Introduction
Breast cancer is one of the most common malignant
neoplasms diagnosed in women worldwide. Metastasis, the single
greatest cause of morbidity and mortality in breast cancer
patients, commonly occurs in the bones, lungs and liver (1–4).
However, the molecular mechanisms underlying breast cancer cell
proliferation, migration and metastasis still require further
investigation, and there is still no effective treatment strategy
for breast cancer metastasis.
Bone morphogenetic proteins (BMPs) regulate a wide
range of cellular responses including cell proliferation, adhesion,
migration and apoptosis (5,6). BMPs bind to heterodimers of type I and
II serine/threonine kinase receptors. Upon ligand binding, the
constitutively active type II receptor transphosphorylates and
activates the kinase activity of the type I receptor. Once active,
the type I receptor phosphorylates the intracellular effector
proteins, SMAD1, SMAD5, and SMAD8, which have complex involvement
with a common partner, SMAD4 to accumulate in the nucleus and
regulate the expression of genes involved in cell growth, cell
differentiation, cell apoptosis, cellular homeostasis and other
cellular functions (7,8). This pathway is known as the
SMAD-dependent pathway. BMP ligands bind to different receptors in
different contexts. The physiological association with a specific
receptor depends on both the binding affinity and the actual
availability of the ligand and the receptor in a specific
environment. Type I receptors determine the specificity of the
intracellular response (9).
Activin-like kinase 2 (ALK2; ACVR1), ALK3 (BMPR1A), and ALK6
(BMPR1B) are type I receptors known to mediate BMP signaling.
Research has shown that type I receptors (ALK3 and
ALK6) are expressed in various breast cancer cell lines and primary
tumor samples. Expression of dominant-negative ALK3 in a mouse
xenograft model was found to result in decreased invasiveness and
metastasis (10). ALK6 is
associated with the poor prognosis of breast cancer patients. In
ER-positive breast cancer specimens, ALK6 expression was found to
correlate with high tumor grade and high tumor proliferation.
Reduced expression of ALK6 was found to correlate with poor
prognosis and increased proliferation of breast cancer cells
(11).
ALK2 belongs to the type I receptors, but the
function of ALK2 in breast cancer is unknown. Research has reported
that ALK2 is generally expressed in various types of cells
(12). ALK2 gene mutation was found
to lead to ossificans progressiva fibrodysplasia (FOP) in bone
disease (13), and led to Down's
syndrome. The reason for congenital heart defects in Down's
syndrome patients is due to ALK2 gene mutation which alters the
BMP/SMAD pathway (14). In
mesenchymal stem cells, ALK2 is necessary for the signaling pathway
of BMP9-induced osteogenic (15).
It has also been reported that ALK2 promotes the proliferation of
lens cells in the early stage of mouse embryonic development, but
inhibits proliferation in late stage (16). ALK2 exhibits various functions in
different types of cells and even in the same type of cells, yet
the reason is unknown (17).
Recently, research has focused on the relationship between ALK2 and
tumors. BMP9 binds to ALK2 to promote ovarian cancer by activating
the SMAD1/4 signaling pathway (18,19).
ALK2 mutant was found to promote the growth of prostate cancer
PC3-M cells (20). However, the
effect of ALK2 on the proliferation and metastasis of breast cancer
cells is still unknown.
Dominant-negative mutation uses engineering
technology to mutate the specific receptor and overexpress the
dominant-negative receptor to produce a negative regulatory effect.
Our group cloned the ALK2 extracellular domain cDNA to the
adenovirus vector, and successfully constructed the
dominant-negative mutant ALK2 adenovirus vector (DNALK2). DNALK2
receptors can compete with wild-type ALK2 receptors by binding to
ligands, and inhibit ligands to active cell signaling pathways.
Materials and methods
Cell culture and infection
The human breast cancer cell lines MDA-MB-231 and
MDA-MB-468 (Shanghai Institute for Biological Sciences, Chinese
Academy of Sciences, Shanghai, China) were maintained in L-15
medium supplemented with 10% fetal bovine serum (FBS; Gibco, Grand
Island, NY, USA) at 37°C without CO2. Other breast
cancer cell lines, MCF-7 and SK-BR-3 (Shanghai Institute for
Biological Sciences, Chinese Academy of Sciences) were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS
at 37°C with 5% CO2.
Log-phase MDA-MB-231 cells were seeded at a density
of 2×106/bottle. The cells were infected with
RFP-expressing and recombinant DNALK2 adenovirus vehicles 24 h
later. After 8–12 h of cultivation, the medium was replaced with
fresh medium. The fluorescence was then observed 24 h later. The
recombinant MDA-MB-231/DNALK2 cells were used for subsequent
experiments. The experimental cells were divided into the
MDA-MB-231 and MDA-MB-231/RFP control groups as well as the
MDA-MB-231/DNALK2 group.
RNA isolation and semi-quantitative
RT-PCR
Total RNA was extracted from artificially induced
breast cancer cell samples using TRIzol (Invitrogen Life
Technologies, Carlsbad, CA, USA).
The first-strand cDNA was synthesized from 1 µg of
total RNA using the M-MLV kit (Promega Corp., Madison, WI, USA).
ALK2 gene expression was quantified by semi-quantitative
reverse transcription-polymerase chain reaction (RT-PCR).
GAPDH was used as an endogenous control. Gene expression
analysis was performed with Quantity One software (Bio-Rad,
Berkeley, CA, USA). The PCR conditions were 94°C for 5 min,
followed by 30 cycles at 94°C for 30 sec, 52°C for 20 sec, and 72°C
for 10 sec. The mRNA expression levels of the target gene were
normalized to those of GAPDH.
The specific primers for ALK2 were
5′-GGCTGCTTCCAGGTTTAT-3′ (forward) and 5′-CATACTGCGAACACTACAGA-3′
(reverse). Those for GAPDH were 5′-CAGCGACACCCACTCCTC-3′
(forward) and 5′-TGAGGTCCACCACCCTGT-3′ (reverse).
Western blot analysis
The protein concentrations in the cell lysates were
determined using a kit (RIPA, P0013B; Bi Yuntian Biological
Technology Institution, Shanghai, China). Equal amounts of proteins
were separated by sodium dodecyl sulphate-polyacrylamide gel
electrophoresis and blotted onto nitrocellulose membranes. The
proteins were then probed with rabbit polyclonal anti-SMAD1/5/8
(sc-6031; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
rabbit polyclonal anti-phospho-SMAD1/5/8 (AB3848; Millipore Corp.,
Billerica, MA, USA), rabbit monoclonal anti-inhibitor of
differentiation 1 (ID1) (ab134163), and rabbit polyclonal
anti-connective tissue growth factor (CTGF) antibodies (ab6992)
(both from Abcam) and peroxidase-conjugated secondary antibodies. A
GAPDH protein sample was used as the control for equal protein
loading. Protein bands were visualized using Quantity One
software.
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR was performed as
previously described (21). All
samples were run in duplicate for each experiment. The following
primer sequences were used for CTGF (sense, 5′-GCGGCTTACCGACTGGA-3′
and antisense, 5′-AGGCGGCTCTGCTTCTC-3′); ID1 (sense,
5′-CGGTCTCATTTCTTCTCG-3′ and antisense, 5′-TCGGTCTTGTTCTCCCTC-3′);
and GAPDH (sense, 5′-CAGCGACACCCACTCCTC-3′ and antisense,
5′-TGAGGTCCACCACCCTGT-3′). The relative expression of target mRNAs
was normalized to the reference gene GAPDH using the
2−ΔΔCT method and is expressed as the fold-change
relative to the control (0.1% or DharmaFECT1) (21).
Cell viability assay
The MTT assay was performed in quintuplicate to
assess the viability of the recombinant MDA-MB-231/DNALK2 cells.
Approximately 1–3×104 of cells from each group in 500 µl
of medium were seeded in 24-well plates. Approximately 50 µl of MTT
reagent (5 mg/ml; Promega Corp.) was added, and the mixture was
incubated for 4 h. After addition of 450 µl of dimethyl sulfoxide,
absorbance was measured daily for the following 5 days at 492 nm
using a microplate reader. Then, a growth curve was drawn.
Colony forming assay
Log-phase cells were collected and seeded in
triplicate in a soft agar medium at 200 cells/culture dish for
10–14 days. When the clones were able to be observed, the cells
were washed twice by PBS and stained by the Wright method. The
colony-forming rate was calculate as: (Colony number/seeded cell
number) × 100%. Each experiment was repeated thrice.
Wound closure assay
Log-phase cells were collected and seeded at
5×105/well in 6-well plates. On the following day, the
cells were infected with recombinant DNALK2 adenovirus for 24 h,
and a wound was created at the center of the culture using a
pipette tip. Photographs were taken under a microscope immediately
and 36 h after the incision was made. The incision width of the
different sites was measured, and the average wound closure rate
was calculated. The wound-closure rate was calculated as: [(0-h
incision width - 36-h incision width)/0-h incision width] ×
100%.
Transwell invasion assay
The invasion assay was performed as previously
described (15). The cells were
seeded at 2×105/well in duplicate in the upper chamber
of type I collagen-coated 24-well culture inserts. After 24 h, the
cells were dried for 5 min, fixed with dehydrated alcohol, and
stained with hematoxylin and eosin. The cells that invaded the
collagen-coated inserts were counted. Mean values for five randomly
selected fields were obtained for each well. The experiments were
performed 4-fold and repeated thrice. Values are expressed as means
± standard errors.
Animals
Four-week-old female nude mice (Balb/c, Beijing,
China) were used. All experiments were approved by the
Institutional Animal Care and Use Committee of Chongqing Medical
University as well as regional authorities [reference no.
SYXK(YU)2007-0001] in accordance with the ‘Guidelines for the
Welfare of Animals in Experimental Neoplasia’ (the China
Coordinating Committee on Cancer Research). The animals were housed
in individual ventilated cages under sterile conditions, and were
given free access to food and water. At the end of the experimental
period, the animals were sacrificed by cervical dislocation.
Animal models
Female nude mice were randomly divided into three
groups of five mice each. Two groups were implanted subcutaneously
with 2×106 control MDA-MB-231 and MDA-MB-231/RFP cells,
respectively. The third group was implanted subcutaneously with
2×106 MDA-MB-231/DNALK2 cells. After 13 days, when
tumors were observable, the tumor diameters were recorded every
three days. Tumor volume (V, in cm3) was then calculated
as: (4π/3) × [(a + b)/4]3, where π=3.14. The mice were
sacrificed after three weeks, and the tumors were collected. The
tumors were then dissected and processed for further
histomorphometrical and immunohistochemical analyses.
Immunohistochemistry
Paraffin-embedded nude mouse xenograft breast tumors
were sliced into 4-µm sections and deparaffinized. The sections
were rehydrated and heat-treated for antigen-retrieval with citric
acid buffer as previously described. The sections were then
incubated with rabbit anti-SMAD1/5/8, rabbit
anti-phospho-SMAD1/5/8, rabbit anti-ID1 and rabbit anti-CTGF
antibodies and rabbit anti-PCNA for 1 h following standard
protocol. Staining procedures were performed under standardized
conditions. The sections were counterstained with Gill's
hematoxylin, mounted, and coverslipped. Staining intensity was
independently assessed by the authors. SMADD1/5/8,
phospho-SMAD1/5/8, ID1 and CTGF expression were determined with
integrated optical density (IOD) using Image-Pro Plus 5.1. The
nuclear staining revealed PCNA-positive cells, and PCNA expression
was determined as: (PCNA-positive cells/total number of tumor
cells) × 100%.
Statistical analysis
Results are expressed as means ± standard
deviations. Relative CTGF and ID1 expression data were analyzed
using real-time quantitative PCR and the 2−ΔΔCT method.
All statistical analyses were performed by SPSS 19.0 using the
independent sample t-test for comparing two sample groups. For all
tests, P<0.05 was considered statistically significant.
Results
ALK2 mRNA is detected in breast cancer
cells
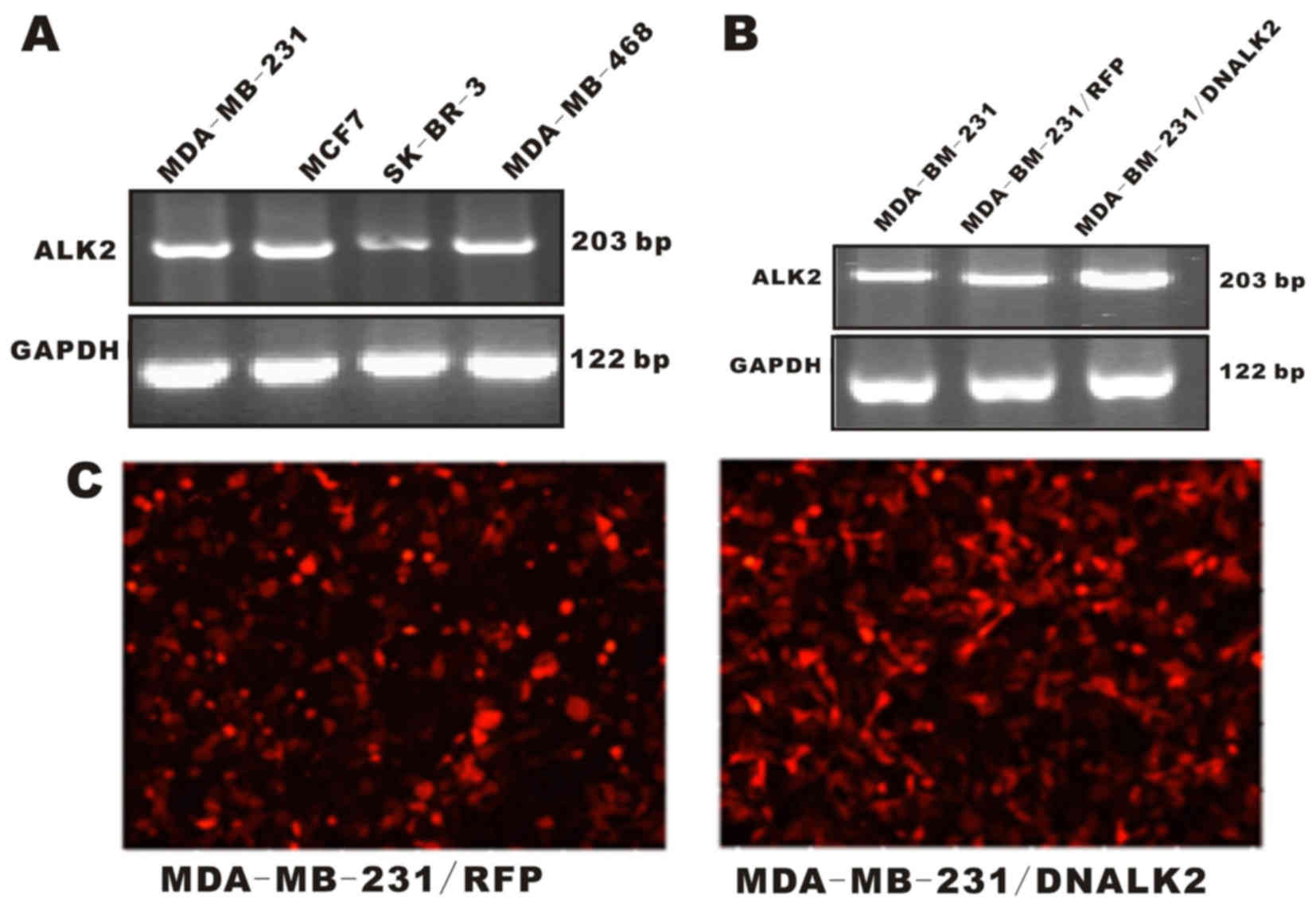
In the present study, four breast cancer cell lines
were used to investigate ALK2 expression. Our group wanted to
determine whether ALK2 expression in the human breast cancer cell
lines is associated with tumorigenic potentials. ALK2 mRNA was
detected in the four breast cancer cell lines by RT-PCR
amplification (Fig. 1A). The result
suggests that ALK2 expression is correlated with the progression
and metastasis of breast cancer.
Recombinant MDA-MB-231/DNALK2 cells
are established
MDA-MB-231 cells were used in the present study to
generate a dominant-negative ALK2 recombinant cell line to examine
the roles of ALK2 in the proliferation and invasiveness of breast
cancer cells. The dominant-negative ALK2 recombinant
MDA-MB-231/DNALK2 cells were established by infection with the
DNALK2 adenovirus. MDA-MB-231/RFP cells infected with an empty
adenovirus vector were also constructed as control cells. The
RT-PCR results showed that these recombinant cells were well
established for use in the subsequent experiments (Fig. 1B and C).
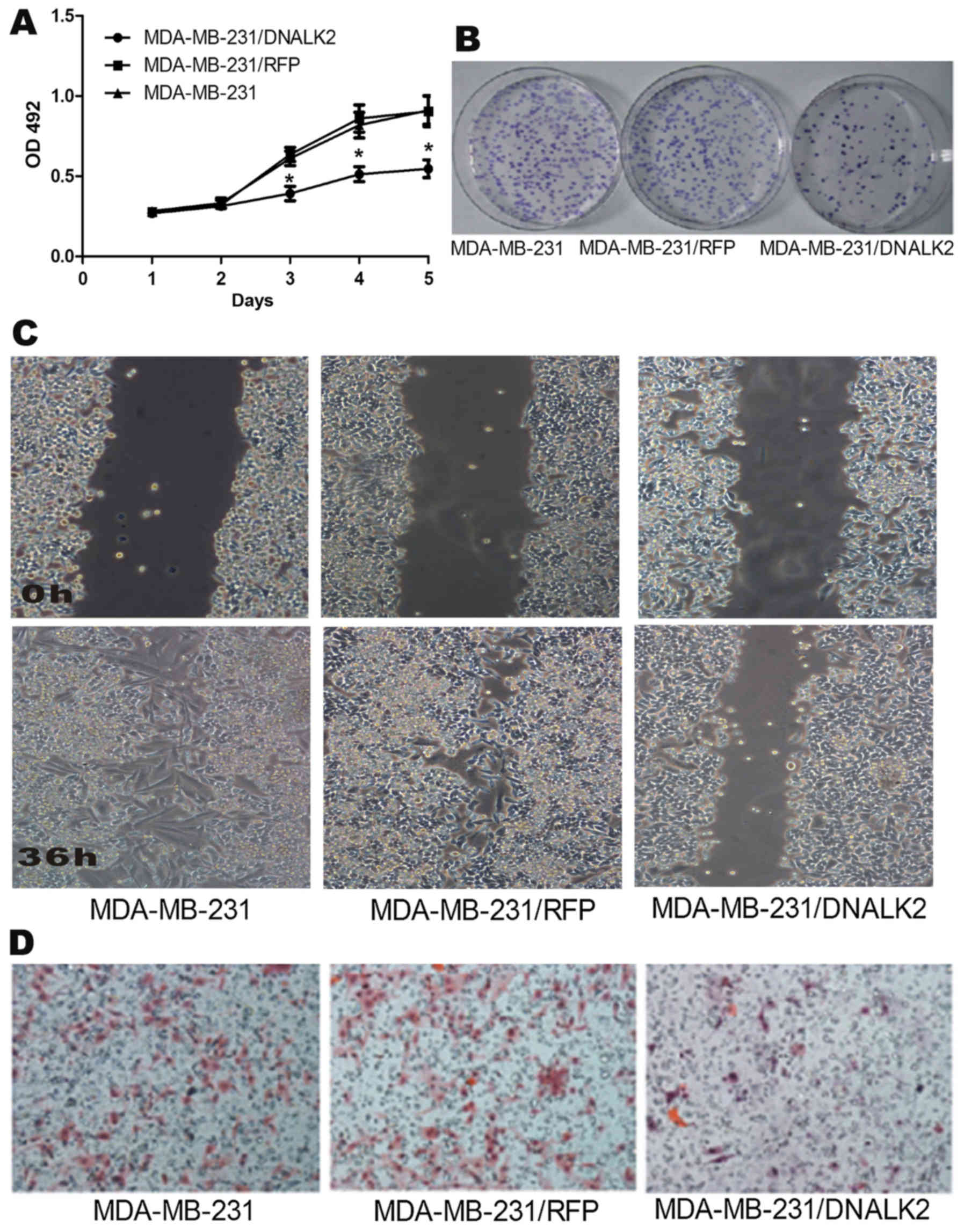
DNALK2 inhibits the proliferation and
invasiveness of breast cancer cells in vitro
ALK2 was reported to bind to BMP9 to promote the
proliferation of ovarian cancer cells but ALK2 was found to inhibit
the growth and to induce the apoptosis of prostate cancer cells. In
the present study, we elucidated the effects of ALK2 on breast
cancer cells in vitro by analyzing cell proliferation,
migration, and invasion abilities. MTT and colony-forming assays
were used to detect the cell proliferation ability. The results
showed that the proliferation of MDA-MB-231/DNALK2 cells was
decreased from 0.8951±0.0902 (MDA-MB-231/RFP) and 0.9012±1.0021
(MDA-MB-231) to 0.5563±0.0617 (MDA-MB-231/DNALK2) (Fig. 2A). Accordingly, Fig. 2B shows that the colony-forming rate
of MDA-MB-231/DNALK2 cells was decreased from 91.3±3.6%
(MDA-MB-231/RFP) and 93.9±5.6% (MDA-MB-231) to 34.0±5.3%
(MDA-MB-231/DNALK2). These results indicated that DNALK2 inhibited
the growth of MDA-MB-231 breast cancer cells.
Wound closure and Transwell invasion assays were
used to detect the invasion and migration abilities of the
MDA-MB-231/DNALK2 cells. Fig. 2C
shows that the wound closure rate decreased from 94.4±3.6%
(MDA-MB-231) and 91.7±2.9% (MDA-MB-231/RFP) to 41.2±5.7%
(MDA-MB-231/DNALK2) (P<0.05). Fig.
2D shows that invasive cell numbers decreased from 142.7±16.1
(MDA-MB-231) and 135.3±15.8 (MDA-MB-231/RFP) to 35.5±6.7
(MDA-MB-231/DNALK2) (P<0.05).
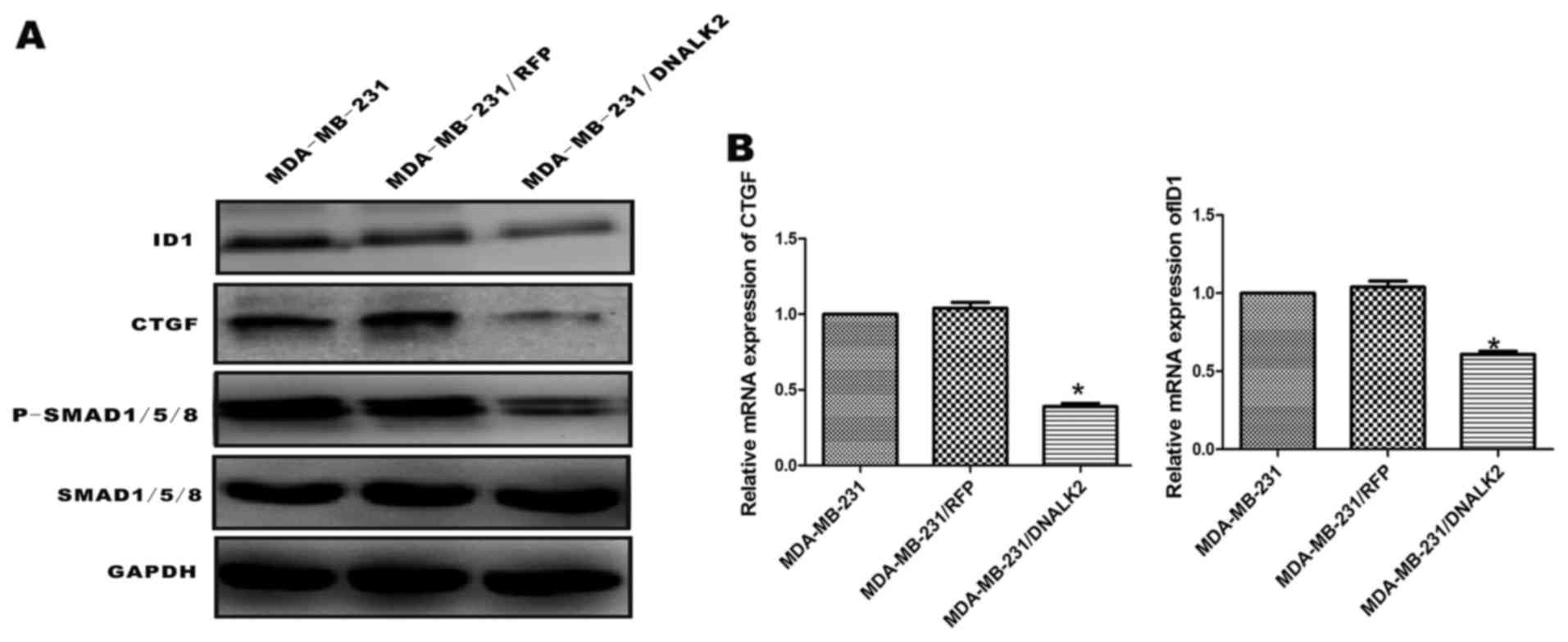
DNALK2 inhibits the BMP/SMAD signaling
pathway and downregulates ID1 and CTGF expression in MDA-MB-231
cells
In a previous study, DNALK2 was found to inhibit the
growth, migration, and invasiveness of MDA-MB-231 breast cancer
cells. To establish whether the BMP/SMAD signaling pathway is
functional, the MDA-MB-231 cells were stimulated with DNALK2, and
phosphorylation of SMAD1/5/8 was assessed using specific
anti-phosphosite antibodies via immunoblot analysis. The
recombinant MDA-MB-231/DNALK2 cells exhibited lower levels of
phosphorylated SMAD1/5/8 than levels noted in the MDA-MB-231/RFP
and MDA-MB-231 cells, while the total SMAD1/5/8 protein was similar
in the three groups (Fig. 3A). ID1
and CTGF expression levels in the recombinant MDA-MB-231/DNALK2
cells were assessed using western blot analysis and real-time
RT-PCR, which are reportedly related with the proliferation and
invasion of tumor cells. CTGF protein (40 kDa) and ID1 protein (16
kDa) expression levels were also significantly lower in the
MDA-MB-231/DNALK2 cells than these levels in the MDA-MB-231/RFP and
MDA-MB-231 cells (Fig. 3A). ID1 and
CTGF mRNA was downregulated in the MDA-MB-231/DNALK2 cells
(Fig. 3B) (CTGF,
2−ΔΔCT=0.39, P<0.05; ID1, 2−ΔΔCT=0.63,
P<0.05).
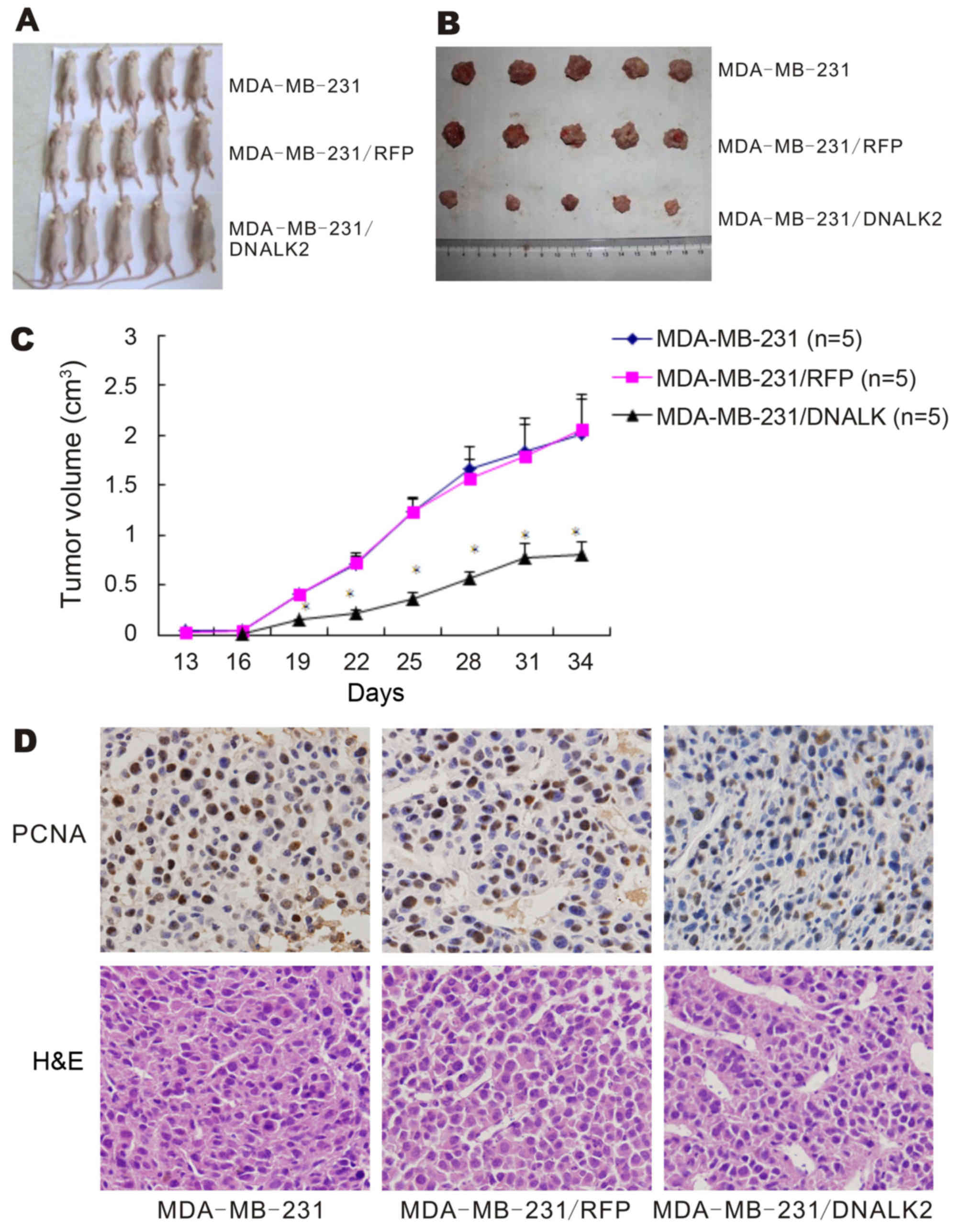
DNALK2 inhibits tumor formation
through the SMAD-dependent pathway in vivo
To investigate the effects of DNALK2 on the growth
of breast cancer cells in vivo, three groups of cells
(MDA-MB-231/DNALK2 as well as the two control cells MDA-MB-231/RFP
and MDA-MB-231) were subcutaneously implanted into nude mice. After
13 days, tumors were observed in the mice implanted with the two
control cell lines, but not in the mice implanted with the
MDA-MB-231/DNALK2 cells. After 16 days, tumors were observed in the
mice implanted with the MDA-MB-231/DNALK2 cells (Fig. 4A). Fig.
4B and C shows that the tumor volume was decreased from
2.005±0.351 (MDA-MB-231) and 2.054±0.273 (MDA-MB-231/RFP)to
0.804±0.135 (MDA-MB-231/DNALK2 group) on day 31 (P<0.001).
Hence, dominant-negative ALK2 decreased the proliferation of breast
cancer cells in nude mice and significantly decreased tumor burden
over time. These results were confirmed by immunohistochemical
analysis of PCNA expression in the tumor sections. The
PCNA-positive cell rate decreased from 55.9±6.5 (MDA-MB-231) and
53.7±4.8 (MDA-MB-231/RFP) to 21.4±4.9% (MDA-MB-231/DNALK2 group)
(P<0.001) as shown in Fig.
4D.
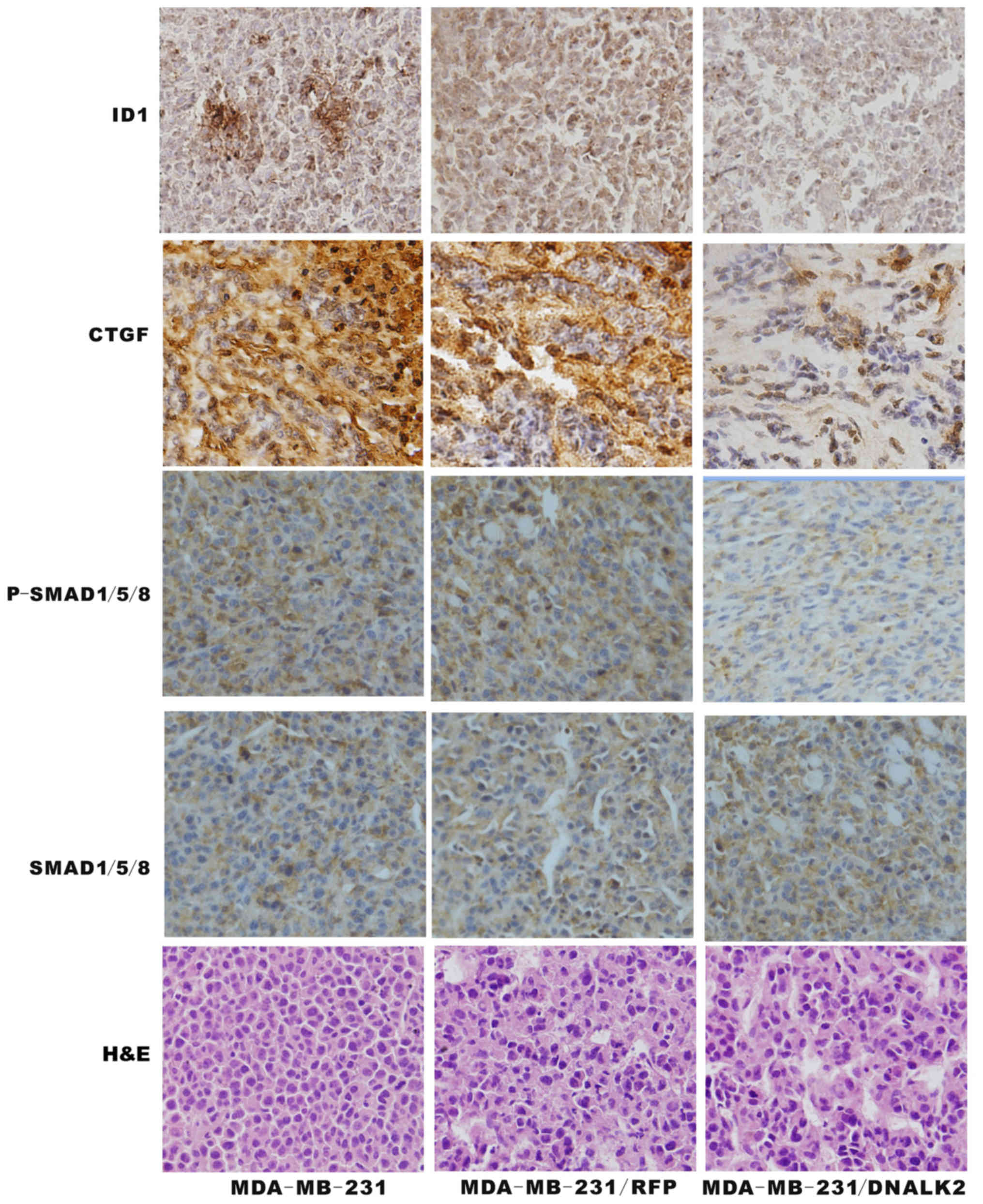
To establish whether the BMP/SMAD signaling pathway
was functional in the MDA-MB-231/DNALK2 cells, total SMAD1/5/8,
p-SMAD1/5/8, CTGF and ID1 was assessed using specific antibodies
via immunohistochemistry analysis. The p-SMAD1/5/8, CTGF and ID1
protein expression was decreased in the MDA-MB-231/DNALK2 group
compared with the MDA-MB-231/RFP and MDA-MB-231 groups as determine
by immunochemical staining (Fig.
5). These data suggest that DNALK2 is a potent inhibitor of the
growth and metastasis of breast cancer cells.
Discussion
ALK2 belongs to the TGF-β superfamily type I
receptors, which is expressed in various carcinoma cell lines of
ovarian, prostate, pancreatic, and breast origin (5,9,14).
More recently, ALK2 has been linked to carcinogenesis and tumor
progression. ALK2 has been described as a pleiotropic receptor in
carcinoma cell regulation, dependent on the cell type,
developmental stage, and microenvironment of the carcinoma cells.
For example ALK2 acts as a tumor suppressor in prostate cancer and
myeloma cells, but as an oncogene in ovarian and lung cancer cell
lines (8,18,19,22).
BMP7 binds to ALK2 to induce mesenchymal-epithelial transition
(MET) in melanoma tumor cells, and ultimately inhibits metastasis
by regulating downstream of Twist protein expression (23). BMP9 binds to ALK2 to activate the
BMP/SMAD signaling pathway to enhance the proliferation of ovarian
cancer cells (18).
Type I receptors (ALK3 and ALK6) are expressed in
primary breast cancer samples even in several breast cancer cell
lines. Dominant-negative ALK3 was found to result in decreased
invasiveness and metastasis in a mouse xenograft model (16). Reduced expression of ALK6 increased
proliferation of breast cancer cells (11). ALK2 belongs to the type I receptors,
but the function of ALK2 in breast cancer is unknown. There are few
studies concerning its function in the development of breast
cancer.
In the present study, MDA-MB-231 cells were infected
with AdDNALK2. MTT results showed that the number of MDA-MB-231
cells began to decline on the third day after DNALK2 adenovirus
infection, obviously decreased on the fifth day; we used a colony
forming assay to determine that the cell colony number in the
MDA-MB-231/DNALK2 group was significantly decreased compared to the
control group, which further confirmed the MTT results. Wound
closure assay showed that the wound healing rate of the
MDA-MB-231/DNALK2 cell group was significantly decreased compared
to the MDA-MB-231/RFP cells. Transwell invasion assay showed that
the number of invasive MDA-MB-231 cells pretreated with AdDNALK2
were obviously reduced compared with the control group, in
vitro. These results showed that the cell proliferation,
invasion and migration abilities of MDA-MB-231 cells were reduced
after infection with the DNALK2 adenovirus. ALK2 is an important
factor influencing breast cancer growth and metastasis and acts as
a potential target for inhibiting the metastasis of breast
cancer.
CTGF, a new induced fibrosis factor, is a CCN (CTGF,
Cyr61 and Nov) family member, and is associated with tumor
proliferation and metastasis. It is involved in the occurrence of
many diseases and tumors such as system scleroderma,
atherosclerosis, liver fibrosis and biliary atresia (24–26),
but the distribution and biological function of CTGF are different
in different types of tumor tissues. It can regulate tumor
progression positively or negatively. ALK2 acts as an oncogene in
liver cell carcinoma, gastric cancer, glioma, breast cancer,
esophageal adenocarcinoma resulting in reduced survival and poor
patient prognosis (27), while in
pancreatic cancer, esophageal squamous cell carcinoma, colorectal
cancer, laryngeal squamous cell carcinoma, thyroid cancer and lung
cancer, ALK2 acts as a tumor suppressor to inhibit tumor growth
(28). CTGF and ID1 exhibit high
expression in breast cancer, and their expression is correlated
with tumor size, lymph node status and age; expression change in
cancer cells and tissues is of great significance (28,29).
We found that CTGF and ID1 expression at the mRNA and protein
levels was significantly reduced after DNALK2 infection in the
MDA-MB-231 cells, suggesting that DNALK2 is likely to inhibit tumor
proliferation, invasion and migration by downregulation of CTGF and
ID1.
Animal models are important tools with which to
research the biological behavior of tumor cells in vivo.
Thus, we successfully constructed a breast cancer subcutaneous
tumor model in nude mice and found that DNALK2 significantly
inhibited the growth of breast cancer cells in nude mice. The
expression of CTGF and ID1 was also downregulated in the
MDA-MB-231/DNALK2 group at the same time. The specific signaling
pathway and related mechanism warrant further study. In the present
study, one of the critical mechanisms underlying the inhibiiton of
the growth and metastasis of breast cancer cells by DNALK2 is by
reducing CTGF and ID1 expression to inhibit the BMP/SMAD cell
signaling pathway.
In conclusion, ALK2 is an important factor that can
inhibit breast cancer growth, invasion and migration through
classical BMP/SMAD cell signaling (BMPs, BMPRs Smads - gene
transcription in the nucleus - corresponding protein production)
(30), ALK2 has profound potential
in the prognosis and therapy of breast cancer metastasis. Further
investigation may lead to a novel therapeutic approach for breast
cancer. Yet, we need further understanding of the processes
involved in breast cancer metastasis and we must develop effective
preventive strategies.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172017), the
Natural Science Foundation Project of Chongqing Dducation Committee
(grant no. KJ1500202) and the Natural Science Foundation Project of
Yong Chuan Hospital (grant no. YJQN201401/YJZQN201517).
References
|
1
|
Raida M, Clement JH, Leek RD, Ameri K,
Bicknell R, Niederwieser D and Harris AL: Bone morphogenetic
protein 2 (BMP-2) and induction of tumor angiogenesis. J Cancer Res
Clin Oncol. 131:741–750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lian WJ, Liu G, Liu YJ, Zhao ZW, Yi T and
Zhou HY: Downregulation of BMP6 enhances cell proliferation and
chemoresistance via activation of the ERK signaling pathway in
breast cancer. Oncol Rep. 30:193–200. 2013.PubMed/NCBI
|
|
3
|
Buijs JT, Petersen M, van der Horst G and
van der Pluijm G: Bone morphogenetic proteins and its receptors;
therapeutic targets in cancer progression and bone metastasis? Curr
Pharm Des. 16:1291–1300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boeuf S, Bovée JV, Lehner B, van den Akker
B, van Ruler M, Cleton-Jansen AM and Richter W: BMP and TGFbeta
pathways in human central chondrosarcoma: Enhanced endoglin and
Smad 1 signaling in high grade tumors. BMC Cancer. 12:4882012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ye L, Mason MD and Jiang WG: Bone
morphogenetic protein and bone metastasis, implication and
therapeutic potential. Front Biosci (Landmark Ed). 16:865–897.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo D, Huang J and Gong J: Bone
morphogenetic protein 4 (BMP4) is required for migration and
invasion of breast cancer. Mol Cell Biochem. 363:179–190. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lim J, Tu X, Choi K, Akiyama H, Mishina Y
and Long F: BMP-Smad4 signaling is required for precartilaginous
mesenchymal condensation independent of Sox9 in the mouse. Dev
Biol. 400:132–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olsen OE, Wader KF, Misund K, Våtsveen TK,
Rø TB, Mylin AK, Turesson I, Størdal BF, Moen SH, Standal T, et al:
Bone morphogenetic protein-9 suppresses growth of myeloma cells by
signaling through ALK2 but is inhibited by endoglin. Blood Cancer
J. 4:e1962014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lei R, Zhang K, Liu K, Shao X, Ding Z,
Wang F, Hong Y, Zhu M and Li H and Li H: Transferrin receptor
facilitates TGF-β and BMP signaling activation to control
craniofacial morphogenesis. Cell Death Dis. 7:e22822016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brederlau A, Faigle R, Elmi M, Zarebski A,
Sjöberg S, Fujii M, Miyazono K and Funa K: The bone morphogenetic
protein type Ib receptor is a major mediator of glial
differentiation and cell survival in adult hippocampal progenitor
cell culture. Mol Biol Cell. 15:3863–3875. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bokobza SM, Ye L, Kynaston HE, Mansel RE
and Jiang WG: Reduced expression of BMPR-IB correlates with poor
prognosis and increased proliferation of breast cancer cells.
Cancer Genomics Proteomics. 6:101–108. 2009.PubMed/NCBI
|
|
12
|
Alsamarah A, LaCuran AE, Oelschlaeger P,
Hao J and Luo Y: Uncovering molecular bases underlying bone
morphogenetic protein receptor inhibitor selectivity. PLoS One.
10:e01322212015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herrera-Esparza R, Pacheco-Tovar D,
Bollain-Y-Goytia JJ, Del Muro F Torres, Ramírez-Sandoval R,
Pacheco-Tovar MG, Castañeda-Ureña M and Avalos-Díaz E: An activin
receptor IA/activin-like kinase-2 (R206H) mutation in
fibrodysplasia ossificans progressiva. Case Rep Genet.
2013:2603712013.PubMed/NCBI
|
|
14
|
Joziasse IC, Smith KA, Chocron S, van
Dinther M, Guryev V, van de Smagt JJ, Cuppen E, Ten Dijke P, Mulder
BJ, Maslen CL, et al: ALK2 mutation in a patient with Down's
syndrome and a congenital heart defect. Eur J Hum Genet.
19:389–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song T, Wang W, Xu J, Zhao D, Dong Q, Li
L, Yang X, Duan X, Liang Y, Xiao Y, et al: Fibroblast growth factor
2 inhibits bone morphogenetic protein 9-induced osteogenic
differentiation of mesenchymal stem cells by repressing Smads
signaling and subsequently reducing Smads dependent up-regulation
of ALK1 and ALK2. Int J Biochem Cell Biol. 45:1639–1646. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chakkalakal SA, Zhang D, Culbert AL,
Convente MR, Caron RJ, Wright AC, Maidment AD, Kaplan FS and Shore
EM: An Acvr1 R206H knock-in mouse has fibrodysplasia ossificans
progressiva. J Bone Miner Res. 27:1746–1756. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomas PS, Sridurongrit S, Ruiz-Lozano P
and Kaartinen V: Deficient signaling via Alk2 (Acvr1) leads to
bicuspid aortic valve development. PLoS One. 7:e355392012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Herrera B, van Dinther M, Ten Dijke P and
Inman GJ: Autocrine bone morphogenetic protein-9 signals through
activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian
cancer cell proliferation. Cancer Res. 69:9254–9262. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsai CL, Tsai CN, Lin CY, Chen HW, Lee YS,
Chao A, Wang TH, Wang HS and Lai CH: Secreted stress-induced
phosphoprotein 1 activates the ALK2-SMAD signaling pathways and
promotes cell proliferation of ovarian cancer cells. Cell Rep.
2:283–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Romero D, Terzic A, Conley BA, Craft CS,
Jovanovic B, Bergan RC and Vary CP: Endoglin phosphorylation by
ALK2 contributes to the regulation of prostate cancer cell
migration. Carcinogenesis. 31:359–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang K, Feng H, Ren W, Sun X, Luo J, Tang
M, Zhou L, Weng Y, He TC and Zhang Y: BMP9 inhibits the
proliferation and invasiveness of breast cancer cells MDA-MB-231. J
Cancer Res Clin Oncol. 137:1687–1696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Langenfeld E, Hong CC, Lanke G and
Langenfeld J: Bone morphogenetic protein type I receptor
antagonists decrease growth and induce cell death of lung cancer
cell lines. PLoS One. 8:e612562013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Na YR, Seok SH, Kim DJ, Han JH, Kim TH,
Jung H, Lee BH and Park JH: Bone morphogenetic protein 7 induces
mesenchymal-to-epithelial transition in melanoma cells, leading to
inhibition of metastasis. Cancer Sci. 100:2218–2225. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leask A, Denton CP and Abraham DJ:
Insights into the molecular mechanism of chronic fibrosis: the role
of connective tissue growth factor in scleroderma. J Invest
Dermatol. 122:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cozzolino M, Biondi ML, Banfi E, Riser BL,
Mehmeti F, Cusi D and Gallieni M: CCN2 (CTGF) gene polymorphism is
a novel prognostic risk factor for cardiovascular outcomes in
hemodialysis patients. Blood Purif. 30:272–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim GJ, Rhee H, Yoo JE, Ko JE, Lee JS, Kim
H, Choi JS and Park YN: Increased expression of CCN2, epithelial
membrane antigen, and fibroblast activation protein in
hepatocellular carcinoma with fibrous stroma showing aggressive
behavior. PLoS One. 9:e1050942014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lacle MM, van Diest PJ, Goldschmeding R,
van der Wall E and Nguyen TQ: Expression of connective tissue
growth factor in male breast cancer: clinicopathologic correlations
and prognostic value. PLoS One. 10:e01189572015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jacobson A and Cunningham JL: Connective
tissue growth factor in tumor pathogenesis. Fibrogenesis Tissue
Repair. 5:(Suppl 1). S82012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wazir U, Jiang WG, Sharma AK, Newbold RF
and Mokbel K: The mRNA expression of inhibitors of DNA binding-1
and −2 is associated with advanced tumour stage and adverse
clinical outcome in human breast cancer. Anticancer Res.
33:2179–2183. 2013.PubMed/NCBI
|
|
30
|
Clementi C, Tripurani SK, Large MJ, Edson
MA, Creighton CJ, Hawkins SM, Kovanci E, Kaartinen V, Lydon JP,
Pangas SA, et al: Activin-like kinase 2 functions in
peri-implantation uterine signaling in mice and humans. PLoS Genet.
9:e10038632013. View Article : Google Scholar : PubMed/NCBI
|