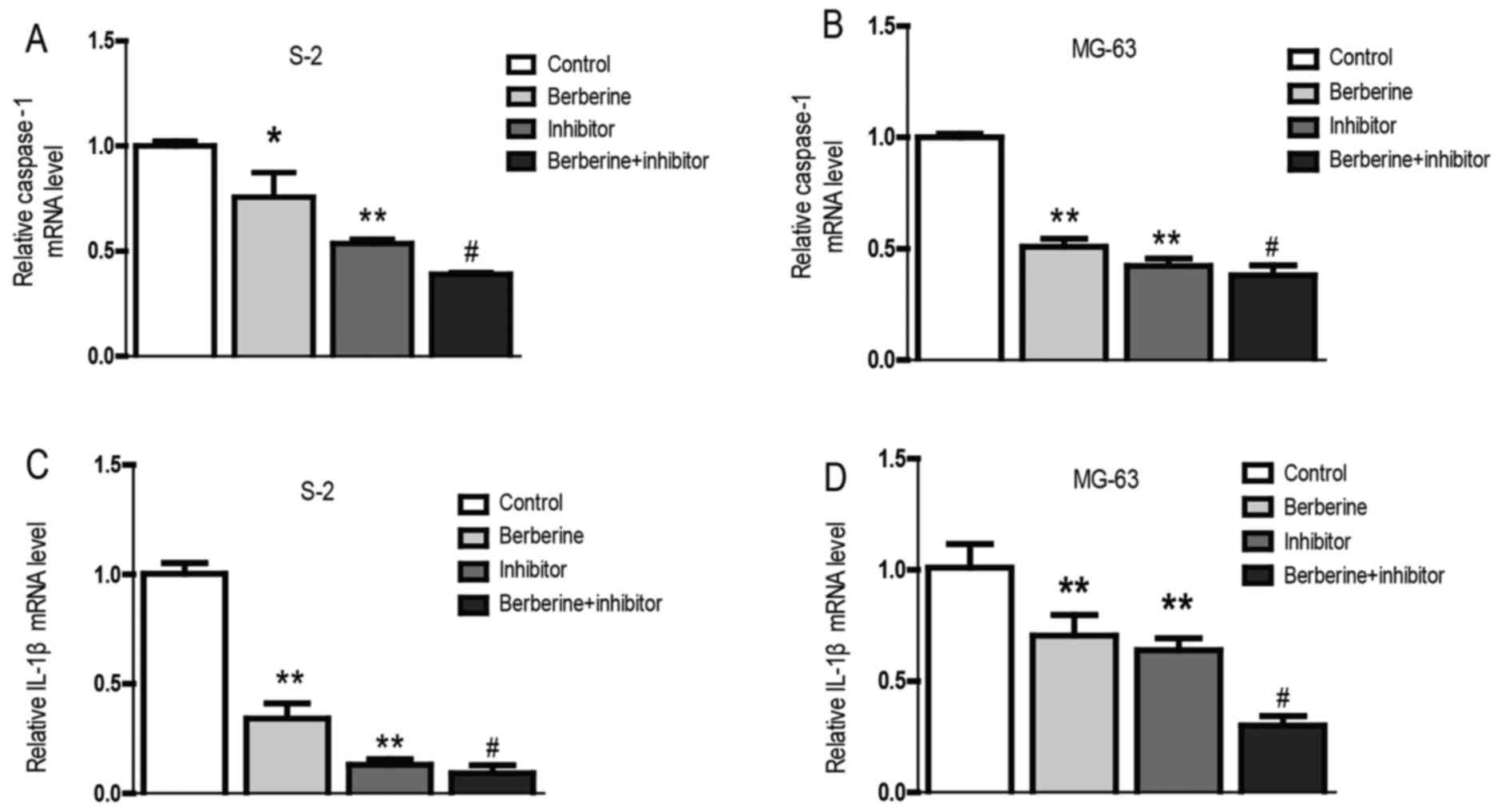
Berberine exerts anti-osteosarcoma
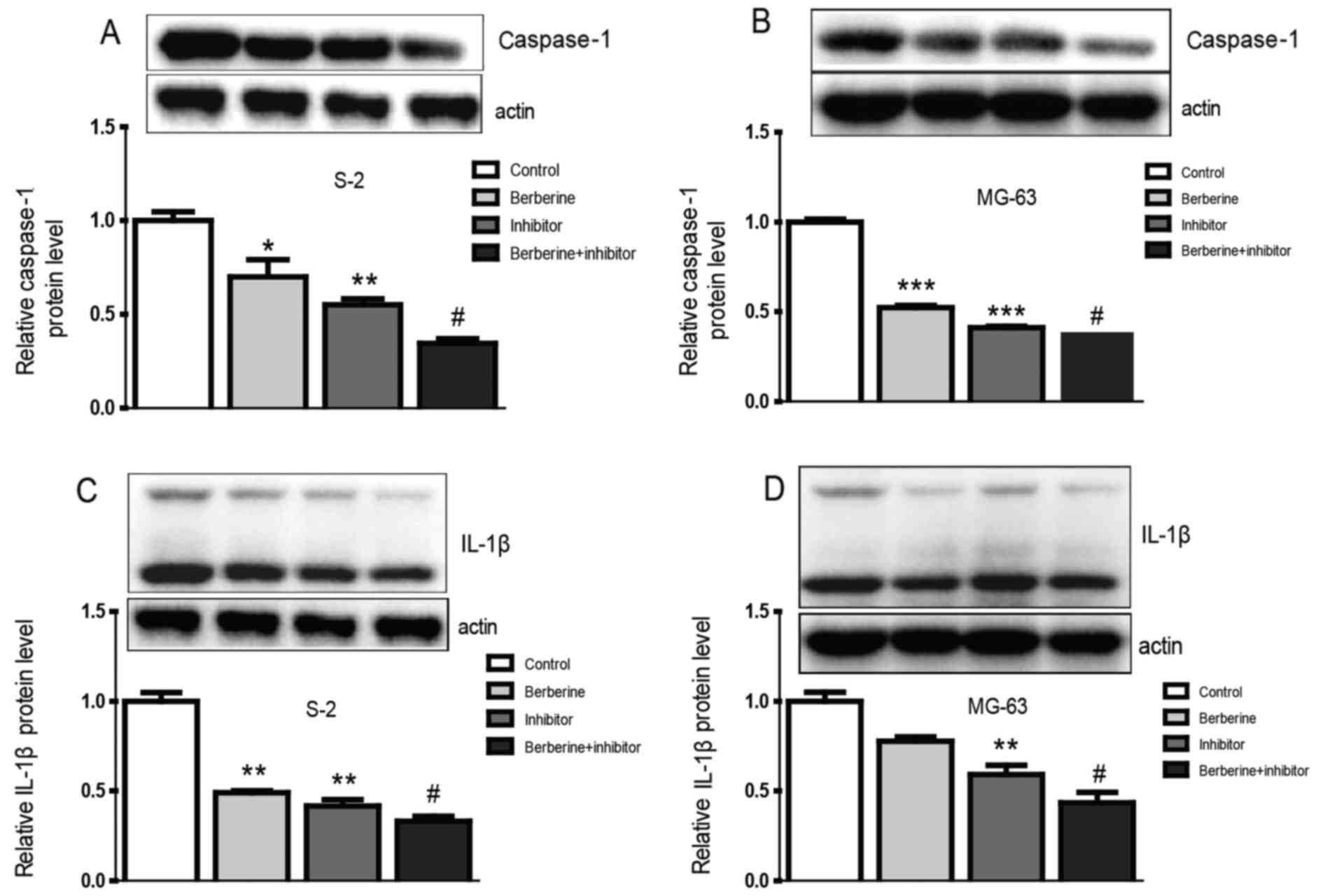
property through reducing caspase-1 and IL-1β expression
Currently, the role of inflammation in cancers has
caused widespread concern. It is believed that inflammation could
promote the occurrence and development of cancer (21,22).
Real-time PCR and western blot assay was performed to explore the
molecular mechanism of berberine on the anti-osteosarcoma property.
As we can see from the bar graphs (Figs. 4 and 5), caspase-1 mRNA and protein expression
level were both downregulated in Saos-2 and MG-63 osteosarcoma
cells after treated with berberine; caspase-1 inhibition extert
similar effect to berberine on caspase-1 expression level. At the
same time, to gain further insights into the mechanism of
anti-osteosarcoma of berberine, we analyzed the expression level of
caspase-1 downstream target IL-1β, which plays a decisive role in
the formation of tumor inflammatory microenvironment. The results
show that the expression of IL-1β was consistent with caspase-1.
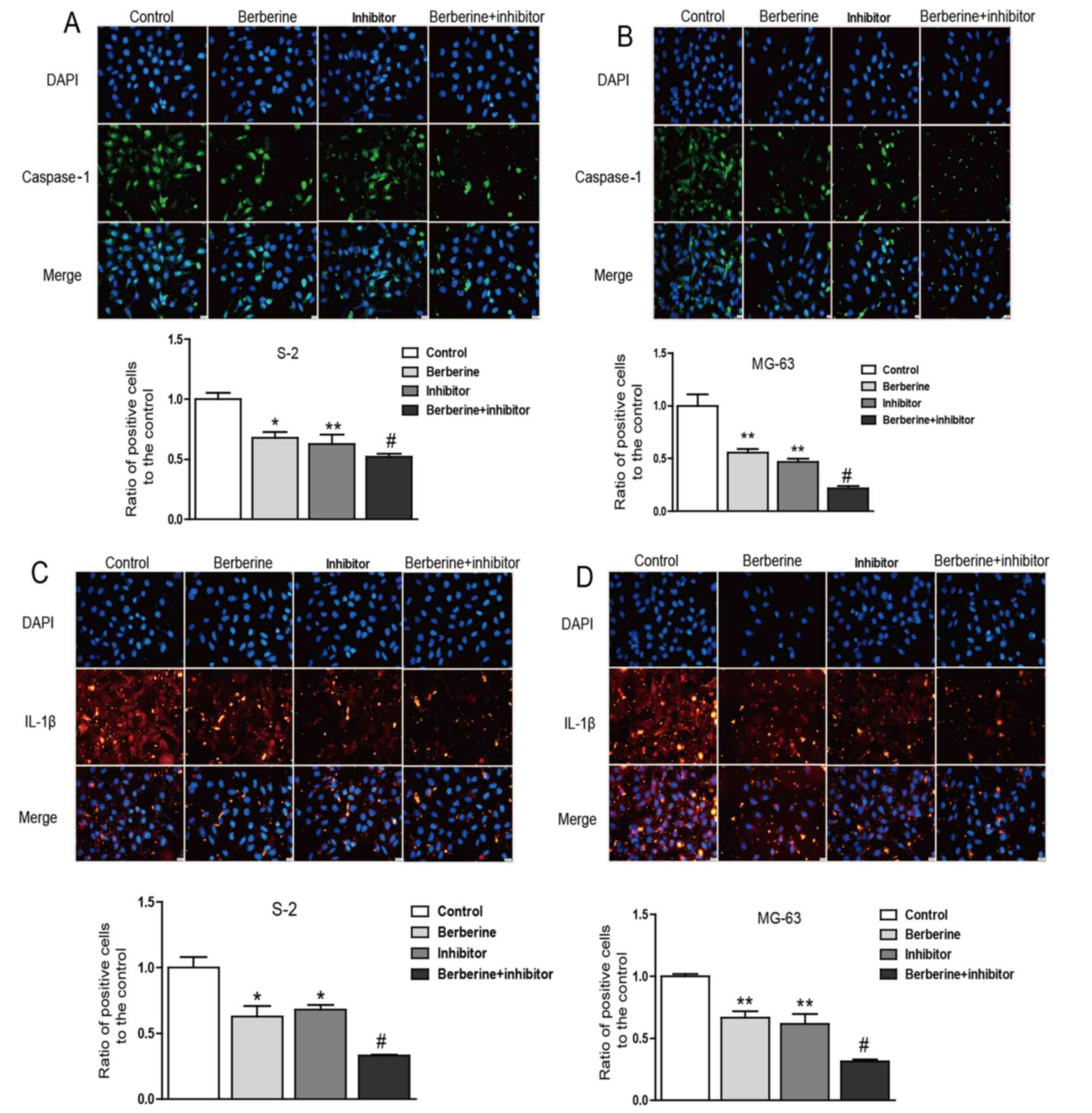
Furthmore, immunofluorescence staining analysis was used to further
confirm the anti-osteosarcoma property of berberine. Accordingly,
immunofluorescence results showed that the expression of caspase-1
and IL-1β were both downregulated compared with normal groups.
Caspase-1 inhibition exterts similar effect to berberine on
osteosarcoma cells (Fig. 6).
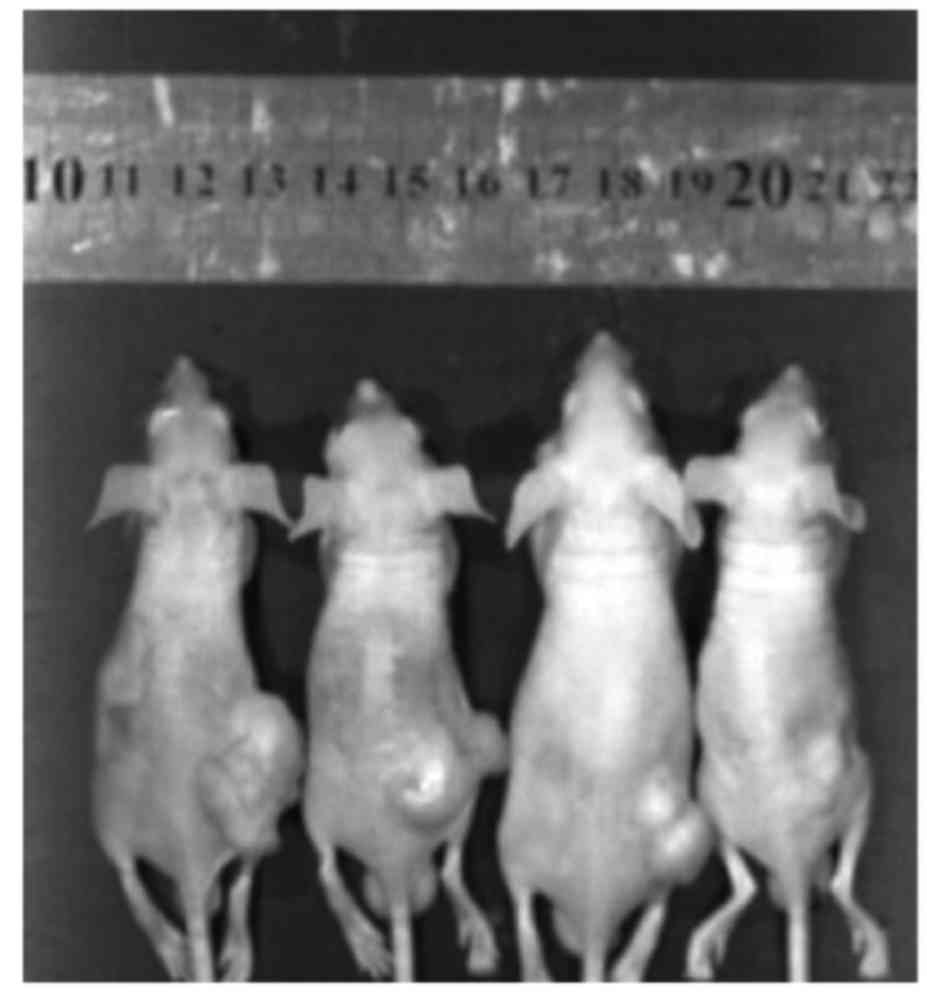
Berberine inhibits the growth of
osteosarcoma tumor in tumor-bearing mice
After eight days of post-implantation of the
osteosarcoma cells, the mice were treated differently. Fig. 7 shows that the size of the
osteosarcoma shrinks obviously after administration of berberine by
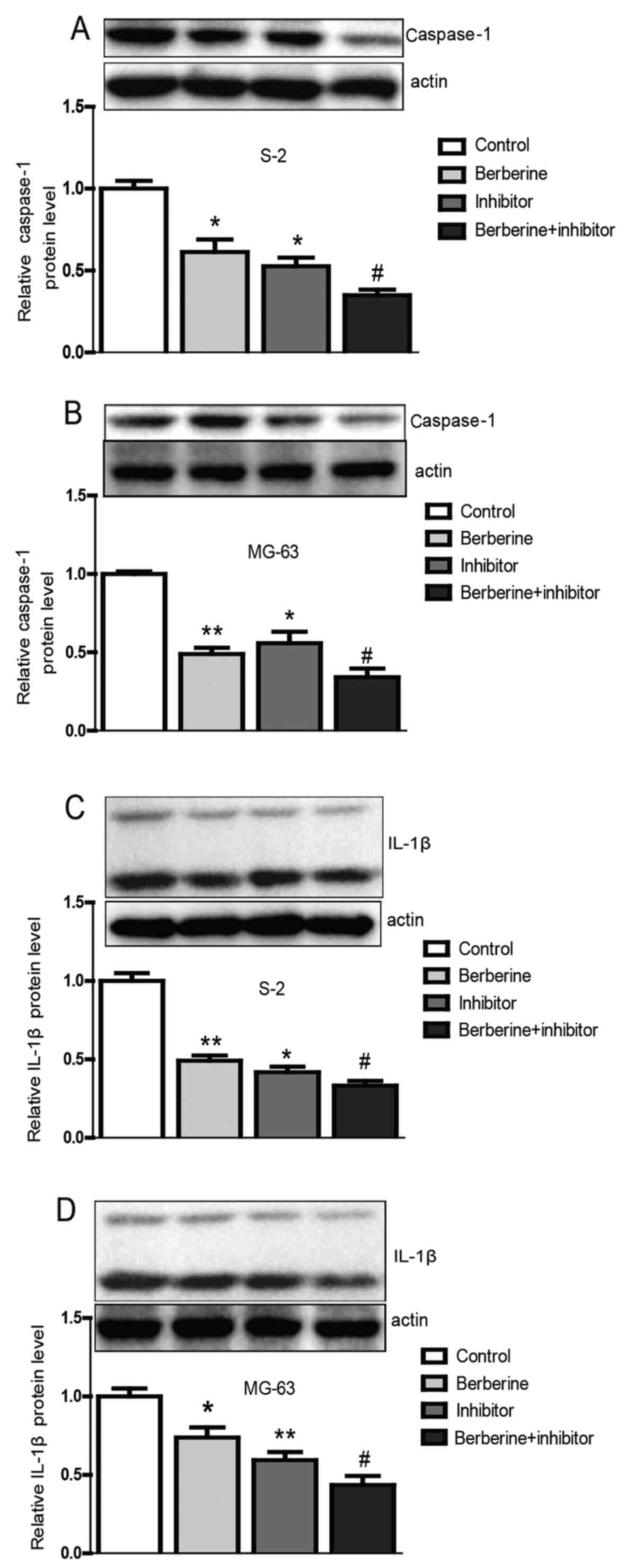
oral gavage compared with the control group. Then, the tumor
tissues were isolated for further mRNA and protein detection. The
results from the tumor tissues were in accordance with the results
from Saos-2 and MG-63 osteosarcoma cells (Fig. 8), which illustrate that berberine
attenuates the activation of caspase-1/IL-1β signal pathway. In
conclusion, these observations demonstrate that berberine could
probably relieve the inflammation in tumor microenvironment and
then results in apoptosis of osteosarcoma cells.
Discussion
Chronic inflammation occurring within the
microenvironment of tumor lesions is now thought to either drive
the first malignant-conferring genetic mutations and/or induce them
as a result of the oncogene expression (23). It is believed that inflammation
could promote the occurrence and development of tumors (24,25).
However, whether relieving the inflammation can attenuate the
viability of cancer cells remains unknown. Caspases are a family of
cysteine proteases that play essential roles in inflammation
(18). Among the caspase family,
caspase-1 is a unique protease because it activates the
proinflammatory cytokines IL-1β and IL-18 into their active mature
peptides, which play a decisive role in the formation of tumor
inflammatory microenvironment and lead to a downstream inflammatory
response (26,27).
Over the past decades, interest in the
pharmacological effects of natural bioactive compounds with respect
to application in cancer treatments and for cancer prevention has
greatly increased (28). Natural
products represent a rich reservoir of potential small chemical
molecules exhibiting various pharmacological effects. Accumulating
evidence has demonstrated a dramatic correlation between the
natural compounds and cancer prevention and treatment (29–31).
Berberine is found in plants from the protoberberine group, such as
Berberis, Berberis vulgaris and Berberis aristata.
This kind of plant is traditionally used as a broad-spectrum
anti-microbial medicine. During the last few decades, many studies
have demonstrated that berberine has anticancer and
anti-inflammatory activities. Berberine has drawn extensive
attention towards its antineoplastic effects. It seems to suppress
the growth of a wide variety of tumor cells, including breast
cancer, lung cancer, melanoma and glioma. It has been reported that
berberine induces cell circle arrest and apoptosis in human
osteosarcoma cells (17,32). The effects of berberine on the
osteosarcoma cells have not been systematically investigated and
the underlying mechanism is controversial. We tried to explain the
mechanism of berberine on osteosarcoma with respect to inflammation
and cancer.
In the present study, we investigated the effect of
berberine on the osteosarcoma cells, and the mechanism underlying
the inhibition of osteosarcoma cell viability after being treated
with berberine. Based on our previous study, caspase-1 was
significantly elevated in the tissues of osteosarcoma patients
(Fig. 1), and the complicated
relationship between inflammation and cancer, we a explored whether
caspase-1/IL-1β was involved in the molecular mechanisms underlying
the anticancer property of berberine. Thus, the expression of
caspase-1 and IL-1β were assessed. The present results show that
caspase-1 and IL-1β in osteosarcoma cells were both downregulated
after being treated with berberine in vivo and in
vitro. These results were further confirmed by the
adiministration of caspase-1 inhibitor. Caspase-1 inhibition exerts
similar effect to berberine. Simultaneously, we established
xenograft mouse model to further confirm the anticancer property of
berberine. The results confirmed previous findings. Further studies
are required to evaluate how berberine alleviates the inflammation
in the tumor microenvironment causing apoptosis.
In conclusion, the present study investigated the
effect of berberine on osteosarcoma cells, and the relationship
between caspase-1/IL-1β signaling pathway and osteosarcoma cell
survival implying that inflammation microenvironment could
influence the viability of osteosarcoma cells to a great extent.
This study suggests that caspase-1/IL-1β could be a new therapeutic
target and berberine could be used or as an adjuvant agent in the
treatment of osteosarcoma.
References
|
1
|
Sampson VB, Kamara DF and Kolb EA:
Xenograft and genetically engineered mouse model systems of
osteosarcoma and Ewings sarcoma: Tumor models for cancer drug
discovery. Expert Opin Drug Discov. 8:1181–1189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tabatabaei SH, Jahanshahi G and Marvasti F
Dehghan: Diagnostic challenges of low-grade central osteosarcoma of
jaw: A literature review. J Dent (Shiraz). 16:62–67.
2015.PubMed/NCBI
|
|
3
|
Li S, Sun W, Wang H, Zuo D, Hua Y and Cai
Z: Research progress on the multidrug resistance mechanisms of
osteosarcoma chemotherapy and reversal. Tumour Biol. 36:1329–1338.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y and Teng JS: Increased multi-drug
resistance and reduced apoptosis in osteosarcoma side population
cells are crucial factors for tumor recurrence. Exp Ther Med.
12:81–86. 2016.PubMed/NCBI
|
|
5
|
Dmitrieva OS, Shilovskiy IP, Khaitov MR
and Grivennikov SI: Interleukins 1 and 6 as main mediators of
inflammation and cancer. Biochemistry. 81:80–90. 2016.PubMed/NCBI
|
|
6
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen XW and Zhou SF: Inflammation,
cytokines, the IL-17/IL-6/STAT3/NF-κB axis, and tumorigenesis. Drug
Des Devel Ther. 9:2941–2946. 2015.PubMed/NCBI
|
|
8
|
Jearaphunt M, Noonin C, Jiravanichpaisal
P, Nakamura S, Tassanakajon A, Söderhäll I and Söderhäll K:
Caspase-1-like regulation of the proPO-system and role of ppA and
caspase-1-like cleaved peptides from proPO in innate immunity. PLoS
Pathog. 10:e10040592014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ataide MA, Andrade WA, Zamboni DS, Wang D,
Souza MC, Franklin BS, Elian S, Martins FS, Pereira D, Reed G, et
al: Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation
mediates inflammation and hypersensitivity to bacterial
superinfection. PLoS Pathog. 10:e10038852014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Exline MC, Justiniano S, Hollyfield JL,
Berhe F, Besecker BY, Das S, Wewers MD and Sarkar A: Microvesicular
caspase-1 mediates lymphocyte apoptosis in sepsis. PLoS One.
9:e909682014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miao EA, Rajan JV and Aderem A:
Caspase-1-induced pyroptotic cell death. Immunol Rev. 243:206–214.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu D, Fu S, Cao Z, Bao M, Zhang G, Pan Y,
Liu W and Zhou Q: Unraveling the novel anti-osteosarcoma function
of coptisine and its mechanisms. Toxicol Lett. 226:328–336. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su K, Hu P, Wang X, Kuang C, Xiang Q, Yang
F, Xiang J, Zhu S, Wei L and Zhang J: Tumor suppressor berberine
binds VASP to inhibit cell migration in basal-like breast cancer.
Oncotarget. Jun 13–2016.doi: 10.18632/oncotarget.9968.
|
|
14
|
Zhang XZ, Wang L, Liu DW, Tang GY and
Zhang HY: Synergistic inhibitory effect of berberine and d-limonene
on human gastric carcinoma cell line MGC803. J Med Food.
17:955–962. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin X, Yan TH, Yan L, Li Q, Wang RL, Hu
ZL, Jiang YY, Sun QY and Cao YB: Design, synthesis, and anticancer
activity of novel berberine derivatives prepared via CuAAC ‘click’
chemistry as potential anticancer agents. Drug Des Devel Ther.
8:1047–1059. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu Y, Ma N, Li HX, Tian L, Ba YF and Hao
B: Berberine induces apoptosis and DNA damage in MG-63 human
osteosarcoma cells. Mol Med Rep. 10:1734–1738. 2014.PubMed/NCBI
|
|
17
|
Liu Z, Liu Q, Xu B, Wu J, Guo C, Zhu F,
Yang Q, Gao G, Gong Y and Shao C: Berberine induces p53-dependent
cell cycle arrest and apoptosis of human osteosarcoma cells by
inflicting DNA damage. Mutat Res. 662:75–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Man SM and Kanneganti TD: Converging roles
of caspases in inflammasome activation, cell death and innate
immunity. Nat Rev Immunol. 16:7–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin Y, Wu W, Zhang W, Zhao Y, Wu Y, Ge G,
Ba Y, Guo Q, Gao T, Chi X, et al: Involvement of EGF receptor
signaling and NLRP12 inflammasome in fine particulate
matter-induced lung inflammation in mice. Environ Toxicol. Jul
5–2016.(Epub ahead of print) doi.org/10.1002/tox.22308. View Article : Google Scholar
|
|
20
|
Heymann D, Ory B, Blanchard F, Heymann MF,
Coipeau P, Charrier C, Couillaud S, Thiery JP, Gouin F and Redini
F: Enhanced tumor regression and tissue repair when zoledronic acid
is combined with ifosfamide in rat osteosarcoma. Bone. 37:74–86.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sansone P and Bromberg J: Environment,
inflammation, and cancer. Curr Opin Genet Dev. 21:80–85. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vakkila J and Lotze MT: Inflammation and
necrosis promote tumour growth. Nat Rev Immunol. 4:641–648. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Persidsky Y, Hill J, Zhang M, Dykstra H,
Winfield M, Reichenbach NL, Potula R, Mukherjee A, Ramirez SH and
Rom S: Dysfunction of brain pericytes in chronic neuroinflammation.
J Cereb Blood Flow Metab. 36:794–807. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cerretti DP, Kozlosky CJ, Mosley B, Nelson
N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T,
Cannizzaro LA, et al: Molecular cloning of the interleukin-1 beta
converting enzyme. Science. 256:97–100. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mariathasan S, Newton K, Monack DM, Vucic
D, French DM, Lee WP, Roose-Girma M, Erickson S and Dixit VM:
Differential activation of the inflammasome by caspase-1 adaptors
ASC and Ipaf. Nature. 430:213–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scott EN, Gescher AJ, Steward WP and Brown
K: Development of dietary phytochemical chemopreventive agents:
Biomarkers and choice of dose for early clinical trials. Cancer
Prev Res (Phila). 2:525–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mantena SK, Sharma SD and Katiyar SK:
Berberine inhibits growth, induces G1 arrest and apoptosis in human
epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin
cascade, disruption of mitochondrial membrane potential and
cleavage of caspase 3 and PARP. Carcinogenesis. 27:2018–2027. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Katiyar SK, Meeran SM, Katiyar N and
Akhtar S: p53 Cooperates berberine-induced growth inhibition and
apoptosis of non-small cell human lung cancer cells in vitro and
tumor xenograft growth in vivo. Mol Carcinog. 48:24–37. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Letasiová S, Jantová S, Miko M, Ovádeková
R and Horváthová M: Effect of berberine on proliferation,
biosynthesis of macromolecules, cell cycle and induction of
intercalation with DNA, dsDNA damage and apoptosis in Ehrlich
ascites carcinoma cells. J Pharm Pharmacol. 58:263–270. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu H, Zhao X, Liu X, Xu P, Zhang K and Lin
X: Antitumor effects of traditional Chinese medicine targeting the
cellular apoptotic pathway. Drug Des Devel Ther. 9:2735–2744.
2015.PubMed/NCBI
|