Introduction
Lung cancer is the most prevalent histological
cancer subtype and is the leading cause of cancer-related death
(1). Current standard treatment for
lung cancer contains chemotherapy and radiotherapy. However, major
problems with the above treatment modalities are the lack of tumor
specificity giving rise to dose-limiting toxicity, and resistance
to the treatments, as well as predicted poor outcomes for lung
metastatic treatment (2–4). Thus, novel therapeutic strategies are
in high demand.
Gene therapy has been proved to be a promising
strategy for the treatment of genetically based diseases, such as
lung cancer (5–8). Using this strategy, cancer
cytotoxicity can be obtained by replacing mutated genes with
functional analogues or introducing a suicide gene into the
malignant cells (2,9). However, one of the major challenges of
gene-based cancer therapy is to achieve specific, efficient and
safe systemic delivery of genes in vivo (10). Several delivery strategies have been
established to overcome the hurdles of in vivo gene delivery
and enhance the efficacy of cancer therapy: modified
oligonucleotides, nanocarriers, and tumor-targeted nanocarriers
(11–13). The third-generation delivery
strategy (tumor-targeting approach) has recently emerged to add
surface modifications to the nanocarriers, which allow specific
binding to the target cancer cells and deliver the gene into the
cancer cells through receptor-mediated endocytosis.
Nanocarriers such as cationic liposomes, lipoplexes,
nanoparticles, micelles, solid lipid nanoparticles (SLN),
nanostructured lipid carriers (NLC), exhibit many advantages as
potential candidates for efficient non-viral gene delivery systems
(14–16). Among them, NLC, composed of a blend
of the solid lipid and the liquid lipid, exhibit superior
advantages over other colloidal carriers mentioned above: an
increase in chemical stability of the incorporated drugs, higher
drug loading capacity, lower toxicity and controlled release
(17). In order to enhance the
tumor target, transferrin (Tf) and hyaluronic acid (HA) dual
ligand-decorated NLC for targeted gene delivery were designed in
the current study.
Tf, a 698-residual protein, has been used as a
cancer-targeting agent in multiple delivery systems since the
transferring receptor is overexpressed in most cancer cells
containing lung carcinoma cells (18). HA is a biodegradable, biocompatible,
and polyanionic glycol amino glycan (19). It has an important biological role
in the cell adhesion, migration, invasion, proliferation,
differentiation and angiogenesis by binding to cell specific
receptors such as glycoprotein CD44 (20). It has already been reported that the
CD44 receptors are highly expressed in majority of non-small cell
lung cancers (NSCLC) (21). Pan
et al constructed HA and Tf co-modified
Fe3O4 nanoparticles for dual-targeting
magnetic resonance imaging of tumors in vivo. Results showed
that this system had a high targeting ability towards tumor cells
and excellent biocompatibility (22). To date, no studies have reported HA
and Tf dual ligand-decorated NLC for targeted gene delivery to lung
carcinoma cells.
Lung adenocarcinoma A549 cells are adenocarcinomic
human alveolar basal epithelial cells (23). The A549 cell line was first
developed in 1972 by Giard et al through the removal and
culturing of cancerous lung tissue in the explanted tumor of a
58-year-old Caucasian male. In nature, these cells are squamous and
responsible for the diffusion of some substances, such as water and
electrolytes, across the alveoli of lungs. When A549 cells are
cultured in vitro, they grow as monolayer cells, adherent or
attaching to the culture flask. Another characteristic of these
cells is that they are able to synthesize lecithin and contain high
level of unsaturated fatty acids, which are important to maintain
the membrane phospholipids in cells. A549 cells are widely used as
an in vitro model for a type II pulmonary epithelial cells
for drug metabolism and as a transfection host. Thus, A549 cells
were used also in this work.
The aim of the present study was to construct dual
ligand- (Tf and HA) modified NLC, the novel nanocarrier for
delivery of a gene, to achieve the tumor target. Plasmid-enhanced
green fluorescent protein was used as the model DNA (pDNA). We
evaluated the systemic delivery efficiency using human lung
adenocarcinoma A549 cell-bearing mouse model.
Materials and methods
Materials
Plasmid encoding enhanced green fluorescent protein
(pEGFP) was obtained from Clontech (Palo Alto, CA, USA).
Transferrin (Tf), glycerol monostearate (GM),
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
(MTT), soybean phosphatidylcholine (SPC) were purchased from
Sigma-Aldrich Co. Ltd. (Shanghai, China). Soybean oil (SO) was
purchased from Guangzhou Hanfang Pharmaceutical Co., Ltd.
(Guangzhou, China). Hyaluronic acid (HA, MW 5 kDa) was provided by
Shandong Freda Biochem Co., Ltd. (Ji'nan, China).
1,2-dioleoyl-3-trimethylammonium propane (DOTAP) was purchased from
Avanti Polar Lipids (Alabaster, AL, USA). Polyethylene
glycol-distearoylphosphatidylethanolamine (PEG-DSPE) was purchased
from CordenPharma International (Plankstadt, Germany). Quant-iT™
PicoGreen® dsDNA quantitation reagent was obtained from
Invitrogen by Life Technologies (Carlsbad, CA, USA).
Lipofectamine® 3000 transfection reagent was obtained
from Thermo Fisher Scientific (Waltham, MA, USA). All of the other
chemicals were of analytical or high performance liquid
chromatography (HPLC) reagent grade.
Cells
A549 cells were obtained from American Type Culture
Collection (ATCC, Manassas, VA) and cultured in Dulbecco's modified
Eagle's medium (DMEM, Sigma, St. Louis, MO, USA) supplemented with
10% fetal bovine serum (FBS) (Fisher Chemicals, Fairlawn, NJ, USA)
in a 5% CO2 fully humidified atmosphere.
Animals
BALB/c nude mice (4–6 weeks old, 18–22 g weight)
were purchased from Beijing Vital River Experimental Animal
Technical Co., Ltd (Beijing, China). Mice were maintained under
25°C and 55% of humidity with free access to standard water and
chow. Lung cancer-bearing mice were prepared by subcutaneous
inoculating a suspension of A549 cells (1×107 cells)
into the right armpit of BALB/c mice (24). Tumors were 8–10 mm in diameter
before initiation of the in vivo studies. Animal experiments
were complied with the Animal Management Rules of the Ministry of
Health of the People's Republic of China.
Synthesis of Tf-PEG-DSPE and
HA-PEG-DSPE
Tf-PEG-DSPE ligands were synthesized using the
method reported previously (24).
Briefly, Tf was first modified with one equivalent of Traut's
reagent to complete thiolation of Tf. The PEG-DSPE was then added
into one equivalent of thiolated Tf solution, and the mixture was
incubated for 2 h at room temperature, with gentle stirring. The
product was dialyzed against Milli-Q water for 24 h to form
Tf-PEG-DSPE solution. The mixture was centrifuged at 10,000 × g for
30 min at 4°C, and then resuspended in PBS (pH 7.4).
HA-PEG-DSPE ligands were synthesized as follows
(25). Briefly, PEG-DSPE and one
equivalent of HA were dissolved in 20 ml of N,N-dimethylformamide
(DMF), followed by adding 2 equivalent of
1-ethyl-3-(3-dimethylamino-propyl) carbodimide (EDC) and one
equivalent of N-hydroxysuccinimide (NHS). The reaction proceeded
overnight at room temperature under the protection of nitrogen.
Then, the resulting solution was concentrated under reduced
pressure and dialyzed using a dialysis bag (molecular weight
cut-off 12,000-14,000). Finally, the HA-PEG-DSPE was obtained after
lyophilization using a freeze dryer.
Chemical structures of Tf-PEG-DSPE and HA-PEG-DSPE
were analyzed by 1H-NMR spectroscopy. Tf-PEG-DSPE, δ
(ppm): 0.89 (CH3), 1.21–1.86 (CH2 of
PEG-DSPE), 2.45 (CH2CO, NHCO), 3.28 (CH2N),
3.36–3.48 (OCH2), 4.03 (NHCO), and 6.54 (NH).
HA-PEG-DSPE, δ (ppm): 0.95 (CH3), 1.31–1.95
(CH2 of PEG-DSPE), 2.01 (OH of HA), 2.29 (NHCO), 2.65
(CH2CO), 3.03–3.51 (CH of HA), 3.83 (OCH2),
4.16 (NHCO), and 10.84 (OH).
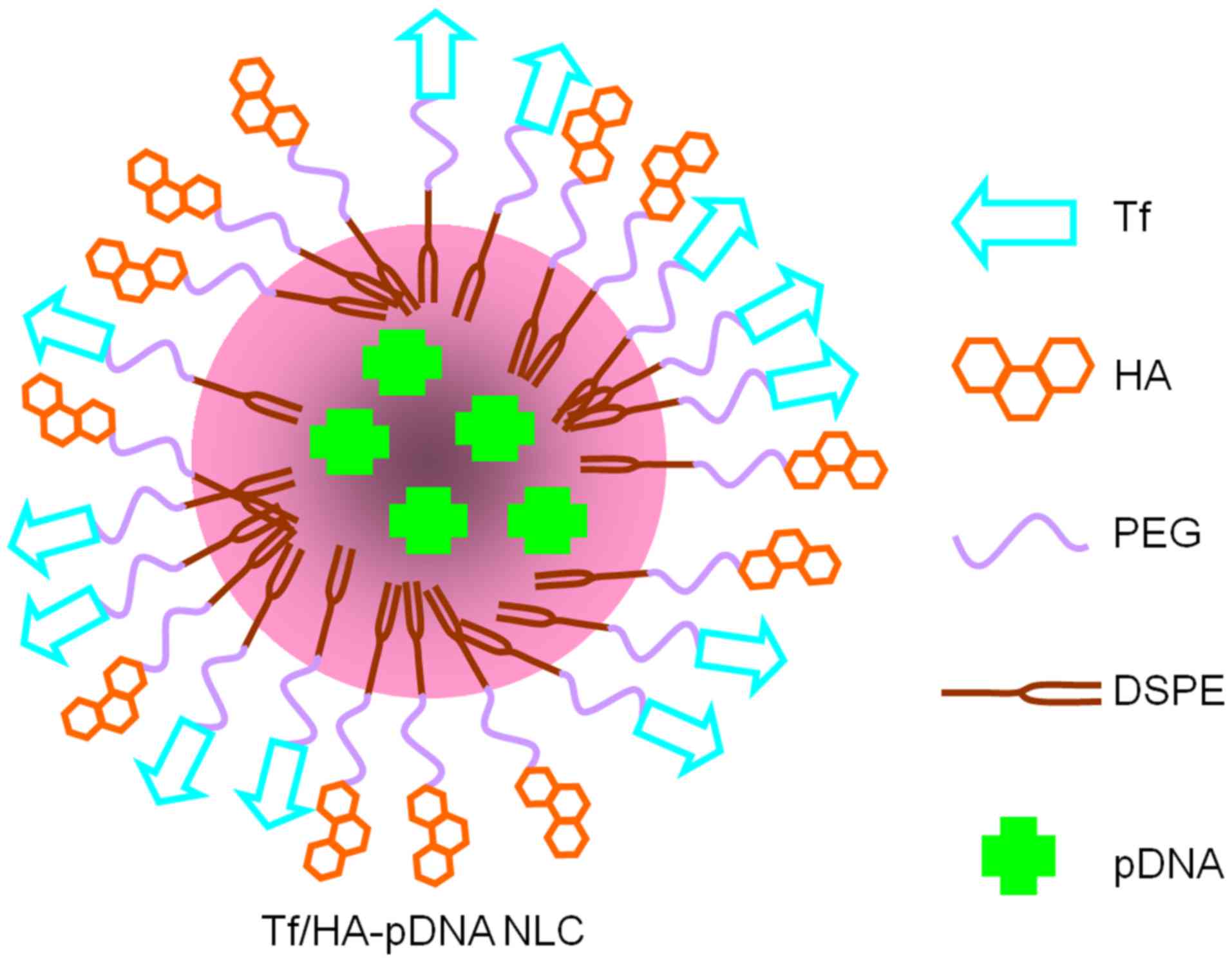
Preparation of NLC and incorporation
of pDNA
The Tf-PEG-DSPE and HA-PEG-DSPE modified, pDNA
incorporated NLC (Tf/HA-pDNA NLC) were prepared according to
previous studies (26). Briefly,
500 mg GM, 200 mg SPC were dissolved in 10 ml warm SO (70°C) to
obtained the oil phase. Ten milliliters (1 mg/ml) pDNA, 1 g
Tf-PEG-DSPE, 1 g HA-PEG-DSPE, and 50 mg DOTAP were dissolved in 30
ml deionized water to form an aqueous phase. The resultant oil
phase was quickly dispersed into aqueous phase under mechanical
stirring with 400 rpm in water bath (70°C) for 5 min. The obtained
complex solution was cooled to room temperature until Tf/HA-pDNA
NLC was obtained (Fig. 1).
Tf-PEG-DSPE modified, pDNA incorporated NLC (Tf-pDNA
NLC) was prepared as described above only without HA-PEG-DSPE in
aqueous phase, using 2 g of Tf-PEG-DSPE in total. HA-PEG-DSPE
modified, pDNA incorporated NLC (HA-pDNA NLC) was prepared as
described above only without Tf-PEG-DSPE in aqueous phase, using 2
g of HA-PEG-DSPE in total. Non-modified pDNA incorporated NLC (pDNA
NLC) was prepared as described above only without Tf-PEG-DSPE and
HA-PEG-DSPE in aqueous phase, using 2 g of PEG-DSPE in total. Blank
NLC was prepared as described above without Tf-PEG-DSPE,
HA-PEG-DSPE, and pDNA in aqueous phase, using 2 g of PEG-DSPE
dissolved in 40 ml deionized water instead.
Preparation of Lipofectamine 3000
incorporated pDNA
Lipofectamine 3000 incorporated pDNA (pDNA LP) was
prepared (27). Briefly, 100 µl of
pDNA (1 mg/ml) was mixed with 200 µl of Lipofectamine 3000 by
vortexing for 30 sec. The mixture was then incubated for 30 min at
room temperature-facilitated formation of the pDNA LP.
Characterization
Morphology of Tf/HA-pDNA NLC
Morphology of Tf/HA-pDNA NLC was characterized by
transmission electron microscopy (TEM) (28). Samples were placing onto copper grid
and air-drying, followed by negative staining with one drop of a 3%
aqueous solution of sodium phosphotungstate. The air-dried samples
were subsequently examined under the transmission electron
microscope (JEM-2100; JEOL, Tokyo, Japan).
Particle size and ζ-potential
Particle size, polydispersity index (PDI), and
ζ-potential of each sample was measured at room temperature by Zeta
Sizer Nano ZS apparatus (Malvern, Southborough, MA, USA) (29). Samples were prepared in disposable
capillary cells without dilution. The measurements were performed
under conditions of low ionic strength where the surface charge of
the particles can be measured accurately. The average particle size
was reflected in volume mean diameter.
Gene loading capacity
Gene loading capacity (GL) of each sample was
determined by the PicoGreen-fluorometry method (30). Briefly, pDNA was separated from the
carriers by centrifugation at 10,000 rpm and 4°C for 20 min. The
supernatant was collected and the concentration of pDNA was
assessed using a fluorescence spectrophotometer (F-4500, Hitachi
Science and Technology, Tokyo, Japan).
Serum stability
In terms of serum protection test, samples were
immersed in cell culture medium containing 10% fetal bovine serum
and co-incubated at 37°C for 24 h. Then samples were tested in
terms of size and GL (31,32). At the same time, the naked pDNA was
also treated by the same method as a control.
In vitro release studies
The in vitro release studies of NLC were
performed in TE buffer (Tris-HCl 10 mM, EDTA 1 mM, pH 7.4)
(33). Typically, pDNA loaded NLC
solution (equivalent to 2 µg pDNA) were suspended in 1 ml TE buffer
in Eppendorf® tubes at 37°C shaking water bath at 100
rpm. Separate tubes were used for each data point. At predetermined
time intervals, the NLC suspensions were centrifuged (10,000 rpm,
20 min) and the amount of DNA released in the supernatant was
analyzed by PicoGreen-fluorometry method as indicated above.
Background readings were obtained using the supernatants from blank
NLC.
In vitro cell viability
In vitro cell viability of each sample in
A549 cells was ascertained by MTT colorimetric assay (16). Cells were seeded in a 96-well cell
culture plate at an initial density of 105 cells/well
and incubated in 200 µl of RPMI-1640 supplemented with 10% FBS and
antibiotics in 5% CO2 incubator at 37°C. After 24 h, the
culture medium was replaced by 200 µl of fresh serum-free RPMI-1640
medium with different concentrations (10, 20, 50, 100, 150, 200
µg/ml) of the samples. After incubating for 24 h, the effect of
different treatments on cell viability was evaluated by MTT assay.
Typically, 5 mg/ml of MTT in PBS were added to each well reaching a
final concentration of 0.5 mg MTT/ml and incubated for 4 h. Then
the supernatants were removed and the formazan crystals were
dissolved in 100 µl DMSO. Aliquots were drawn from each well and
the absorbance at 570 nm was determined with a microplate reader
(US/680, Bio-Rad, Hercules, CA, USA). Untreated cells were taken as
control with 100% viability and cells without addition of MTT were
used as blank to calibrate the spectrophotometer to zero
absorbance. The relative cell viability (%) compared to control
cells was calculated by Asample/Acontrol
×100.
In vitro gene transfection
In vitro gene transfection efficiency studies
were evaluated using flow cytometry method. Flow cytometry is a
laser- or impedance-based, biophysical technology employed in cell
counting, cell sorting, biomarker detection and protein
engineering, by suspending cells in a stream of fluid and passing
them by an electronic detection apparatus. It allows simultaneous
multiparametric analysis of the physical and chemical
characteristics of up to thousands of particles per second.
In this study for the cells that were successfully
transfected should express green fluorescence and could be detected
and quantified after transfection (34). Cells were grown in 6-cm Petri dishes
seeding the cells at 105 cells/dish. After plating the
cells were incubated at 37°C for 24 h and transfection was
conducted at approximately 80% confluence. The culture medium was
aspirated from each dish and replaced with 2 ml of serum-free
RPMI-1640 medium, containing different samples (each sample
contained 10 µg pDNA). The cells were incubated in 5%
CO2 incubator at 37°C for 12 h. Then, the transfected
cells were washed with PBS and incubated at 37°C in 5 ml of fresh
serum-free RPMI-1640 medium containing FBS for 48 h to allow the
expression of the protein. Cells transfected by 10 µg of naked pDNA
were utilized as negative control. HA-PTX SLN with no pDNA was used
to exclude fluorescence which may be caused by SLN, PTX or HA.
After 36 and 72 h of incubation, the fluorescent
cells were quantified by flow cytometry. The cells were washed with
500 µl of PBS and detached with 500 µl of 0.25% trypsin added with
EDTA (1%, v/v). Then the cells were centrifuged at 1000 rpm, at 4°C
for 5 min, the supernatant was discarded, and the cells were washed
once with 1 ml of PBS, centrifuged again (1000 rpm, 4°C, 5 min),
the supernatant was discarded, and the cells were re-suspended in
300 µl of PBS and directly introduced to a BD FACSCalibur flow
cytometer (Becton Dickinson Medical Device Co. Ltd., Franklin
Lakes, NJ, USA).
In vivo gene delivery and expression
For in vivo gene delivery, lung
cancer-bearing BALB/c nude mice were randomly divided into several
groups and different samples were injected intravenously (24). The untreated group was used as
control. The mice were then euthanized at 72 h after injection, and
the tumor tissue samples were removed. The tumor tissues were
homogenized by pressing the samples through a 30-µm cell mesh with
the plunger of a 10-ml syringe. Erythrocyte lysis buffer was added
during homogenization to lyse the red blood cells. The homogenates
were washed three times with PBS containing 0.5% bovine serum
albumin and then filtered. The cells were obtained after
centrifugation (1000 rpm, 4°C, 5 min) and were seeded into 96-well
plates in 1 ml of DMEM with 10% FBS. The fluorescent cells were
washed twice with PBS and cells were directly observed using an
inversion fluorescence microscope (BX40, Olympus, Tokyo, Japan).
The cells were also quantified by flow cytometry the same way as
the ‘in vitro gene transfection’ section.
Data analysis
The experimental data were analyzed using the
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterization of Tf/HA-pDNA
NLC
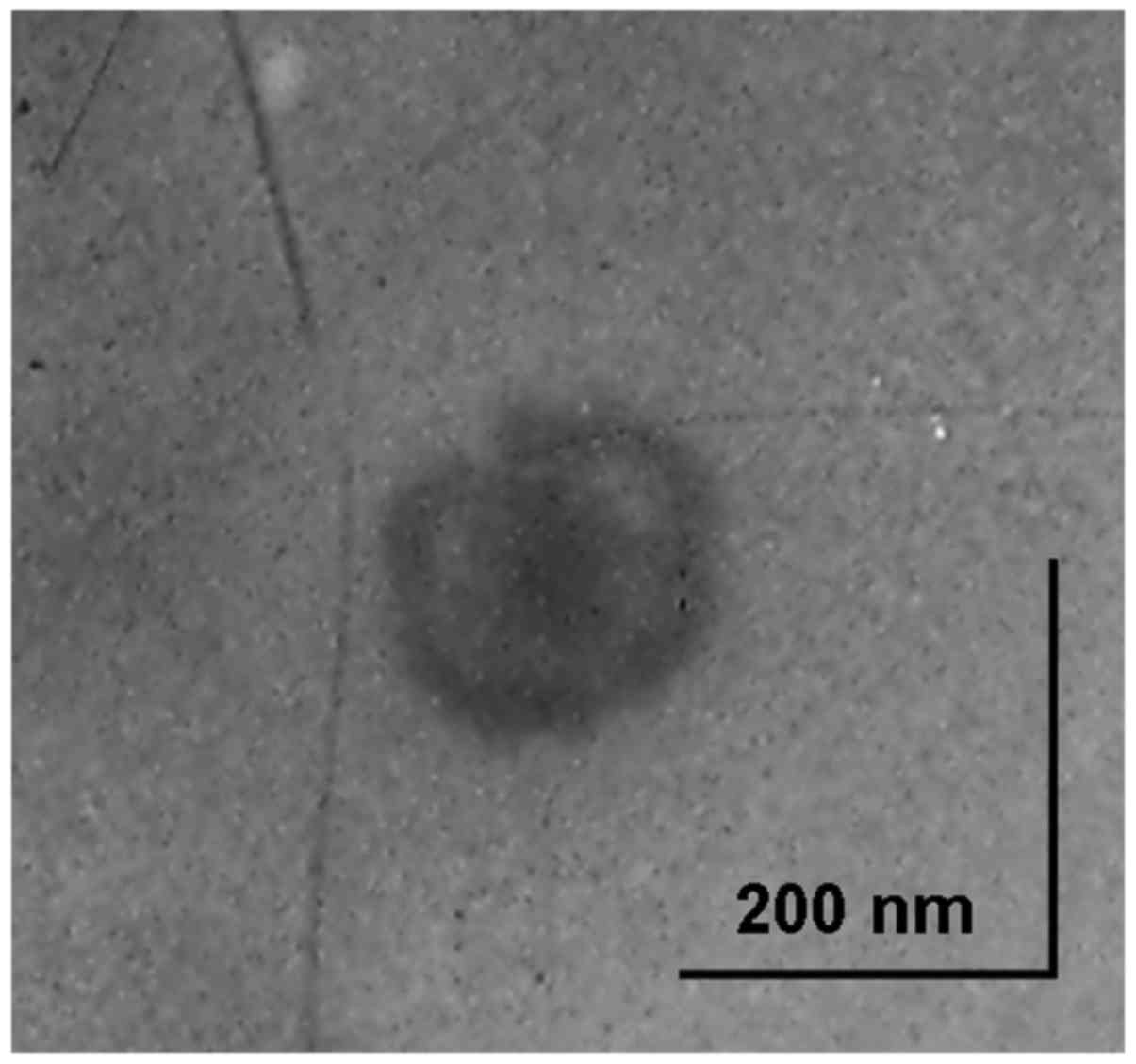
Morphology of Tf/HA-pDNA NLC is exhibited in
Fig. 2. Tf/HA-pDNA NLC revealed a
core-shell structured spherical morphology with grey coating
outside the core, which may be evidence of the modification of Tf
and HA ligands.
Table I showed that
Tf/HA-pDNA NLC had a larger particle size (189 nm) than pDNA NLC
(153 nm). After the decoration of Tf and HA, ζ-potential of NLC
system decreased from +38 mV (Tf/HA-pDNA NLC) to +20 mV (Tf/HA-pDNA
NLC). The GL of all pDNA loaded systems was ~90%.
 | Table I.Particle size, polydispersity index
(PDI), ζ-potential, and GL characterization. |
Table I.
Particle size, polydispersity index
(PDI), ζ-potential, and GL characterization.
| Characteristic | Particle size
(nm) | PDI | ζ-potential
(mV) | GL (%) |
|---|
| Tf/HA-pDNA NLC | 189.1±6.9 | 0.18 | +24.3±3.4 | 89.6±2.6 |
| Tf-pDNA NLC | 183.4±6.1 | 0.16 | +31.5±4.1 | 91.1±2.1 |
| HA-pDNA NLC | 191.6±7.2 | 0.19 | +20.3±3.9 | 88.7±3.0 |
| pDNA NLC | 153.4±5.3 | 0.14 | +38.4±3.2 | 89.4±2.4 |
| Blank NLC | 149.4±3.8 | 0.08 | +46.2±5.5 | N/A |
| pDNA LP | 176.1±5.4 | 0.12 | +35.9±4.4 | 90.3±2.8 |
Serum stability
Table II described
the changes of NLC systems in size and GL in the presence of serum.
The NLC systems remained stable up to 24 h without significant size
or GL changes. The results suggest that the NLC was stable in the
serum and this may help in the performance of the system in
vivo.
 | Table II.Serum stability in 10% FBS. |
Table II.
Serum stability in 10% FBS.
| Systems | Particle size
(nm) | GL (%) |
|---|
| Tf/HA-pDNA NLC | 192.3±8.9 | 87.2±3.8 |
| Tf-pDNA NLC | 187.1±9.4 | 88.6±3.4 |
| HA-pDNA NLC | 194.6±10.1 | 85.9±4.2 |
| pDNA NLC | 156.9±7.3 | 86.3±5.1 |
| Blank NLC | 152.4±6.3 | N/A |
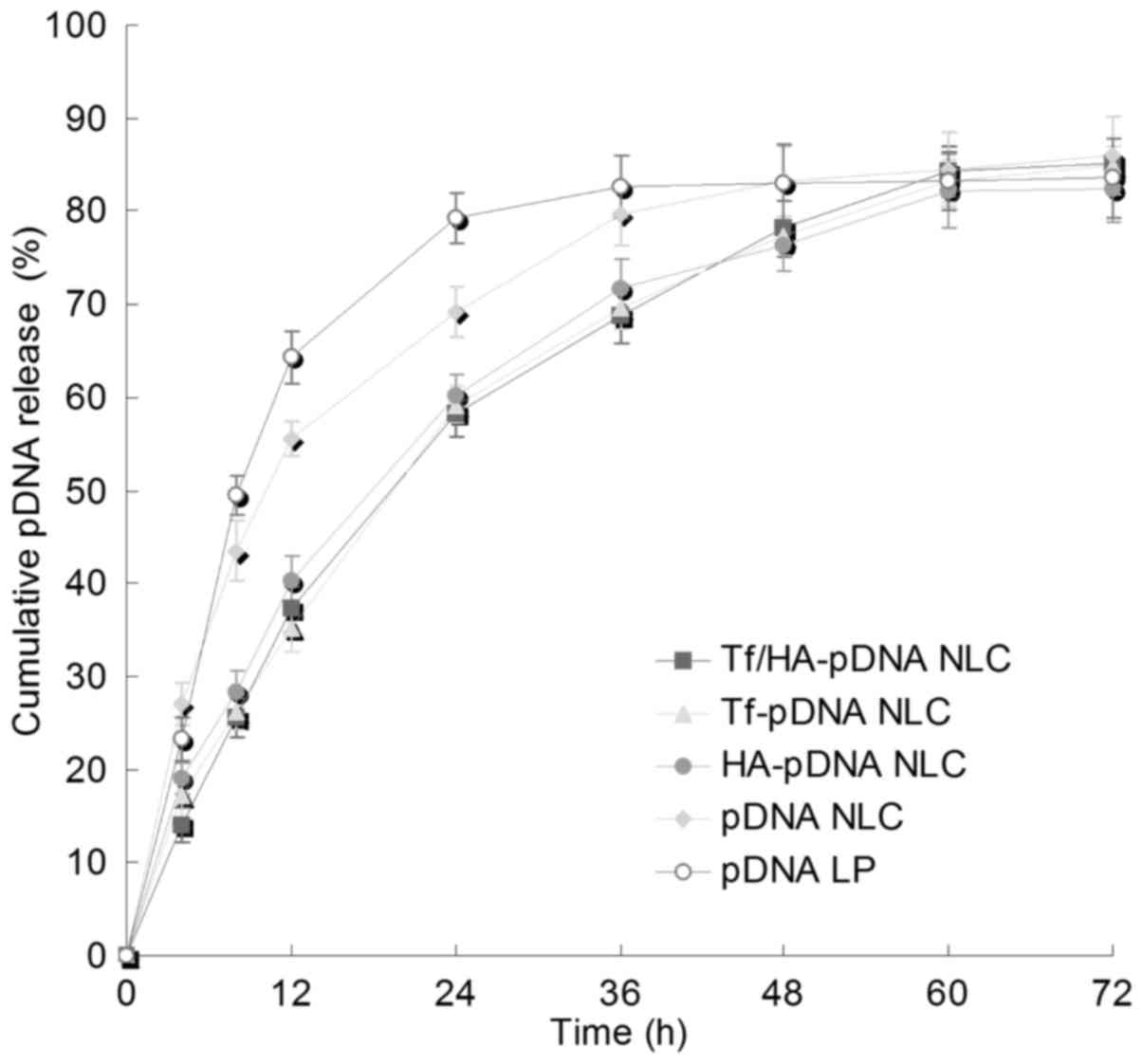
In vitro release
Fig. 3 illustrates
the pDNA release profiles of NLC systems. The pDNA release of Tf
and/or HA decorated NLC was slower than undecorated NLC. The
release of pDNA from different decorated NLC did not appear
significantly different. The release of pDNA LP was faster than the
NLC systems.
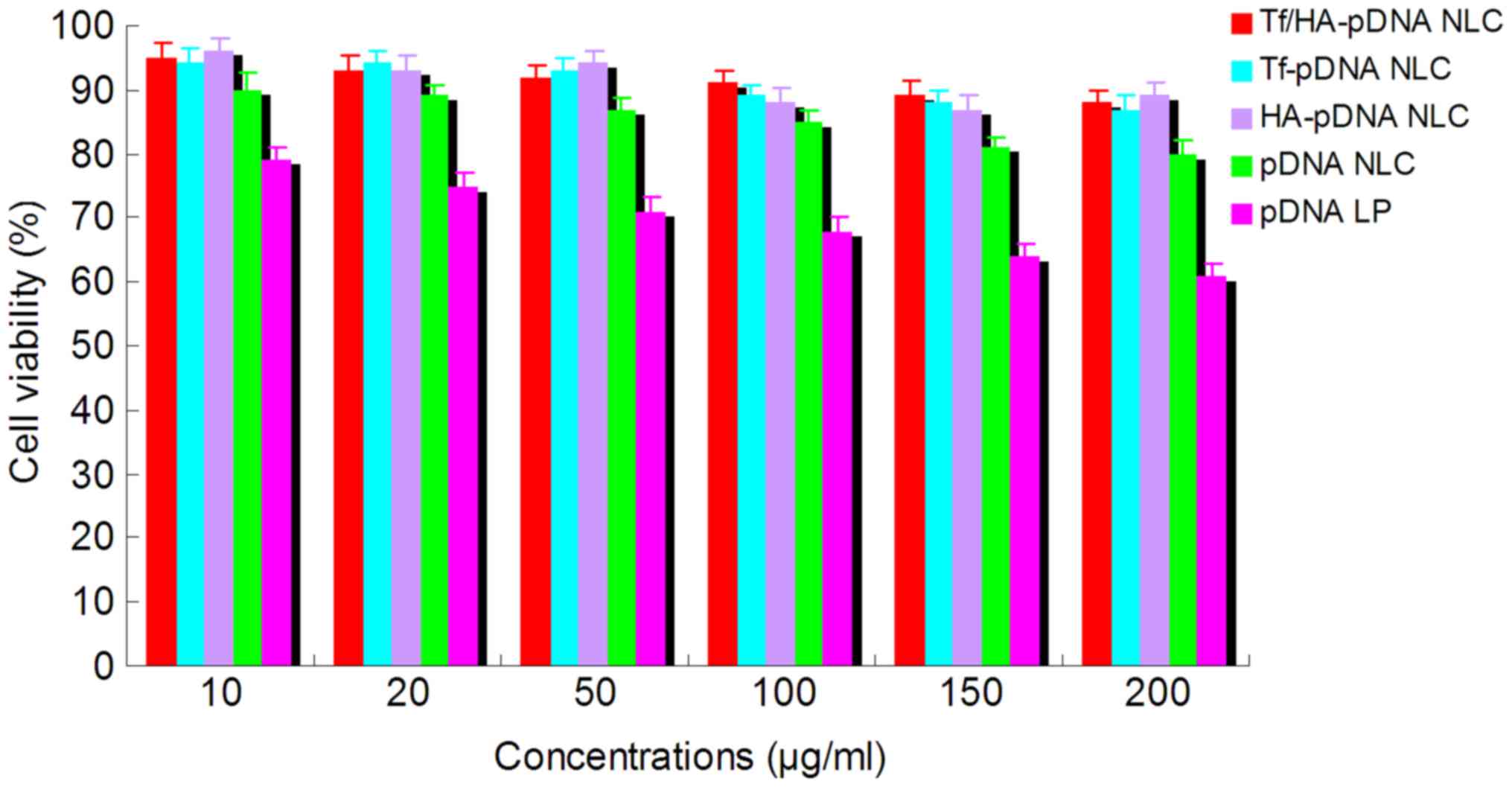
In vitro cell viability
Fig. 4 showed the
viability of A549 cells after treated with different NLC systems.
The cell viabilities in the presence of NLC systems over the
studied concentration range were >80%. NLC systems exhibited
lower cytotoxicity than pDNA LP at all concentrations (P<0.05).
Tf and/or HA decorated NLC showed higher cell viability than
undecorated NLC at the concentrations of 150 and 200 µg/ml.
In vitro gene transfection
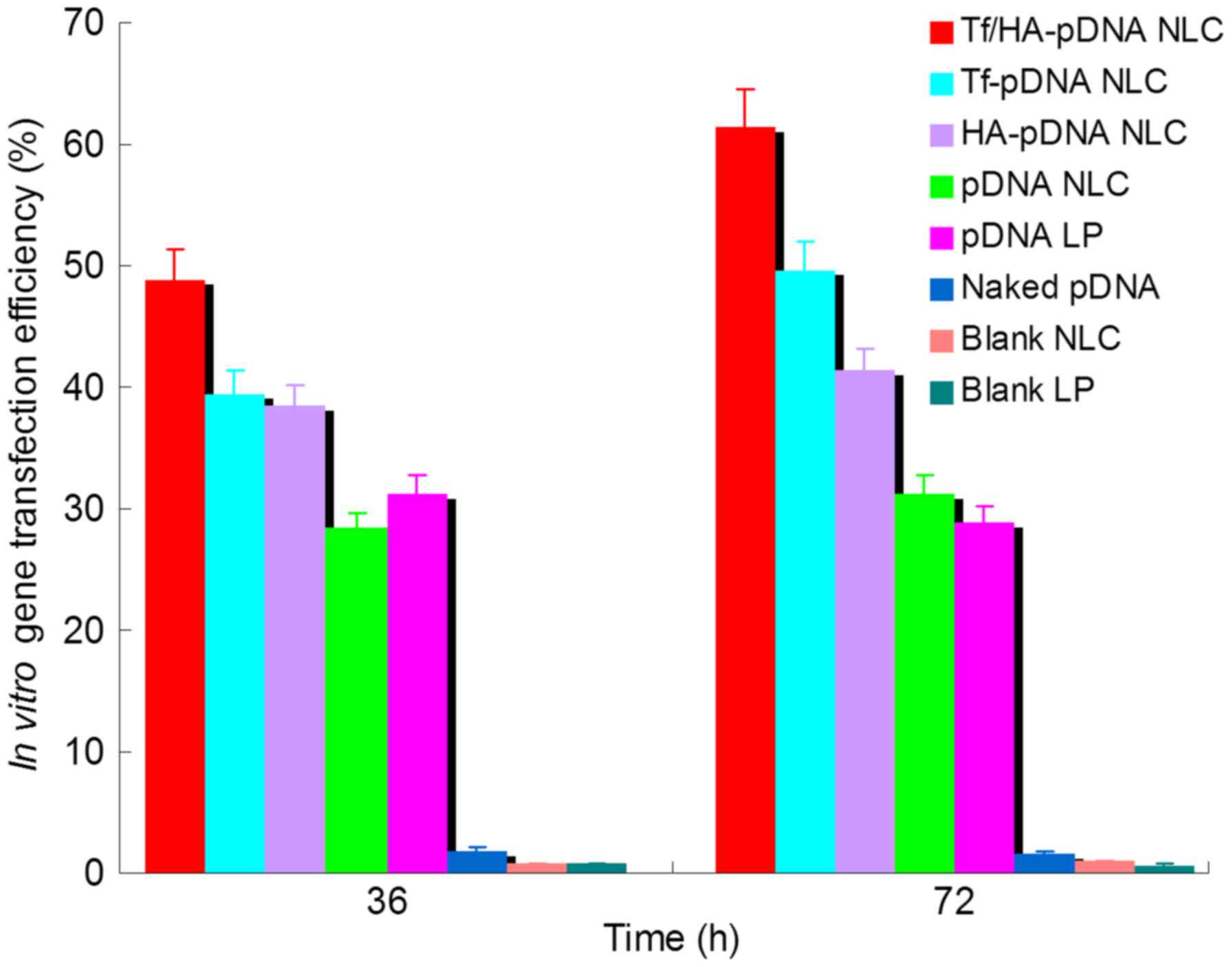
As showed in Fig. 5,
Tf/HA-pDNA NLC exhibited significantly higher gene transfection
efficiency than Tf-pDNA NLC and HA-pDNA NLC at both 36 and 72 h
after administration (P<0.05). Tf and/or HA decorated pDNA
loaded NLC systems had obviously better ability than undecorated
pDNA NLC at 36 and 72 h (P<0.05). pDNA NLC displayed no obvious
deference to pDNA LP (P>0.05). Tf-pDNA NLC displayed better
capacity than HA-pDNA NLC at 72 h (P<0.05). Naked pDNA and blank
NLC did not have any outcome in the quantitation study.
In vivo gene delivery and
expression
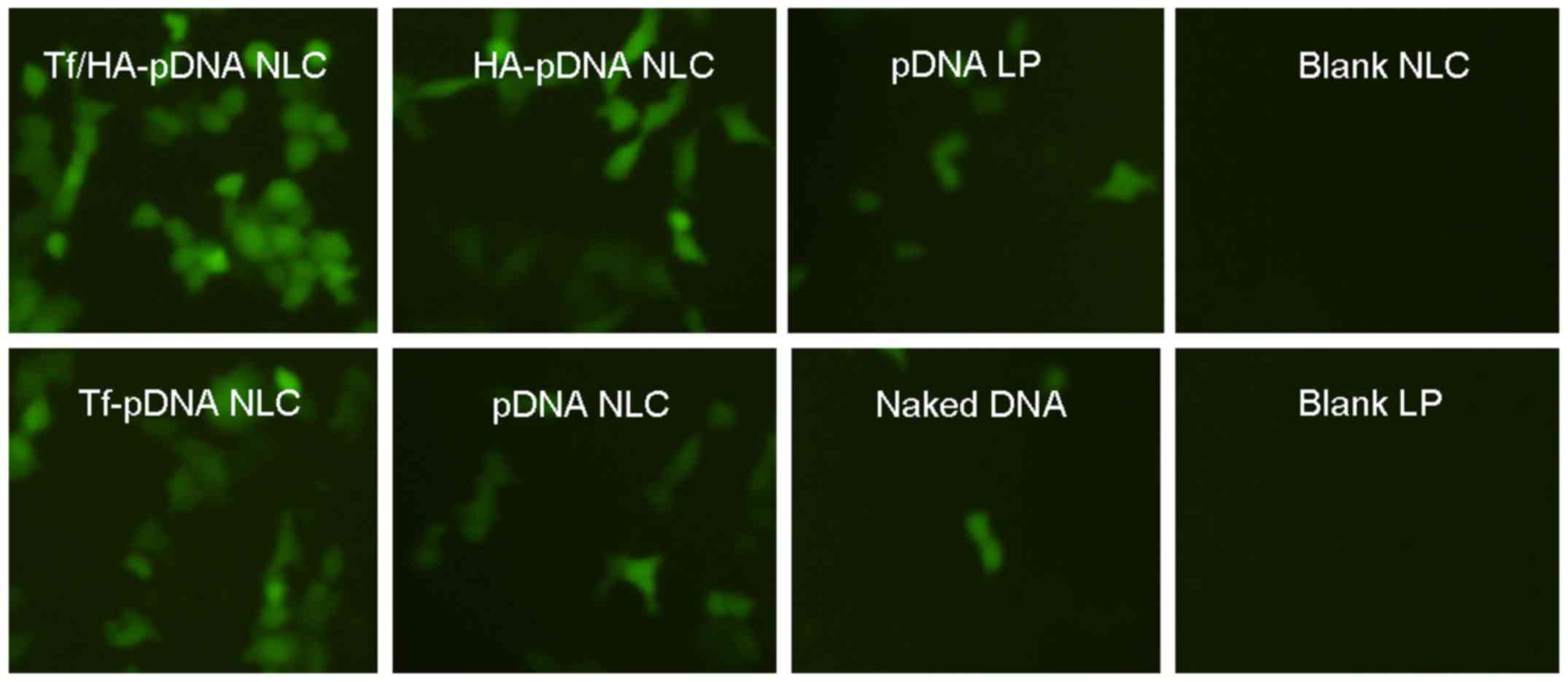
Fig. 6 illustrates
the fluorescence images of A549 cells when the different systems
were delivered in vivo. The best green fluorescence
expression was obtained by Tf/HA-pDNA NLC according to the images.
Decorated pDNA loaded NLC systems had better performance than
undecorated NLC and pDNA LP. Naked pDNA and blank NLC had no green
fluorescence in the images.
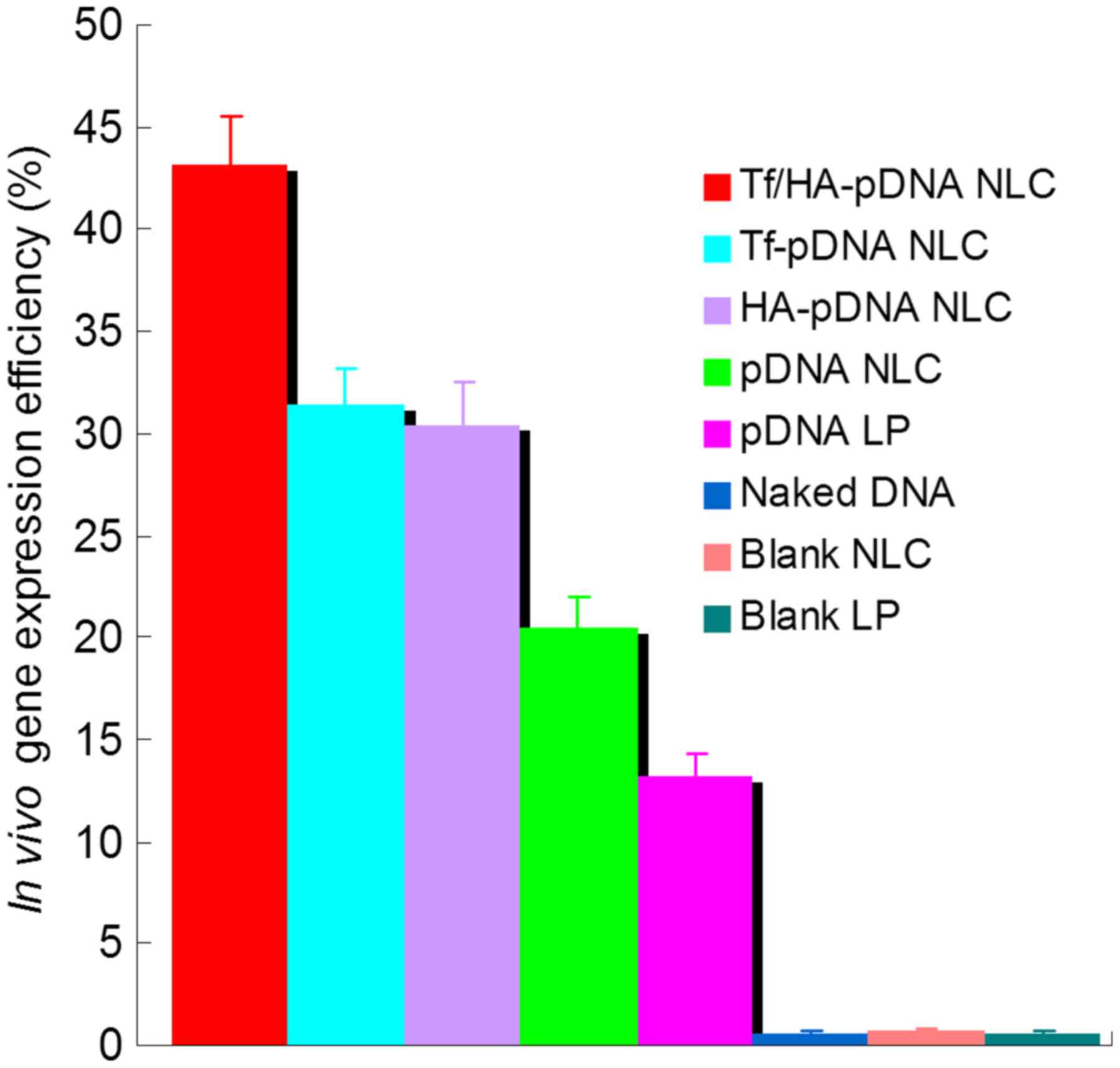
The in vivo transfection efficiency of the
fluorescent cells was quantified using flow cytometry, as shown in
Fig. 7. Tf/HA-pDNA NLC displayed
higher transfection efficiency than Tf-pDNA NLC, which was higher
than HA-pDNA NLC (P<0.05). pDNA loaded NLC systems had obviously
better ability than pDNA LP (P<0.05). The in vivo
quantification results were in accordance with the qualitative
images and the in vitro quantification results.
Discussion
In this study, we used dual ligand-modified NLC as
nanocarrier for the targeted delivery of gene to tumor site.
Firstly, Tf-PEG-DSPE and HA-PEG-DSPE were synthesized and analyzed.
Then Tf/HA-pDNA NLC was prepared and characterized.
TEM was used to explore the structure and morphology
of Tf/HA-pDNA NLC (Fig. 2). The
images reveal that Tf/HA-pDNA NLC has a core-shell spherical
structure with grey coating outside the core. The shell structure
may prove the existence of Tf and HA ligands. Surface morphology
has been demonstrated to be important in determining the uniformity
and stability of the system (35).
The size and ζ-potential of NPs not only determine their colloidal
stability but also influence the effectiveness of their interaction
with cell membranes, which is the key step for successful cellular
uptake (36). Tf/HA-pDNA NLC had a
larger size and lower ζ-potential than that of pDNA NLC, indicating
that anionic Tf and HA successfully decorated the surface of NLC,
enlarged the size and neutralized the charge of the pDNA NLC. All
the NLC samples tested have positive surface charge, which is
necessary to ensure the uptake of complexes by cells due to
electrostatic interactions between the carriers and the negatively
charged cellular membranes.
The GL of pDNA loaded NLC systems was ~90%. pDNA was
anionic materials, cationic materials of NLC system could
efficiently adsorb and incorporate anionic plasmid, primarily via
ionic interaction, which depends on the charge attraction (32,37).
Serum stability of NLC systems was evaluated in 10% FBS in order to
evaluate the stability of the NLC intravenous administration. The
results illustrated that these carriers would not collapse or
accumulate in blood circulation (38). pDNA loaded in the systems was well
protected and was not damaged by the serum. This behavior could be
helpful with the in vivo effect of the gene delivery
system.
Sustained-release property of the nanocarriers is
significant for gene therapy (39).
More sustained pDNA release behavior of Tf/HA-pDNA NLC than
undecorated pDNA NLC could be explained by the modification of
ligands influencing the release property of the NLC system. The
sustained release can give continuous protection of the pDNA and
lead to the persistent therapeutic effect.
Lipid carriers have cytotoxic drawbacks, especially
when cationic lipids are involved (40). Cationic formulations have been
described to affect cell proliferation, differentiation, and
pro-apoptotic genes in human epithelial cells. Therefore,
evaluation of the cytotoxicity of the cationic gene delivery
systems is essential. The cell viabilities in the presence of NLC
systems were high. Lower cytotoxicity of NLC systems than pDNA LP
system suggested the favorable tolerance of the NLC. Tf and/or HA
decorated NLC showed higher cell viability than undecorated ones,
indicating the decoration of ligands may have influence on the
surface character of the NLC system and provide better cell
viability.
In vitro and in vivo gene transfection
study intended to evaluate the ability of ligands decorated NLC
systems to transfer the pDNA to A549 lung cancer cells and lung
cancer-bearing mouse model was reported (30). Intracellular trafficking, gene
expression and subsequent protein synthesis are required for an
efficient gene delivery system to be accomplish (41,42).
The commercial cationic liposome based reagent, Lipofectamine 3000,
well known to provide high transfection efficiency and high level
of transgene expression in a range of mammalian cell types was
chosen as positive control in our study. Higher gene transfection
efficiency achieved by Tf/HA-pDNA NLC than Tf-pDNA NLC and HA-pDNA
NLC could be evidence of better target ability of the dual
ligand-decorated NLC system. Better ability exhibited by pDNA
loaded NLC systems than pDNA LP in the animal model showed the
superiority of the NLC vectors compared to the liposomes when
delivered in vivo. Tf-pDNA NLC displayed better capacity
than HA-pDNA NLC at 72 h could be proof of better affinity of Tf
than HA ligands after a period of administration. The test of naked
pDNA and blank NLC samples influenced the gene and NLC materials on
the transfection result. As expected, significant gene transfer
effects of Tf/HA-pDNA NLC in lung cancer cells through intravenous
injection were observed without toxicity. Tf/HA-pDNA NLC could be a
promising gene delivery system for lung cancer gene therapy.
In summary, Tf and HA containing ligands were
synthesized and used for the decoration of lipid carriers.
Tf/HA-pDNA NLC was developed as an efficient and safe gene delivery
system. The dual ligand-decorated, pDNA loaded NLC system had low
cytotoxicity and exhibited enhanced gene transfer ability in
vitro as well as in vivo. Therefore, Tf/HA-pDNA NLC has
the potential to be a safe and efficient gene carrier for lung
cancer gene therapy.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Poulsen TT, Pedersen N and Poulsen HS:
Replacement and suicide gene therapy for targeted treatment of lung
cancer. Clin Lung Cancer. 6:227–236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stuckey DW and Shah K: Stem cell-based
therapies for cancer treatment: Separating hope from hype. Nat Rev
Cancer. 14:683–691. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang TY, Huang B, Wu HB, Wu JH, Li LM, Li
YX, Hu YL, Han M, Shen YQ, Tabata Y, et al: Synergistic effects of
co-administration of suicide gene expressing mesenchymal stem cells
and prodrug-encapsulated liposome on aggressive lung melanoma
metastases in mice. J Control Release. 209:260–271. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho WY, Hong SH, Singh B, Islam MA, Lee S,
Lee AY, Gankhuyag N, Kim JE, Yu KN, Kim KH, et al: Suppression of
tumor growth in lung cancer xenograft model mice by
poly(sorbitol-co-PEI)-mediated delivery of osteopontin siRNA. Eur J
Pharm Biopharm. 94:450–462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim I, Byeon HJ, Kim TH, Lee ES, Oh KT,
Shin BS, Lee KC and Youn YS: Doxorubicin-loaded porous PLGA
microparticles with surface attached TRAIL for the inhalation
treatment of metastatic lung cancer. Biomaterials. 34:6444–6453.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Merdan T, Kopecek J and Kissel T:
Prospects for cationic polymers in gene and oligonucleotide therapy
against cancer. Adv Drug Deliv Rev. 54:715–758. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie RL, Jang YJ, Xing L, Zhang BF, Wang
FZ, Cui PF, Cho MH and Jiang HL: A novel potential biocompatible
hyperbranched polyspermine for efficient lung cancer gene therapy.
Int J Pharm. 478:19–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Christensen CL, Zandi R, Gjetting T,
Cramer F and Poulsen HS: Specifically targeted gene therapy for
small-cell lung cancer. Expert Rev Anticancer Ther. 9:437–452.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Gao DY and Huang L: In vivo
delivery of miRNAs for cancer therapy: Challenges and strategies.
Adv Drug Deliv Rev. 81:128–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Y, Zhu X, Zhang X, Liu B and Huang L:
Nanoparticles modified with tumor-targeting scFv deliver siRNA and
miRNA for cancer therapy. Mol Ther. 18:1650–1656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lennox KA and Behlke MA: Chemical
modification and design of anti-miRNA oligonucleotides. Gene Ther.
18:1111–1120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tivnan A, Orr WS, Gubala V, Nooney R,
Williams DE, McDonagh C, Prenter S, Harvey H, Domingo-Fernández R,
Bray IM, et al: Inhibition of neuroblastoma tumor growth by
targeted delivery of microRNA-34a using anti-disialoganglioside GD2
coated nanoparticles. PLoS One. 7:e381292012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heger Z, Gumulec J, Cernei N, Tmejova K,
Kopel P, Balvan J, Masarik M, Zitka O, Beklova M, Adam V, et al:
17β-estradiol-containing liposomes as a novel delivery system for
the antisense therapy of ER-positive breast cancer: An in vitro
study on the MCF-7 cell line. Oncol Rep. 33:921–9. 2015.PubMed/NCBI
|
|
15
|
de Jesus MB and Zuhorn IS: Solid lipid
nanoparticles as nucleic acid delivery system: Properties and
molecular mechanisms. J Control Release. 201:1–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Sha X, Shen A, Wang Y, Sun Z, Gu
Z and Fang X: Polycation nanostructured lipid carrier, a novel
nonviral vector constructed with triolein for efficient gene
delivery. Biochem Biophys Res Commun. 370:478–482. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doktorovova S, Souto EB and Silva AM:
Nanotoxicology applied to solid lipid nanoparticles and
nanostructured lipid carriers - a systematic review of in vitro
data. Eur J Pharm Biopharm. 87:1–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han Y, Zhang Y, Li D, Chen Y, Sun J and
Kong F: Transferrin-modified nanostructured lipid carriers as
multifunctional nanomedicine for codelivery of DNA and doxorubicin.
Int J Nanomed. 9:4107–4116. 2014.
|
|
19
|
Avitabile T, Marano F, Castiglione F,
Bucolo C, Cro M, Ambrosio L, Ferrauto C and Reibaldi A:
Biocompatibility and biodegradation of intravitreal hyaluronan
implants in rabbits. Biomaterials. 22:195–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang XJ, Jiang H, Zhu YQ, Zhang LY, Fan QH
and Tian Y: Doxorubicin induces drug resistance and expression of
the novel CD44st via NF-κB in human breast cancer MCF-7 cells.
Oncol Rep. 31:2735–2742. 2014.PubMed/NCBI
|
|
21
|
Coradini D, Pellizzaro C, Abolafio G,
Bosco M, Scarlata I, Cantoni S, Stucchi L, Zorzet S, Turrin C, Sava
G, et al: Hyaluronic-acid butyric esters as promising
antineoplastic agents in human lung carcinoma: A preclinical study.
Invest New Drugs. 22:207–217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan J, Sun SK, Wang Y, Fu YY, Zhang X,
Zhang Y and Yu C: Facile preparation of hyaluronic acid and
transferrin co-modified Fe3O4 nanoparticles
with inherent biocompatibility for dual-targeting magnetic
resonance imaging of tumors in vivo. Dalton Trans. 44:19836–19843.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kondo H, Miyoshi K, Sakiyama S, Tangoku A
and Noma T: Differential regulation of gene expression of alveolar
epithelial cell markers in human lung adenocarcinoma-derived A549
clones. Stem Cells Int. 2015:1658672015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang W, Zhou F, Ge L, Liu X and Kong F:
Transferrin-PEG-PE modified dexamethasone conjugated cationic lipid
carrier mediated gene delivery system for tumor-targeted
transfection. Int J Nanomed. 7:2513–2522. 2012.
|
|
25
|
Jing L, Shao S, Wang Y, Yang Y, Yue X and
Dai Z: Hyaluronic acid modified hollow Prussian blue nanoparticles
loading 10-hydroxycamptothecin for targeting thermochemotherapy of
cancer. Theranostics. 6:40–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang XG, Miao J, Dai YQ, Du YZ, Yuan H
and Hu FQ: Reversal activity of nanostructured lipid carriers
loading cytotoxic drug in multi-drug resistant cancer cells. Int J
Pharm. 361:239–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Du YZ, Cai LL, Li J, Zhao MD, Chen FY,
Yuan H and Hu FQ: Receptor-mediated gene delivery by folic
acid-modified stearic acid-grafted chitosan micelles. Int J
Nanomed. 6:1559–1568. 2011. View Article : Google Scholar
|
|
28
|
Yu W, Liu C, Liu Y, Zhang N and Xu W:
Mannan-modified solid lipid nanoparticles for targeted gene
delivery to alveolar macrophages. Pharm Res. 27:1584–1596. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang RH, Cao HM, Tian ZJ, Jin B, Wang Q,
Ma H and Wu J: Efficacy of dual-functional liposomes containing
paclitaxel for treatment of lung cancer. Oncol Rep. 33:783–791.
2015.PubMed/NCBI
|
|
30
|
Yu W, Liu C, Ye J, Zou W, Zhang N and Xu
W: Novel cationic SLN containing a synthesized single-tailed lipid
as a modifier for gene delivery. Nanotechnology. 20:2151022009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pepić I, Lovrić J, Hafner A and
Filipović-Grčić J: Powder form and stability of Pluronic mixed
micelle dispersions for drug delivery applications. Drug Dev Ind
Pharm. 40:944–951. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu X, Yang G, Shi Y, Su C, Liu M, Feng B
and Zhao L: Intracellular targeted co-delivery of shMDR1 and
gefitinib with chitosan nanoparticles for overcoming multidrug
resistance. Int J Nanomed. 10:7045–7056. 2015.
|
|
33
|
Zou W, Liu C, Chen Z and Zhang N: Studies
on bioadhesive PLGA nanoparticles: A promising gene delivery system
for efficient gene therapy to lung cancer. Int J Pharm.
370:187–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vighi E, Montanari M, Hanuskova M,
Iannuccelli V, Coppi G and Leo E: Design flexibility influencing
the in vitro behavior of cationic SLN as a nonviral gene vector.
Int J Pharm. 440:161–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hazzah HA, Farid RM, Nasra MM, El-Massik
MA and Abdallah OY: Lyophilized sponges loaded with curcumin solid
lipid nanoparticles for buccal delivery: Development and
characterization. Int J Pharm. 492:248–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang L, Dong J, Ouyang W, Wang X and Tang
J: Anticancer effect and feasibility study of hyperthermia
treatment of pancreatic cancer using magnetic nanoparticles. Oncol
Rep. 27:719–726. 2012.PubMed/NCBI
|
|
37
|
Kumar MN Ravi, Bakowsky U and Lehr CM:
Preparation and characterization of cationic PLGA nanospheres as
DNA carriers. Biomaterials. 25:1771–1777. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Immordino ML, Brusa P, Rocco F, Arpicco S,
Ceruti M and Cattel L: Preparation, characterization, cytotoxicity
and pharmacokinetics of liposomes containing lipophilic gemcitabine
prodrugs. J Control Release. 100:331–346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song RF, Li XJ, Cheng XL, Fu AR, Wang YH,
Feng YJ and Xiong Y: Paclitaxel-loaded trimethyl chitosan-based
polymeric nanoparticle for the effective treatment of gastroenteric
tumors. Oncol Rep. 32:1481–1488. 2014.PubMed/NCBI
|
|
40
|
Torchilin VP: Multifunctional
nanocarriers. Adv Drug Deliv Rev. 58:1532–1555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kamiya H, Tsuchiya H, Yamazaki J and
Harashima H: Intracellular trafficking and transgene expression of
viral and non-viral gene vectors. Adv Drug Deliv Rev. 52:153–164.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang HL, Hong SH, Kim YK, Islam MA, Kim
HJ, Choi YJ, Nah JW, Lee KH, Han KW, Chae C, et al: Aerosol
delivery of spermine-based poly(amino ester)/Akt1 shRNA complexes
for lung cancer gene therapy. Int J Pharm. 420:256–265. 2011.
View Article : Google Scholar : PubMed/NCBI
|