Introduction
Colorectal cancer (CRC) is the most common
gastrointestinal cancer and one of the leading causes of
cancer-related deaths worldwide. Various biomarkers have been
identified for chemotherapy in advanced CRC. Particularly,
KRAS/BRAF mutation status and microsatellite instability
(MSI) status are known to be effective as predictive biomarkers.
One of the important signalling pathways in CRC, activation of the
RAS-RAF-MAPK pathway, which consists of KRAS/BRAF, is known
(1). The pathway lies downstream
from the epidermal growth factor receptor (EGFR), a transmembrane
protein receptor, and contributes to cell proliferation, survival,
growth, apoptosis resistance, invasion and migration (2,3). EGFR
is overexpressed in most CRCs and antibodies against it inhibit
stimulation of several intracellular signalling pathways, such as
RAS-RAF-MAPK pathways (4). However,
previous studies have shown that KRAS-mutant CRC is
resistant to EGFR antibodies (5,6).
KRAS mutation occurs in approximately 40% of CRC cases
(6). Therefore, analysis of
KRAS mutations is important for the selection of anti-EGFR
therapy, and it is necessary before treatment in advanced CRC. In
addition, CRC with wild-type KRAS is not always sensitive to
EGFR antibodies and BRAF-mutant CRC has a poor prognosis
(7). It is suggested that the
efficiency of EGFR antibodies is further restricted to CRC, with
both KRAS/BRAF wild-types.
BRAF, a member of the RAF family of
serin/threonine kinases, is directly downstream from KRAS.
BRAF mutations lead to constitutive activation of a MAPK
pathway. KRAS/BRAF mutations are considered to be mutually
exclusive. BRAF mutations are present in approximately 6%
advanced CRC cases (5,7–9).
Patients with BRAF-mutant advanced CRC are more likely to be
older, of the female gender, have right-sided primary tumours and
show an unusual pattern of metastatic spread, including frequent
peritoneal and distant lymph node involvement. BRAF-mutant
advanced CRC has proven to be a poor prognosis (5,7,9). The
BRAF inhibitor vemurafenib as well as dabrafenib, have
resulted in significantly prolonged progression-free survival and
overall survival in patients with BRAF-mutated advanced
melanoma (10,11). However, in contrast to
BRAF-mutant melanoma, BRAF-mutant advanced CRC has
shown a lack of sensitivity to BRAF inhibitor monotherapy in
previous clinical trials (12).
Nevertheless, FOLFOXIRI + bevacizumab and BRAF inhibitor +
MEK or EGFR inhibitors, might be a reasonable therapy for
BRAF-mutant advanced CRC (13–16).
BRAF is a good biomarker, not only for a poor prognosis but
also for the selection of molecular-targeted therapy.
MSI is a genetic change caused by a deficiency in
the mismatch repair (MMR) system. The MMR system detects and
repairs the mismatches that occur during DNA replication. It has
been reported that approximately 15% of CRC cases show MSI in
western countries, and approximately 6% of CRC cases in Asian
countries (9,17). Recently, advanced CRC with MSI-H
have been shown to have a high response rate to programmed death-1
(PD-1) inhibitor therapy, namely an immune checkpoint inhibitor
(18). MSI status may be a helpful
biomarker for immune therapy.
Based on the above, evaluating KRAS/BRAF
mutation status and MSI status may be important to choose the
regimen and predict the prognosis for advanced CRC. However,
acquiring the various mutations during the CRC progression causes
cancer-cell heterogeneity. The prevalence of intratumoural genetic
heterogeneity was investigated in the cases of resistance to cancer
therapy in previous studies, and the resistance to therapy may be
explained by the presence of intratumoural heterogeneity (19). Evaluation of whether
KRAS/BRAF mutation status and MSI status could change during
the progression of metastatic disease might be useful to decide
appropriate treatment for advanced CRC. KRAS mutation is
recognized as an early event in colorectal carcinogenesis (20,21).
Therefore, concordance of KRAS mutation status between
primary CRC and corresponding metastases should be expected, and
previous studies demonstrated high concordance rate (22–24).
Nevertheless, some other studies reported discordance of
KRAS mutation status between primary CRC and corresponding
metastases. Therefore, there is still conflict about its
concordance. Besides, concordance of BRAF mutation status
and MSI status between primary CRC and corresponding metastases, is
still unclear because of the small number of advanced CRC cases
with BRAF mutation or MSI-H. In the present study, we
assessed the concordance of KRAS/BRAF mutation status and
MSI status in primary CRC and corresponding metastases.
Materials and methods
Patients and tissue samples
A total of 457 patients with surgically resected CRC
at the Saitama Cancer Center, from July 1999 to August 2013, were
enrolled in this study. Four hundred and fifty-seven primary CRCs,
557 corresponding metastases (499 synchronous metastases and 57
metachronous metastases) and seven local recurrences were analysed.
Primary CRCs and corresponding metastatic tissues were paired with
normal colorectal tissues and stored at −80°C. Patients who had a
history of preoperative radiotherapy or chemotherapy, inflammatory
bowel disease, or a history of familial adenomatous polyposis were
excluded. The cases with three or less metastatic lymph nodes were
also excluded. Since our preliminary study demonstrated that
discordant rate of KRAS mutation between primary CRC and
macroscopically suspected metastatic lymph node increased in the
cases with three or less metastatic lymph nodes comparing to the
cases with more.
Informed consent was obtained from all the patients
included in this study. Furthermore, the ethics committee of the
Saitama Cancer Center approved this study.
Analysis of KRAS/BRAF mutations
Genomic DNA was extracted from fresh-frozen tissue
samples using the standard phenol-chloroform extraction method.
KRAS mutations in exon 2 and 3 were detected by denaturing
gradient gel electrophoresis or high resolution melting (HRM)
analysis, using a Rotor-Gene Q (Qiagen, Hilden, Germany), as
previously described (25,26). BRAF mutations in exon 15
(codon 600) were detected using either polymerase chain reaction
(PCR)-restriction fragment length polymorphism or HRM, as
previously described (27).
Analysis of microsatellite status
MSI analysis was performed using fluorescence-based
PCR, as previously described (9).
MSI status was determined using five Bethesda markers (BAT25,
BAT26, D5S346, D2S123 and D17S250). MSI status was graded as MSI-H
when there were two or more unstable markers, MSI-low (MSI-L) when
only one unstable marker, and microsatellite-stable (MSS) when no
unstable markers. MSI-positive markers were re-examined at least
twice to confirm the results. MSI-L was included with MSS in this
study.
Results
Characteristics of primary CRCs and
corresponding metastases
Five hundred and fifty-six corresponding metastases
(499 synchronous and 57 metachronous metastases) and seven local
recurrences that matched primary CRC were included in this study.
The metastatic samples included 343 lymph node metastases (331
synchronous and 12 metachronous), 155 liver metastases (127
synchronous and 28 metachronous), 52 peritoneal metastases (37
synchronous and 15 metachronous), five splenic metastases (4
synchronous and 1 metachronous), one pulmonary metastasis (1
metachronous metastasis) and seven local recurrences. KRAS
exon 2, 3 and BRAF exon 15 mutations were analysed in 457
primary CRC cases and 556 corresponding metastases (499 synchronous
and 57 metachronous metastases) and seven local recurrences
(Figs. 1 and 2). KRAS and BRAF mutations
were detected in 228 and 30 primary CRCs, respectively. MSI status
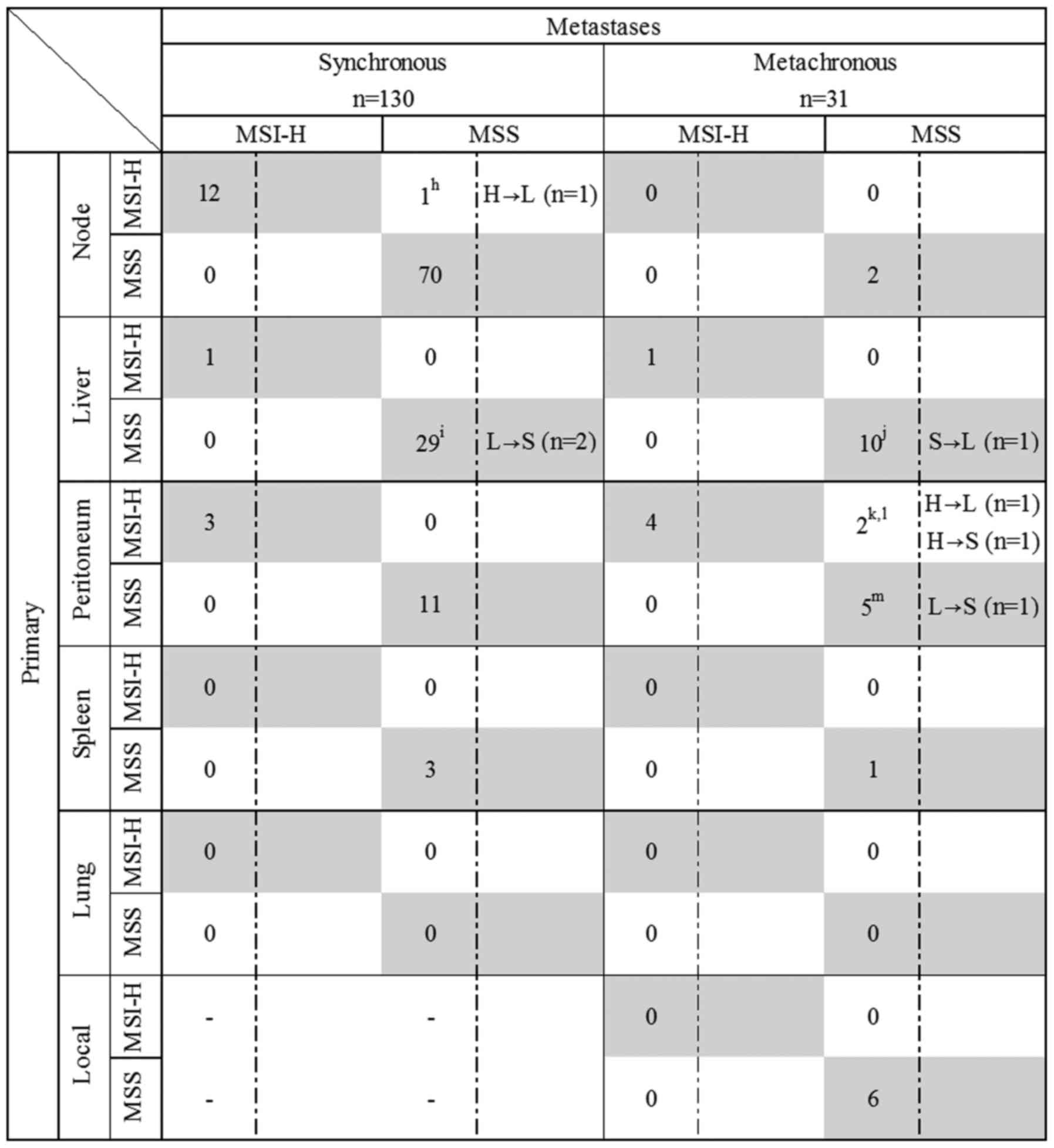
was analysed in 482 primary CRC, 155 corresponding metastases (130
synchronous and 25 metachronous metastases) and six local
recurrences. Four hundred and two metastases were not analysed for
MSI status (Figs. 1 and 3). Eighteen MSI-H CRC cases were
identified in this study and consisted of 3 Lynch Syndrome cases,
10 MLH1 hypermethylated and 5 MLH1 unmethylated cases
without germline mutation (Table
II).
 | Table II.Characteristics of primary CRCs with
MSI-H and corresponding metastases. |
Table II.
Characteristics of primary CRCs with
MSI-H and corresponding metastases.
|
|
| Primary |
| Metastases |
|---|
|
|
|
|
|
|
|---|
| Case no. | Type |
KRAS/BRAF | MSI | Type of
metastases |
KRAS/BRAF | MSI |
|---|
| 403 | LS
(MLH1) | WT | H | Synchronous
node | WT | H |
| 238 | LS
(MSH2) | KRAS
ex2 | H | Synchronous
node | KRAS
ex2 | H |
| 238 | LS
(MSH2) | KRAS
ex2 | H | Synchronous
Liver | KRAS
ex2 | H |
| 238 | LS
(MSH2) | KRAS
ex2 | H | Synchronous
Peritoneum | KRAS
ex2 | H |
| 455 | LS
(MSH6) | WT | H | Synchronous
node | WT | H |
|
8 | MLH1
methylated | WT | H | Synchronous
node | WT | H |
| 65 | MLH1
methylated | KRAS
ex2 | H | Synchronous
node | KRAS
ex2 | H |
| 193 | MLH1
methylated | BRAF | H | Synchronous
node | BRAF | H |
| 396 | MLH1
methylated | KRAS
ex2 | H | Synchronous
node | KRAS
ex2 | H |
| 424 | MLH1
methylated | BRAF | H | Synchronous
node | BRAF | H |
| 342 | MLH1
methylated | WT | H | Synchronous
node | WT | H |
| 227 | MLH1
methylated | KRAS
ex3/BRAF | H | Synchronous
node | KRAS
ex3/BRAF | H |
| 149 | MLH1
methylated | WT | H | Synchronous
Peritoneum | WT | H |
| 397 | MLH1
methylated | BRAF | H | Synchronous
Peritoneum | BRAF | H |
| 397 | MLH1
methylated | BRAF | H | Metachronous
Liver | BRAF | H |
| 397 | MLH1
methylated | BRAF | H | Metachronous
Peritoneum | BRAF | H |
| 397 | MLH1
methylated | BRAF | H | Metachronous
Peritoneum | BRAF | H |
| 191 | MLH1
methylated | KRAS
ex3 | H | Metachronous
Peritoneum | WT | H |
|
4 | MLH1
unmethylated | WT | H | Synchronous
node | WT | H |
| 220 | MLH1
unmethylated | WT | H | Synchronous
node | WT | L |
| 398 | MLH1
unmethylated | KRAS
ex2 | H | Synchronous
node | KRAS
ex2 | H |
| 239 | MLH1
unmethylated | BRAF | H | Metachronous
Peritoneum | KRAS
ex2 | S |
| 253 | MLH1
unmethylated | BRAF | H | Metachronous
Peritoneum | BRAF | L |
| 253 | MLH1
unmethylated | BRAF | H | Metachronous
Peritoneum | BRAF | H |
Concordance rate of KRAS mutation,
BRAF mutation and MSI-H between primary CRCs and corresponding
metastases
The concordance rate of KRAS/BRAF mutation
between primary CRC and corresponding metastases was 94.6%
(243/257). The concordance rates of KRAS mutation,
BRAF mutation or wild-type (KRAS wild-type and
BRAF wild-type) between primary CRC and corresponding
metastases were 93.9% (214/228), 100% (30/30) and 99.3% (304/306),
respectively. High concordance rate was observed in either
synchronous or metachronous metastases (Table I).
 | Table I.Concordance rate of KRAS/BRAF
mutation status and MSI status between primary CRCs and
corresponding metastases. |
Table I.
Concordance rate of KRAS/BRAF
mutation status and MSI status between primary CRCs and
corresponding metastases.
|
| Concordance rate
(%) |
|---|
|
|
|
|---|
| Status | Total | Synchronous | Metachronous |
|---|
|
KRAS/BRAF | 94.6 (243/257) | 95.1 (215/226) | 90.3 (28/31) |
| KRAS | 93.9 (214/228) | 94.6 (194/205) | 87.0 (20/23) |
| BRAF | 100 (30/30) | 100 (22/22) | 100 (8/8) |
| WT | 99.3 (304/306) | 99.6 (272/273) | 97.0 (32/33) |
| MSI-H | 87.5 (21/24) | 94.1 (16/17) | 71.4 (5/7) |
| MSS | 100 (137/137) | 100 (113/113) | 100 (24/24) |
The concordant rates of MSI-H and MSS (included
MSI-L) were 87.5% (21/24) and 100% (137/137), respectively.
Discordance of MSI status was found in 3 cases and all of them were
MLH1 unmethylated cases. KRAS and BRAF
mutation status in primary MSI-H CRC was consistent with that in
metastases except one case (Table
II).
Concordance rate of KRAS/BRAF mutation
or MSI status between primary CRCs and each site of corresponding
metastases
BRAF mutation status of each metastatic
tissue was perfectly consistent with primary CRC. In each
metastatic tissue, a high concordance rate of KRAS mutation
was shown as well. Local recurrences (75.0%) had lower concordance
rates with each metastatic tissue. Regarding MSI status, a high
concordance rate of MSI-H was also observed in each metastatic
tissue. Peritoneal metastases (77.8%) had lower concordance rates
in each metastatic tissue (Table
III).
 | Table III.Concordance rate of KRAS/BRAF
mutation and MSI status between primary CRCs and each site of
corresponding metastases. |
Table III.
Concordance rate of KRAS/BRAF
mutation and MSI status between primary CRCs and each site of
corresponding metastases.
|
| Concordance rate
(%) |
|---|
|
|
|
|---|
| Status | Node | Liver | Peritoneum | Spleen | Lung | Local |
|---|
|
KRAS/BRAF | 93.1 (134/144) | 97.2 (69/71) | 97.1 (34/35) | 100 (2/2) | 100 (1/1) | 75.0 (3/4) |
| KRAS | 92.2 (118/128) | 96.9 (63/65) | 96.0 (24/25) | 100 (2/2) | 100 (1/1) | 75.0 (3/4) |
| BRAF | 100 (17/17) | 100 (4/4) | 100 (9/9) | – | – | – |
| Wild | 100 (197/197) | 97.6 (82/84) | 100 (17/17) | 100 (3/3) | – | 100 (3/3) |
| MSI-H | 92.3 (12/13) | 100 (2/2) | 77.8 (7/9) | 100 (2/2) | – | – |
| MSS | 100 (72/72) | 100 (39/39) | 100 (16/16) | 100 (4/4) | – | 100 (6/6) |
Discordant cases
Twenty-three cases were discordant between primary
CRC and corresponding metastases. Discordant cases were observed in
the 16 cases with KRAS mutation and 3 cases with MSI-H, but
not in BRAF mutation cases. Of the 16 discordant cases with
KRAS mutation, 10 cases were lymph node metastases. Most of
discordant cases in KRAS mutants were lymph node metastases.
Of the three cases with MSI-H, one was in lymph node metastases and
two cases in the peritoneal metastases (Table IV).
 | Table IV.The discordant cases. |
Table IV.
The discordant cases.
| Case no. | Primary | Metastasis | Location | Time |
|---|
| 22a | KRAS exon2
mutant | WT | Node | Synchronous |
|
230a | KRAS exon2
mutant | WT | Node | Synchronous |
|
237a | KRAS exon2
mutant | WT | Node | Synchronous |
|
323a | KRAS exon2
mutant | WT | Node | Synchronous |
|
361a | KRAS exon2
mutant | WT | Node | Synchronous |
|
391a | KRAS exon2
mutant | WT | Node | Synchronous |
|
449a | KRAS exon2
mutant | WT | Node | Synchronous |
|
474a | KRAS exon2
mutant | WT | Node | Synchronous |
|
492a | KRAS exon2
mutant | WT | Node | Synchronous |
|
501a | KRAS exon2
mutant | WT | Node | Synchronous |
|
466b | KRAS exon2
mutant | WT | Liver | Synchronous |
|
371c | WT | KRAS exon2
mutant | Liver | Synchronous |
| 61d | KRAS exon2
mutant | WT | Liver | Metachronous |
|
270e | KRAS exon4
mutant | KRAS exon2
mutant | Liver | Metachronous |
|
191f | KRAS exon3
mutant | WT | Peritoneum | Metachronous |
|
380g | KRAS exon3
mutant | WT | Local | Metachronous |
|
220h | MSI-H | MSI-L | Node | Synchronous |
|
126i | MSI-L | MSS | Liver | Metachronous |
|
108i | MSI-L | MSS | Liver | Metachronous |
|
445j | MSS | MSI-L | Liver | Metachronous |
|
253k | MSI-H | MSI-L | Peritoneum | Metachronous |
|
239l | MSI-H | MSS | Peritoneum | Metachronous |
|
296m | MSI-L | MSS | Peritoneum | Metachronous |
Discussion
High concordance of KRAS/BRAF mutation status
and MSI status was observed between primary CRC and corresponding
metastases in the present study. These high concordance rates were
not different between synchronous and metachronous metastases.
These results are in agreement with the notion that
KRAS/BRAF mutations occur early in CRC carcinogenesis
(20,28). Lymph node metastases showed a
slightly lower concordance rate than other metastatic sites. Mao
et al demonstrated that lymph node metastases indicated a
lower concordance rate with KRAS mutation status (29) and this support our results. However,
concordance rate of KRAS/BRAF mutation and MSI-H was >90%
in lymph node suspected metastases macroscopically, metastatic
lymph node will be useful for mutation analysis after confirmation
of enough tumour cells and content microscopically. With regard to
the other metastatic sites, a high concordance was observed between
primary CRC and corresponding liver metastases. Knijin et al
demonstrated a high concordance, i.e. 96.4%, in 305 liver
metastases (30). In addition, this
study showed high concordance of KRAS/BRAF mutation between
primary tumour and peritoneal metastases. No other studies have
systematically compared the concordance of KRAS/BRAF
mutation status in primary CRC with corresponding peritoneal
metastases.
Regarding MSI status, a high concordance rate was
also shown between primary CRC and corresponding metastases. This
result suggested that cancer cells do not change their MSI status
during progression. MSI-H CRC consists of three types, which
harbours a germline mutation in the MMR gene (e.g. Lynch syndrome),
acquires epigenetic change in the MMR gene (e.g. MLH1
promoter hypermethylation) and uncertified germline mutation
without MLH1 promoter hypermethylation (e.g. Lynch-like
syndrome). Our results indicated perfect concordance of MSI status
was observed in two types, i.e. Lynch syndrome and MLH1
promotor hypermethylation (3 Lynch syndrome cases and 10
MLH1 promoter hypermethylation cases) between primary CRC
and corresponding metastases (Table
II). This is the first study of concordance rate of MSI status
between primary and metastatic CRC using Bethesda markers.
Recently, Haraldsdottir et al reported perfect concordance
of MMR deficiency evaluated by immunohistochemistry (IHC) between
primary CRC and corresponding metastases (31).
In this study, 23 cases showed discordance of
mutation status between primary CRC and corresponding metastases.
Several reasons are conceivable. First, it could be speculated that
discrepancies may depend on the molecular heterogeneity in primary
CRCs. For instance, intratumoural heterogeneity for a KRAS
point mutation was observed within 20–60% CRC cases in previous
studies (32,33). In contrast to KRAS mutation
status, BRAF mutation status did not show heterogeneity in
previous studies (34,35). Mao et al have reported a
higher concordance rate of BRAF mutation status (93.6%)
between primary and lymph node metastases (29). Our results, which showed a perfect
concordance rate in BRAF-mutant cases, is in agreement with
these studies.
Second, discordant results could be explained by the
acquisition of the mutation during the disease progression.
However, KRAS mutation occur in early stage of
carcinogenesis (20,28), it may be rare that CRC acquired
KRAS mutation after metastasis (21,36).
Third, selecting improper samples containing a high
number of normal or necrotic cells, could create discordance
between primary CRC and corresponding metastases. In this study,
samples that were suspected to contain enough cancer cells
macroscopically by surgeons were used for mutation testing.
Consequently, these samples might not include enough cancer cells
especially in the lymph nodes. High concordance rate might be shown
in previous studies that used laser microdissection-collected
cancer cells from lymph node metastases (37,38).
In conclusion, although attention should be paid to
selecting and sampling tissue, high concordance rate of
KRAS/BRAF mutation status and MSI status was observed
between primary CRC and corresponding metastases, regardless of
metastatic sites and synchronous/metachronous types. Therefore, to
choose the appropriate regimen for therapy, either primary or
metastatic CRC can be used for testing KRAS/BRAF mutation
status and MSI status.
Acknowledgements
We would like to thank the staff of the Divisions of
Gastroenterological Surgery and Molecular Diagnosis and Cancer
Prevention, Saitama Cancer Center.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
MSI
|
microsatellite instability
|
|
MSI-H
|
MSI-high
|
|
MMR
|
mismatch repair
|
|
HRM
|
high resolution melting
|
|
PCR
|
polymerase chain reaction
|
|
MSI-L
|
MSI-low
|
|
MSS
|
microsatellite stable
|
References
|
1
|
Rajagopalan H, Bardelli A, Lengauer C,
Kinzler KW, Vogelstein B and Velculescu VE: Tumorigenesis: RAF/RAS
oncogenes and mismatch-repair status. Nature. 418:9342002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scaltriti M and Baselga J: The epidermal
growth factor receptor pathway: A model for targeted therapy. Clin
Cancer Res. 12:5268–5272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McCubrey JA, Steelman LS, Abrams SL, Lee
JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA,
D'Assoro AB, et al: Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT
pathways in malignant transformation and drug resistance. Adv
Enzyme Regul. 46:249–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Normanno N, Maiello MR and De Luca A:
Epidermal growth factor receptor tyrosine kinase inhibitors
(EGFR-TKIs): Simple drugs with a complex mechanism of action? J
Cell Physiol. 194:13–19. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E, Köhne CH, Láng I, Folprecht
G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D,
Tejpar S, et al: Cetuximab plus irinotecan, fluorouracil, and
leucovorin as first-line treatment for metastatic colorectal
cancer: Updated analysis of overall survival according to tumor
KRAS and BRAF mutation status. J Clin Oncol. 29:2011–2019. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amado RG, Wolf M, Peeters M, Van Cutsem E,
Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, et
al: Wild-type KRAS is required for panitumumab efficacy in patients
with metastatic colorectal cancer. J Clin Oncol. 26:1626–1634.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Nicolantonio F, Martini M, Molinari F,
Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L,
Frattini M, Siena S, et al: Wild-type BRAF is required for response
to panitumumab or cetuximab in metastatic colorectal cancer. J Clin
Oncol. 26:5705–5712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tol J, Nagtegaal ID and Punt CJA: BRAF
mutation in metastatic colorectal cancer. N Engl J Med. 361:98–99.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kadowaki S, Kakuta M, Takahashi S,
Takahashi A, Arai Y, Nishimura Y, Yatsuoka T, Ooki A, Yamaguchi K,
Matsuo K, et al: Prognostic value of KRAS and BRAF mutations in
curatively resected colorectal cancer. World J Gastroenterol.
21:1275–1283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sosman JA, Kim KB, Schuchter L, Gonzalez
R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ,
Flaherty KT, et al: Survival in BRAF V600-mutant advanced melanoma
treated with vemurafenib. N Engl J Med. 366:707–714. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chapman PB, Hauschild A, Robert C, Haanen
JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et
al: BRIM-3 Study Group: Improved survival with vemurafenib in
melanoma with BRAF V600E mutation. N Engl J Med. 364:2507–2516.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kopetz S, Desai J, Chan E, Hecht JR,
O'Dwyer PJ, Maru D, Morris V, Janku F, Dasari A, Chung W, et al:
Phase II pilot study of vemurafenib in patients with metastatic
BRAF-mutated colorectal cancer. J Clin Oncol. 33:4032–4038. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cremolini C, Loupakis F, Antoniotti C,
Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M,
Zaniboni A, et al: FOLFOXIRI plus bevacizumab versus FOLFIRI plus
bevacizumab as first-line treatment of patients with metastatic
colorectal cancer: Updated overall survival and molecular subgroup
analyses of the open-label, phase 3 TRIBE study. Lancet Oncol.
16:1306–1315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Loupakis F, Cremolini C, Masi G, Lonardi
S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi
R, et al: Initial therapy with FOLFOXIRI and bevacizumab for
metastatic colorectal cancer. N Engl J Med. 371:1609–1618. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Loupakis F, Cremolini C, Salvatore L, Masi
G, Sensi E, Schirripa M, Michelucci A, Pfanner E, Brunetti I, Lupi
C, et al: FOLFOXIRI plus bevacizumab as first-line treatment in
BRAF mutant metastatic colorectal cancer. Eur J Cancer. 50:57–63.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Corcoran RB, Atreya CE, Falchook GS, Kwak
EL, Ryan DP, Bendell JC, Hamid O, Messersmith WA, Daud A, Kurzrock
R, et al: Combined BRAF and MEK inhibition with dabrafenib and
trametinib in BRAF V600-mutant colorectal cancer. J Clin Oncol.
33:4023–4031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Umar A, Boland CR, Terdiman JP, Syngal S,
de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ,
Hamelin R, et al: Revised Bethesda Guidelines for hereditary
nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite
instability. J Natl Cancer Inst. 96:261–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klein CA: Parallel progression of primary
tumours and metastases. Nat Rev Cancer. 9:302–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vogelstein B, Fearon ER, Hamilton SR, Kern
SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM and Bos
JL: Genetic alterations during colorectal-tumor development. N Engl
J Med. 319:525–532. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Forrester K, Almoguera C, Han K, Grizzle
WE and Perucho M: Detection of high incidence of K-ras oncogenes
during human colon tumorigenesis. Nature. 327:298–303. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paliogiannis P, Cossu A, Tanda F, Palmieri
G and Palomba G: KRAS mutational concordance between primary and
metastatic colorectal adenocarcinoma. Oncol Lett. 8:1422–1426.
2014.PubMed/NCBI
|
|
23
|
Miglio U, Mezzapelle R, Paganotti A,
Allegrini S, Veggiani C, Antona J, Gentilli S, Monga G, Alabiso O
and Boldorini R: Mutation analysis of KRAS in primary colorectal
cancer and matched metastases by means of highly sensitivity
molecular assay. Pathol Res Pract. 209:233–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vakiani E, Janakiraman M, Shen R, Sinha R,
Zeng Z, Shia J, Cercek A, Kemeny N, D'Angelica M, Viale A, et al:
Comparative genomic analysis of primary versus metastatic
colorectal carcinomas. J Clin Oncol. 30:2956–2962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akagi K, Uchibori R, Yamaguchi K, Kurosawa
K, Tanaka Y and Kozu T: Characterization of a novel oncogenic K-ras
mutation in colon cancer. Biochem Biophys Res Commun. 352:728–732.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ogura T, Kakuta M, Yatsuoka T, Nishimura
Y, Sakamoto H, Yamaguchi K, Tanabe M, Tanaka Y and Akagi K:
Clinicopathological characteristics and prognostic impact of
colorectal cancers with NRAS mutations. Oncol Rep. 32:50–56.
2014.PubMed/NCBI
|
|
27
|
Asaka S, Arai Y, Nishimura Y, Yamaguchi K,
Ishikubo T, Yatsuoka T, Tanaka Y and Akagi K: Microsatellite
instability-low colorectal cancer acquires a KRAS mutation during
the progression from Dukes' A to Dukes' B. Carcinogenesis.
30:494–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mao C, Wu XY, Yang ZY, Threapleton DE,
Yuan JQ, Yu YY and Tang JL: Concordant analysis of KRAS, BRAF,
PIK3CA mutations, and PTEN expression between primary colorectal
cancer and matched metastases. Sci Rep. 5:80652015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Knijn N, Mekenkamp LJ, Klomp M,
Vink-Börger ME, Tol J, Teerenstra S, Meijer JW, Tebar M, Riemersma
S, van Krieken JH, et al: KRAS mutation analysis: A comparison
between primary tumours and matched liver metastases in 305
colorectal cancer patients. Br J Cancer. 104:1020–1026. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haraldsdottir S, Roth R, Pearlman R,
Hampel H, Arnold CA and Frankel WL: Mismatch repair deficiency
concordance between primary colorectal cancer and corresponding
metastasis. Fam Cancer. 15:253–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baisse B, Bouzourene H, Saraga EP, Bosman
FT and Benhattar J: Intratumor genetic heterogeneity in advanced
human colorectal adenocarcinoma. Int J Cancer. 93:346–352. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Losi L, Baisse B, Bouzourene H and
Benhattar J: Evolution of intratumoral genetic heterogeneity during
colorectal cancer progression. Carcinogenesis. 26:916–922. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baldus SE, Schaefer KL, Engers R, Hartleb
D, Stoecklein NH and Gabbert HE: Prevalence and heterogeneity of
KRAS, BRAF, and PIK3CA mutations in primary colorectal
adenocarcinomas and their corresponding metastases. Clin Cancer
Res. 16:790–799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Molinari F, Martin V, Saletti P, De Dosso
S, Spitale A, Camponovo A, Bordoni A, Crippa S, Mazzucchelli L and
Frattini M: Differing deregulation of EGFR and downstream proteins
in primary colorectal cancer and related metastatic sites may be
clinically relevant. Br J Cancer. 100:1087–1094. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Uchi R, Takahashi Y, Niida A, Shimamura T,
Hirata H, Sugimachi K, Sawada G, Iwaya T, Kurashige J, Shinden Y,
et al: Integrated multiregional analysis proposing a new model of
colorectal cancer evolution. PLoS Genet. 12:e10057782016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gattenlöhner S, Etschmann B, Kunzmann V,
Thalheimer A, Hack M, Kleber G, Einsele H, Germer C and
Müller-Hermelink HK: Concordance of KRAS/BRAF mutation status in
metastatic colorectal cancer before and after anti-EGFR therapy. J
Oncol. 2009:8316262009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim M-J, Lee HS, Kim JH, Kim YJ, Kwon JH,
Lee J-O, Bang S-M, Park KU, Kim D-W, Kang S-B, et al: Different
metastatic pattern according to the KRAS mutational status and
site-specific discordance of KRAS status in patients with
colorectal cancer. BMC Cancer. 12:3472012. View Article : Google Scholar : PubMed/NCBI
|