Introduction
Cancer cachexia is a multifactorial syndrome
characterized by a progressive loss of skeletal muscle mass that
cannot be fully compensated for by conventional treatments such as
increasing energy intake through nutritional support (1). Approximately 60–80% of all advanced
cancer patients experience cachexia, particularly those with
gastric and pancreatic cancers (2).
Cachexia is associated with decreased physical function, impaired
quality of life, and shortened survival time (3), and is responsible for roughly 20% of
all cancer deaths (4). With growing
incidence and high mortality, it also increases health care
resource utilization and associated hospitalization costs, and has
become a major social health problem (5). Since a significant unmet need exists
for improving patients' quality of life and decreasing the public
health burden, it is critical to clarify the mechanism of this
debilitating condition.
As the most important feature of cancer cachexia,
skeletal muscle atrophy is believed to be due to a significant
increase in proteolysis of myofibrils (6). Although mounting evidence indicates
that the ubiquitin-proteasome pathway (UPP) plays a prominent role
in the process (7,8), the proteasome only degrades peptides
by engulfing them in its catalytic chamber and cannot disassemble
myofibrillar proteins until they have been released from the
myofibrils as myofilaments by the calpain system (6). Furthermore, evaluation of muscle
biopsies in clinical trials suggests that muscle apoptosis exists
in this hypercatabolic state, which contributes to the severity of
the cachexia (9).
Calpain, a member of the calcium-activated cysteine
protease family, is implicated in diverse diseases, including
muscular dystrophy, neurological disorders, cataracts, and
hematonosis (10). Inhibition of
calpain can preserve the function of critical proteins by
suppressing associated proteolysis, thus potentially ameliorating
these diseases. In terms of cachexia-induced muscle wasting,
calpain and UPP have a synergistic effect on muscle protein
proteolysis, as calpains can provide substrates for UPP when
activated (11). In addition,
calpain inhibitors prevent calpain-mediated neuron apoptosis in
several rodent models of nervous system injury (12,13).
These inhibitors thus might also protect skeletal muscle from
cachexia-induced apoptosis.
In this study, we evaluated the effect of three
common calpain inhibitors, calpastatin (an endogenous polypeptide
inhibitor), calpain inhibitor IV, and calpeptin (both irreversible
inhibitors derived from chemical synthesis) on skeletal muscle
proteolysis and apoptosis in a murine model of cancer cachexia.
Materials and methods
Reagents and cell line
Calpastatin peptide (CAST for short), calpain
inhibitor IV (IV for short) and Calpeptin (Merck Millipore, Boston,
MA, USA, catalogue no. 208902, 208724, 03-34-0051) were resolved
with dimethyl sulfoxide (DMSO) as a stock solution and diluted in
sterile saline solution before use. Primary antibodies [rabbit
monoclonal anti-calpain 1 (ab108400; 1:1000); rabbit monoclonal
anti-calpain 2 (ab126600; 1:1000); rabbit polyclonal
anti-calpastatin (ab28252; 1:5000); rabbit monoclonal anti-Bax
(ab32503; 1:1000); rabbit polyclonal anti-Bcl-2 (ab59348; 1:1000);
mouse monoclonal anti-GAPDH (ab9484; 1:1000)] were purchased from
Abcam (Cambridge, UK), rabbit polyclonal anti-cleaved caspase-3
(Asp175) (cat# 9661; 1:1000) was obtained from Cell Signaling
Technology (Danvers, MA, USA), and secondary goat anti-rabbit IgG
(BA1055; 1:3000) and goat anti-mouse IgG (BA1050; 1:3000)
conjugated with horseradish peroxidase were obtained from Boster
(Wuhan, China). Colon carcinoma cell line CT26 was purchased from
American Type Culture Collection (Manassas, VA, USA).
Design of animal experiments
BALB/c male mice (6–8 weeks old, body weight 20–24
g) were purchased from the Animal Center of the Chinese Academy of
Sciences (Shanghai, China; Security processing facility certificate
number SCXK (Hu) 2012-0002). The animals were maintained in a
specific pathogen-free environment at a controlled temperature
(22±1°C) and a 12 h light-dark cycle, which was provided by the
Animal Experiments Center of Fujian Medical University. All animals
were fed with standard laboratory chow and tap ad libitum.
The protocol was approved by the Institutional Animal Care
Committee of the Fujian Medical University.
After 7 days of acclimation, a total of 144 mice
received CT26 cells as subcutaneous injections in the right
armpits, with a homogenate of 50 mg (2–3 mm3) minced
solid murine CT26 adenocarcinoma in 0.1 ml of saline, while 18 mice
were injected with saline alone as healthy controls. The
tumor-inoculated animals were randomly divided into the eight
groups as follows: CAST, IV, calpeptin, CAST+IV, CAST+calpeptin,
IV+calpeptin, CAST+IV+calpeptin, and cachexia control group (n=18
per group). Intraperitoneal administration of CAST (1.5 µg/kg,
daily), IV (0.75 mg/kg, daily), and/or calpeptin (1.75 mg/kg,
daily) at a total volume of 0.2 ml began on the 12th day after
inoculation, when the mice presented signs and symptoms of
cachexia, and lasted for 7 consecutive days. Besides the above
mentioned healthy controls, 18 mice of the cachexia control group
also received an equal volume of DMSO+saline i.p. once daily for
one week in parallel. Physical activity, fur condition, body
weight, food intake, and tumor growth were recorded daily. The
tumor volume was calculated according to the following method:
tumor volume V (cm3) = (axb2)/2, where a is
the length and b is the width.
On the 19th day, ten mice in each group were
randomly sacrificed, and their blood, tumors, and gastrocnemius
muscles were collected for further analysis. After being obtained
from orbital veins and clotting for 1 h, blood samples were
centrifuged at 1200 × g for 10 min, the serum was collected and
stored at −20°C. After the animals were euthanized by cervical
dislocation, both their gastrocnemius muscles from the left leg and
tumors were harvested and weighed. The gastrocnemius muscles were
then snap-frozen in liquid nitrogen and stored at −80°C. Survival
time for the remaining eight mice from each group was monitored and
analyzed.
Bioanalytical assays
Serum glucose, triglyceride, total protein, and
albumin used as biochemical markers of nutritional status were
measured by an Olympus AU2700 automated biochemistry analyzer
(Olympus, Tokyo, Japan).
Calpain activity assay
Calpain activity was measured by a calpain activity
assay kit (Biovision, Mountain View, CA, USA). An appropriate
amount of muscle tissue was ground into powder in liquid nitrogen.
The fine powder was then lysed with extraction buffer in the kit
and placed on ice for 20 min. After centrifuging in a
microcentrifuge at 10,000 × g for 5 min, the supernatants were
collected. After protein quantification using a Bradford protein
assay kit (Wanleibio, Shenyang, China), 100 µg of protein was
incubated with calpain substrate Ac-LLY-AFC in a dark fluorescent
microplate at 37°C for 1 h. The samples were read in a SpectraMax
M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA)
equipped with a 400 nm excitation filter and a 505 nm emission
filter.
Western blot analysis
The muscle specimens were homogenized and lysed in
RIPA buffer mixed with protease inhibitors (150 mM NaCl, 50 mM Tris
pH 7.4, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium
dodecyl sulfate, sodium orthovanadate, sodium fluoride,
ethylenediaminetetraacetic acid, and leupeptin). After
centrifugation at 12,000 × g for 5 min at 4°C, the resulting
proteins were quantified with a bicinchoninic acid protein assay
kit (Beyotime, Shanghai, China). Proteins were separated by
SDS-polyacrylamide gel electrophoresis according to their molecular
weight using a mini-gel electrophoresis system (Bio-Rad, Hercules,
CA, USA) and subsequently transferred onto a polyvinylidene
difluoride membrane (Millipore, Billerica, MA, USA). The membrane
was blocked with 5% bovine serum albumin at room temperature for 1
h, then incubated with primary antibodies overnight at 4°C, and
further incubated with horseradish peroxidase-conjugated
anti-rabbit or mouse IgG for 1 h at room temperature. The protein
bands were illuminated with enhanced chemiluminescence reagents
(Gen-View Scientific, Inc., Calimesa, CA, USA) and detected by the
ChemiDoc XRS+ system (Bio-Rad). Finally, optical density values
were normalized to that of the housekeeping protein
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and analyzed with
ImageJ computer software (NIH, Bethesda, MD, USA). The average
values were obtained from three repeated measures.
Quantitative real-time PCR
RNA of collected tissue was isolated using a tripure
isolation reagent (Roche Diagnostics, Indianapolis, IN, USA). cDNA
was synthesized from 3 µg total RNA using the First Strand cDNA
Synthesis kit (Gen-View Scientific, Inc.) according to the
manufacturer's instructions. Semi-quantitative real-time PCR
analysis was performed using an Applied Biosystems 7500 Real-time
PCR System (Applied Biosystems, Carlsbad, CA, USA) with SYBR-Green
staining of DNA double-strands, while the staining was performed
using the FastStart Universal SYBR Green Master (Roche Diagnostics)
according to the product manual. Primer pairs for the amplification
of E3 ubiquitin ligase genes were the following: mouse MuRF-1
(sense: 5-GGAACACGAAGA CGAGAAAATC-3, antisense: 5-TGGCTATTCTCCTTGG
TCACTC-3); mouse atrogin-1 (sense: 5-GAAGAGAGCAGT ATGGGGTCAC-3,
antisense: 5-CTTGAGGGGAAAGRG AGACG-3); and mouse GAPDH (sense:
5-GGTGAAGGTCG GTGTGAACG-3, anti-sense: 5-CTCGCTCCTGGAAGAT GGTG-3).
The amplification conditions were as follows: 50°C for 2 min, 95°C
for 10 min, and 40 cycles at 95°C for 15 sec and 60°C for 1 min.
Finally, either target gene expression was normalized to the
housekeeping gene mouse GAPDH that was amplified at the same time,
and further analyzed using the 2−∆∆CT method (14).
Statistical analyses
All data were expressed as mean ± standard
deviation. Differences were examined for significance using one-way
ANOVA followed by Tukey's pos-hoc test with SPSS 19.0 software
(SPSS, Chicago, IL, USA). The Kruskal-Wallis test followed by the
Nemenyi test was used when the data distribution was skewed. The
log-rank test was used for survival analysis. All statistical
analyses were two-sided, and P-values of 0.05 were considered
statistically significant.
Results
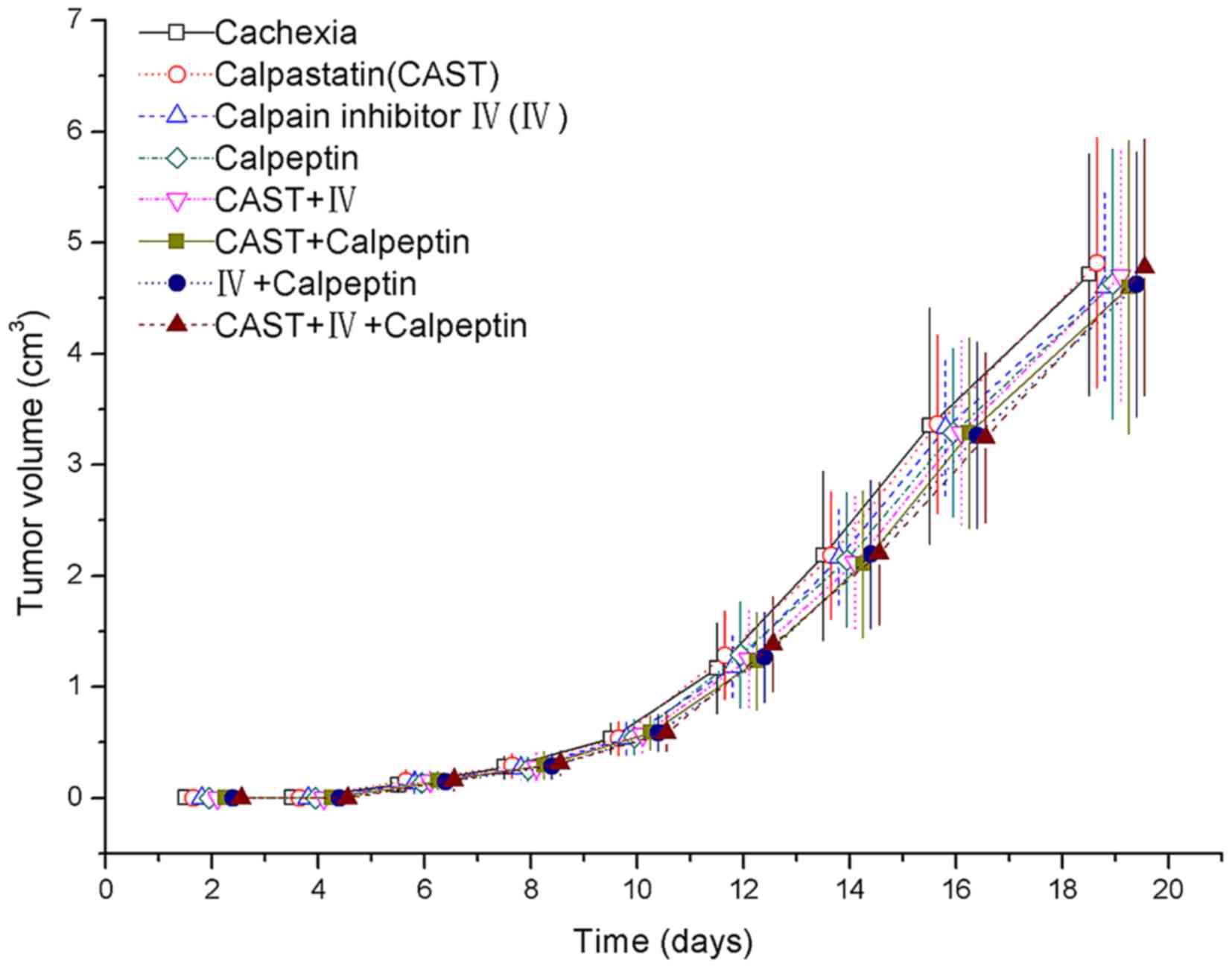
Tumor volume
The tumors of CT26-inoculated mice were perceptible
by day 6, and grew more rapidly from day 10 onward. The mice
exhibited symptoms of cachexia on day 12 after tumor inoculation,
including delayed responsiveness, poor physical activity, scruffy
fur, piloerection, and darkening of dorsal hairs. All the
tumor-bearing groups had a significant increase in tumor volume
over time but no significant differences were observed between the
treatment groups and the cachexia control group (Fig. 1).
Tumor-free body weight
There was no difference in initial body weight among
the nine groups (Fig. 2). As the
body weight of the healthy control group grew during the study
period, the weight of the tumor-bearing groups increased during the
first 8 days then declined from day 9 to the final day, despite a
slight transient increase in some groups (Fig. 2). From days 16–19, the tumor-free
body weight of the IV+calpeptin and CAST+IV+calpeptin groups were
significantly higher than the cachexia group (P=0.03, P=0.016 and
P=0.015, P=0.011, respectively). A significant tumor-free body
weight increase was observed in the calpeptin, CAST+IV, and
CAST+calpeptin groups only on day 19 (P=0.045, P=0.045, P=0.014,
respectively). However, the tumor-free body weight of all
tumor-bearing groups was significantly different from that of the
healthy controls from days 12–19 (P<0.01). Cumulative food
intake of standard chow showed no significant difference during the
experiment (data not shown).
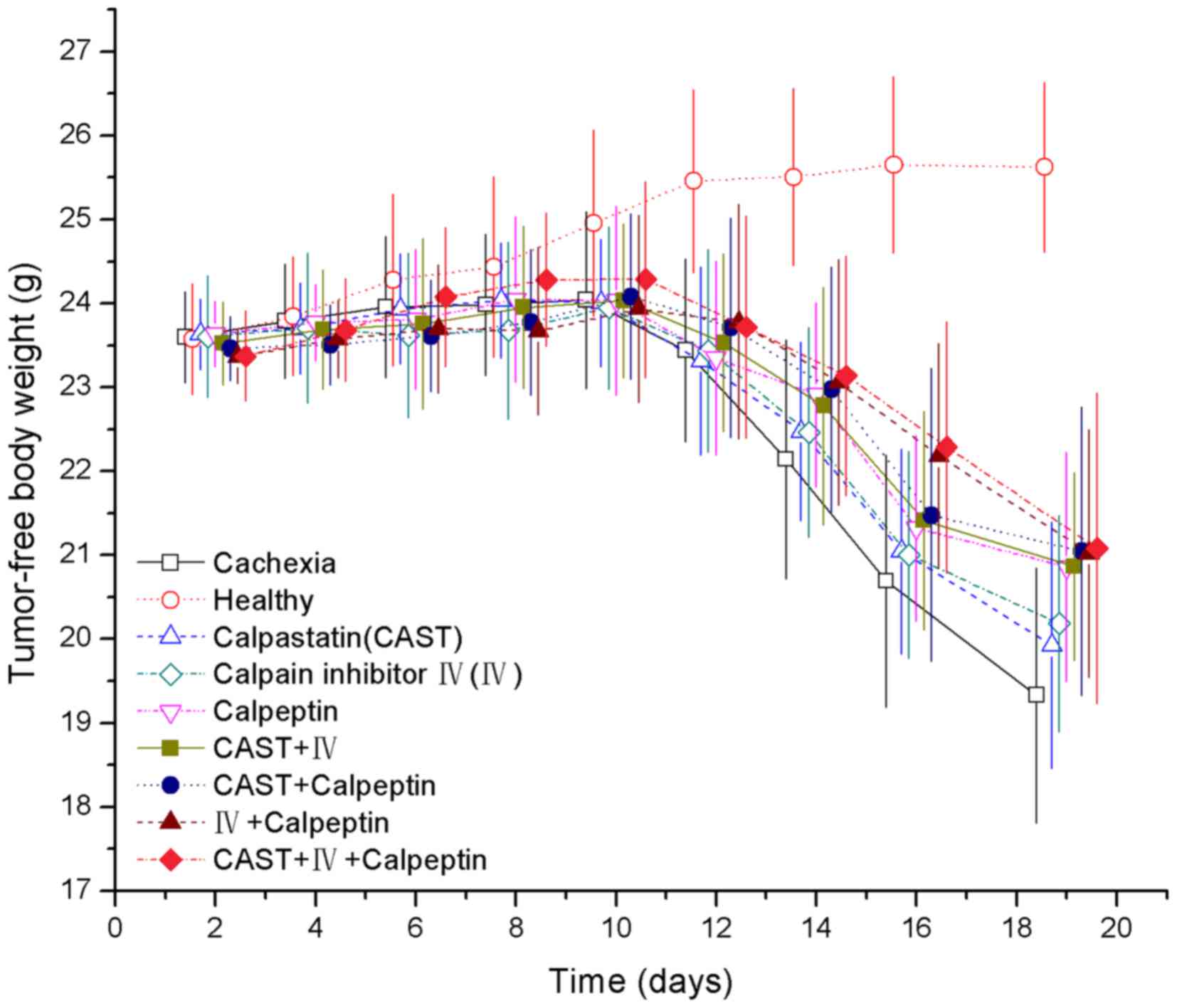 | Figure 2.Effect of CAST, IV, and calpeptin on
tumor-free body weight (g) of mice with cancer cachexia. Bars
represent mean ± SD, n=18 in each group. There were significant
differences across all the groups on days 12, 14, 16 and 19
(P<0.01). Pair-wise comparisons showed that (A) each of the
eight tumor-bearing groups was significantly different from the
healthy group on days 12, 14, 16 and 19 (P<0.01). (B) The
calpeptin group (day 19), CAST+IV group (day 19), CAST+calpeptin
group (day 19), IV+calpeptin group (days 16 and 19) and
CAST+IV+calpeptin group (days 16 and 19) were significantly
different from the cachexia group (P<0.05); and (C) for each
day, there were no significant differences across the treatment
groups or between any two particular groups (P>0.05). |
Muscle weight and serum nutritional
markers
Significant loss of gastrocnemius wet weight was
found in the cachexia controls as compared to the healthy controls
(Table I, P<0.001). There were
also significant differences in gastrocnemius weight between any
treatment group and the cachexia group (P<0.001). Moreover, a
markedly increased gastrocnemius muscle mass was observed in the
IV+Calpeptin and CAST+IV+calpeptin groups compared with the CAST
and IV groups (P=0.002, P=0.001 and P=0.004, P=0.001,
respectively).
 | Table I.Initial body weight of each group and
effect of different calpain inhibitors on gastrocnemius muscle mass
and serum metabolic parameters (mean ± SD), (n=10 each). |
Table I.
Initial body weight of each group and
effect of different calpain inhibitors on gastrocnemius muscle mass
and serum metabolic parameters (mean ± SD), (n=10 each).
|
| Cachexia | Healthy | CAST | IV | Calpeptin | CAST+IV | CAST +Calpeptin | IV+ Calpeptin | CAST+IV
+Calpeptin |
|---|
| Initial body weight
(g) | 23.60±0.54 | 23.58±0.66 | 23.63±0.42 | 23.61±0.73 | 23.63±0.39 | 23.52±0.49 | 23.47±0.38 | 23.37±0.33 | 23.37±0.54 |
| Gastrocnemius muscle
mass (mg) | 104.49±7.48 |
175.00±12.76a |
136.79±10.49a,b |
137.82±12.94a,b |
143.40±10.09a,b |
148.71±11.26a,b |
150.29±10.90a,b |
157.63±10.79a,b,c,d |
159.20±11.11a,c,d |
| TP (g/l) | 49.86±1.35 |
54.23±1.23a |
49.29±2.99b | 51.65±2.70 |
50.66±2.34b | 51.66±1.57 | 51.42±1.41 |
53.04±2.06a,c |
53.80±2.02a,c,e |
| ALB (g/l) | 27.42±1.99 |
35.07±1.34a |
27.90±2.68b |
29.88±2.13b |
28.89±3.95b |
31.62±2.34a,b,c |
32..69±1.91a,c,e |
33.42±2.12a,c,d,e |
32.99±2.13a,c,e |
| GLU (mmol/l) | 3.08±0.78 |
6.09±0.62a |
3.39±1.60b | 4.54±0.99 | 4.83±1.85 | 4.86±1.59 | 4.61±1.46 |
5.33±0.63a | 5.17±1.12 |
| TG (mmol/l) | 3.36±0.90 |
1.53±0.84a |
3.10±1.62b | 2.85±1.12 | 2.71±0.63 | 2.20±0.75 | 2.23±1.31 | 2.45±0.32 | 2.36±0.77 |
To evaluate the effect of treatments with different
calpain inhibitors on the nutritional state of cachectic mice, we
measured the serum levels of serum total protein, albumin, glucose,
and triglycerides in the nine groups. Mice from the cachexia group
had lower levels of total protein, albumin, and glucose, and higher
levels of triglycerides (Table I,
P<0.01) compared with the healthy controls. The administration
of different calpain inhibitors could partially reverse these
metabolic changes, especially the combination treatment with two or
three kinds of inhibitors. However, no significant difference in
these markers was found between any mono-treatment group and the
cachexia controls (between the CAST group and the cachexia group,
P=0.999, P=1.000, P=1.000, P=1.000; between the IV group and the
cachexia group, P=0.581, P=0.352, P=1.000, P=0.961; between the
calpeptin group and the cachexia group, P=0.994, P=0.903, P=0.392,
P=0.862).
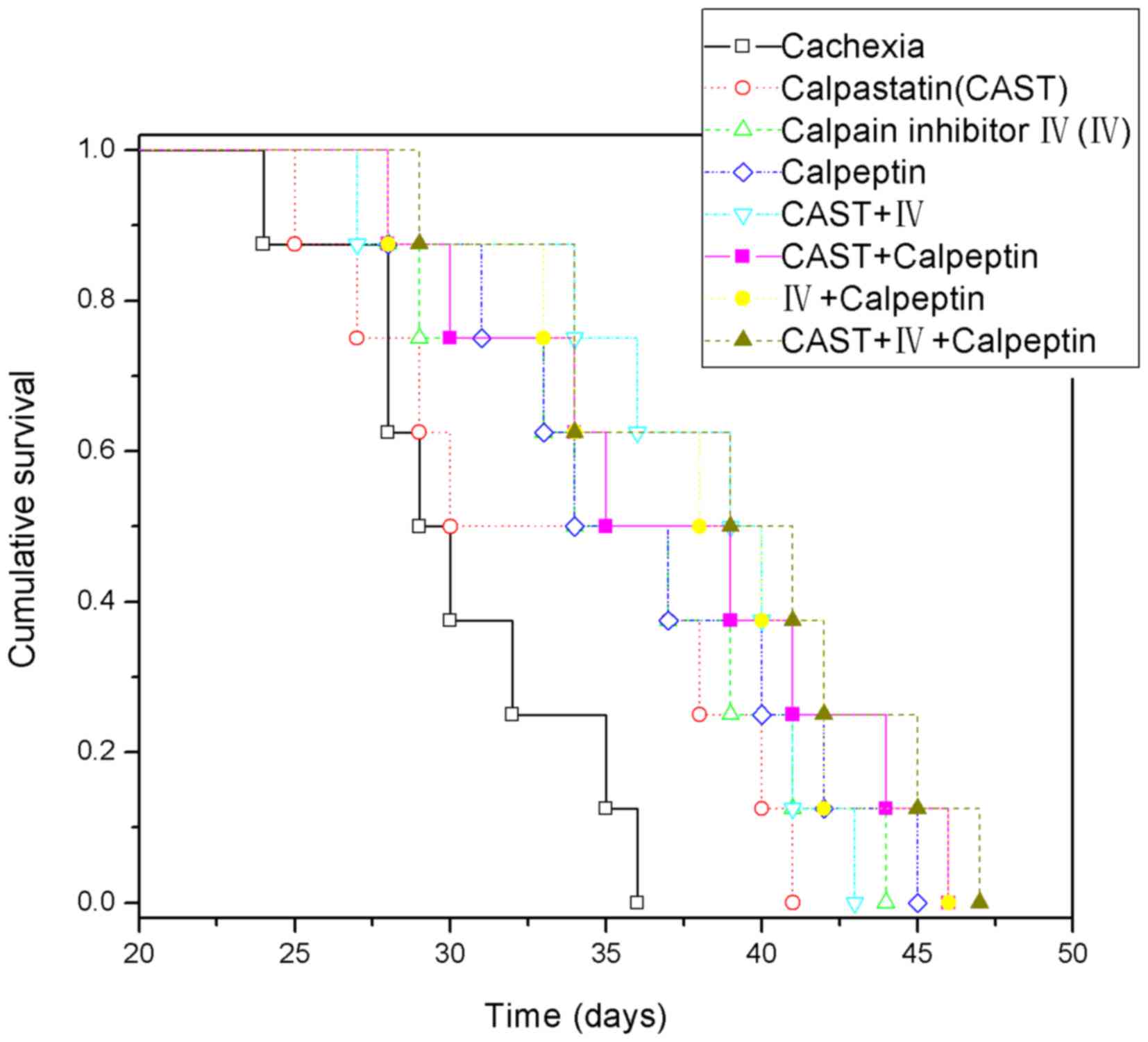
Survival time
To evaluate the effect of the different treatments
on the survival time of mice, we constructed Kaplan-Meier curves
with 8 mice in each group (eight remained alive after the other ten
were sacrificed on day 19). The data demonstrated that most of the
treatment groups had a longer survival time than the cachexia group
(Fig. 3, P<0.05) except for the
calpastatin group (P=0.101). In addition, there were no significant
differences among the different treatment groups (P>0.05). All
mice in the healthy control group survived for 50 days.
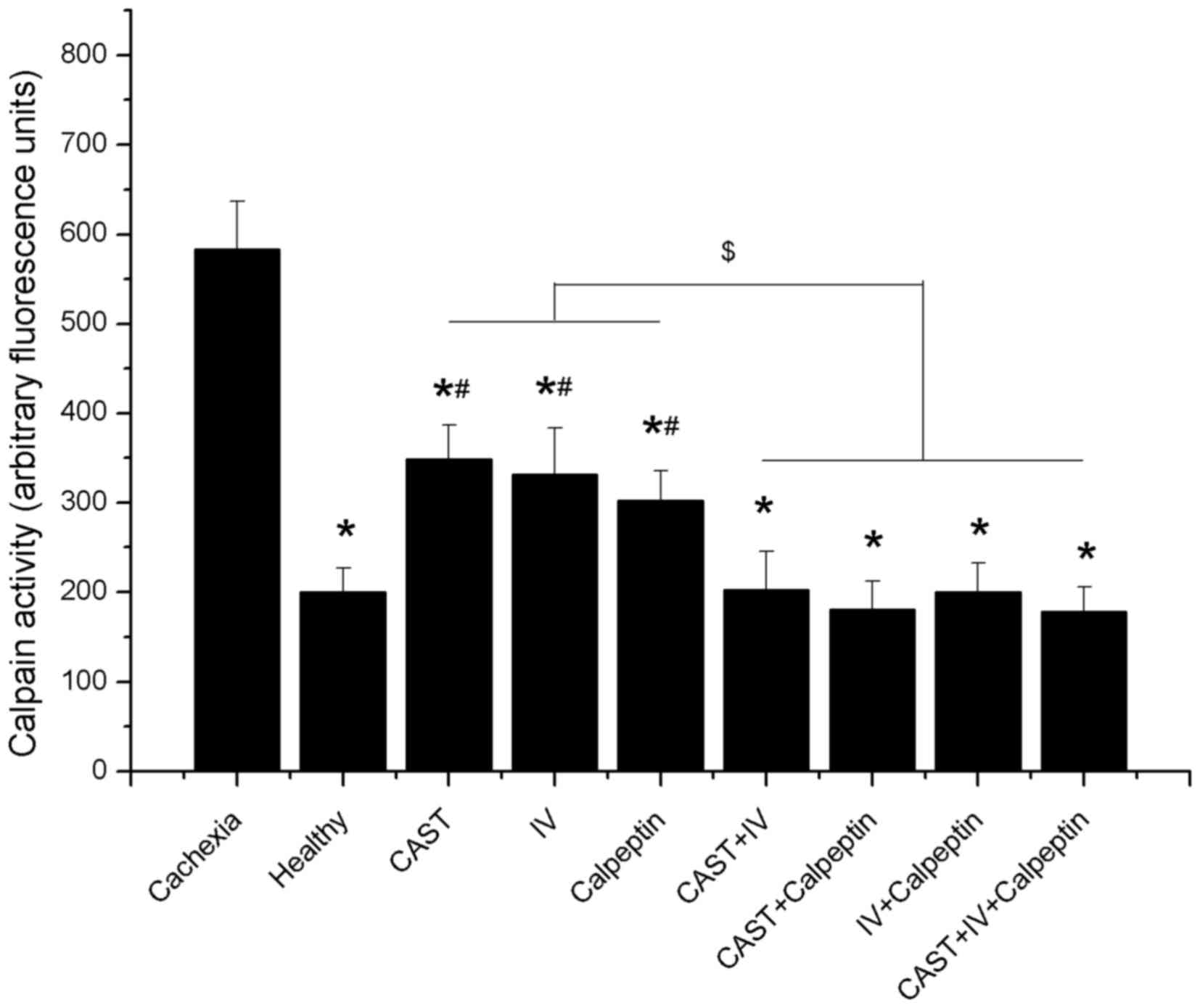
Calpain expression and activity
Previous studies (15) have shown that activation of the
calpain system is ubiquitous in cancer cachexia, and calpain
inhibitors can antagonize the effects induced by activated calpain.
To confirm that treatments with different calpain inhibitors affect
calpain expression and activity in cachectic mice, we measured the
levels of µ-calpain, m-calpain, and calpastatin, and examined the
calpain activity in all nine groups.
There were no significant differences in either
µ-calpain or m-calpain protein expression between the cachexia
controls and the healthy controls. Treatment with calpain inhibitor
IV and calpeptin were associated with lower levels of the two
ubiquitous calpains; however, their levels in the calpastatin group
were higher than the control groups and the other mono-treatment
groups, while their expression could be downgraded when combined
with other calpain inhibitors such as calpain inhibitor IV,
calpeptin, or both (Fig. 4A, B and
D).
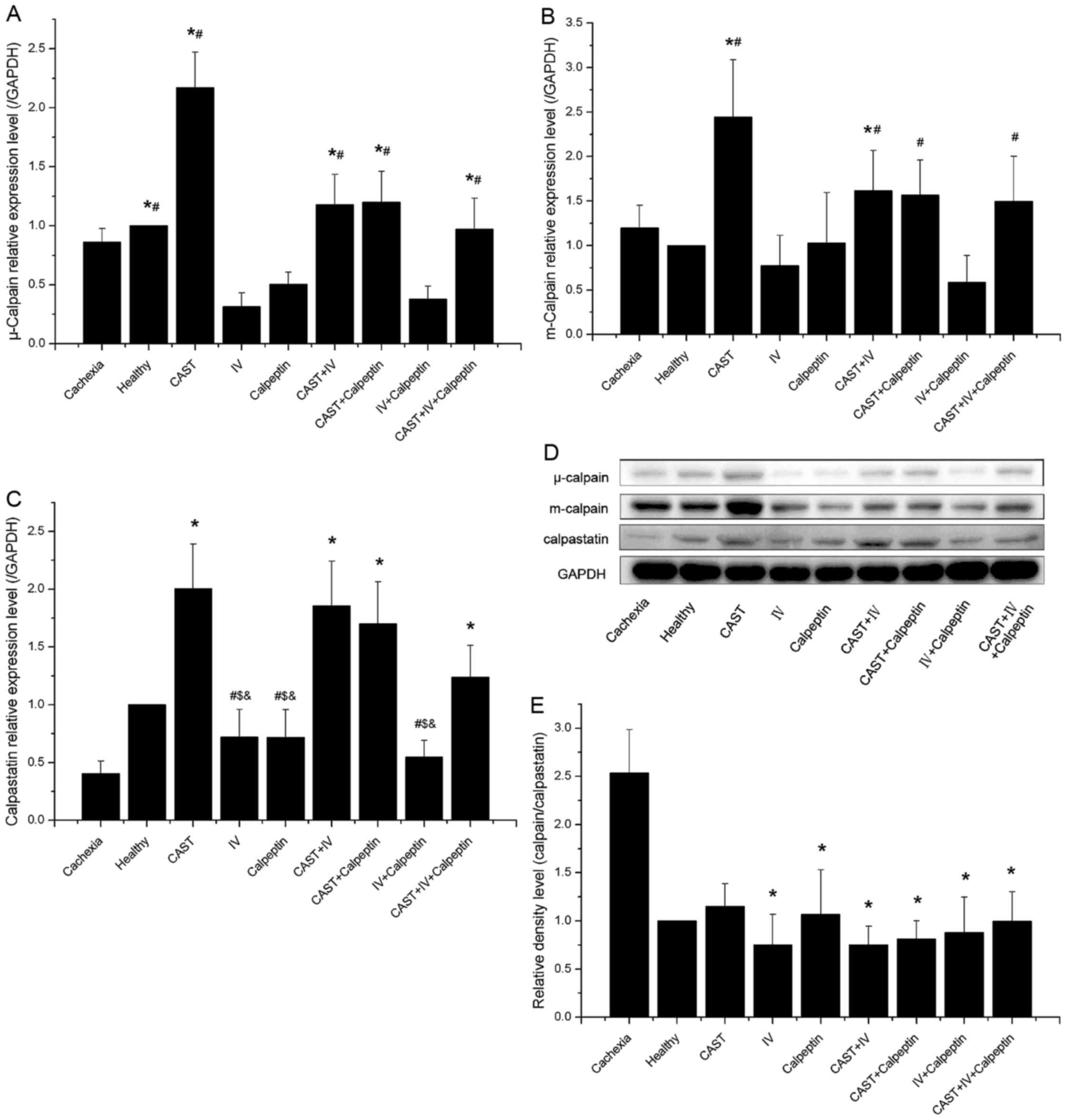 | Figure 4.Effect of different inhibitor
treatments on the calpain system in tumor-bearing mice administered
after CT26 inoculation and control groups (n=10 each). (A) The
level of µ-calpain in gastrocnemius muscle extract. Significant
differences were detected between the IV and the healthy groups
(*P=0.005), between the IV group and the groups involving
calpastatin (*P<0.001; P=0.011 when IV versus
CAST+IV+calpeptin), between the IV+calpeptin and the healthy groups
(#P=0.021), and between the IV+calpeptin group and the
groups involving calpastatin (#P<0.001, P=0.001,
P=0.001, P=0.045, respectively). (B) The level of m-calpain in
gastrocnemius muscle extract. Significant differences were observed
between the IV and the CAST groups (*P<0.001), between the IV
and the CAST+IV groups (*P=0.023), and between the IV+calpeptin
group and the groups involving calpastatin (#P<0.001,
P=0.001, P=0.002, P=0.015, respectively). (C) The level of
calpastatin in gastrocnemius muscle extract. Significant
differences were observed between the cachexia group and the groups
involving calpastatin (*P<0.001; P=0.005 when cachexia versus
CAST+IV+calpeptin), between the CAST group and the treatment groups
not involving calpastatin(#P=0.001, P=0.001, P<0.001,
respectively), between the CAST+IV group and the treatment groups
not involving calpastatin ($P=0.003, P=0.002,
P<0.001, respectively), and between the CAST+calpeptin group and
the treatment groups not involving calpastatin
(&P=0.009, P=0.008, P<0.001, respectively). (D)
The protein bands of µ-calpain, m-calpain, calpastatin, and the
housekeeping protein GAPDH. (E) The calpain-to-calpastatin ratio.
Significant differences were observed between the cachexia group
and the treatment groups (*P<0.001, P=0.018, P<0.001,
P<0.001, P=0.001, P=0.028, respectively) except for the CAST
group (P=0.697). |
Lower levels of calpastatin were observed in the
muscle of tumor-bearing mice compared to the healthy controls,
except for the groups affected by exogenous calpastatin. Compared
with groups not involving calpastatin, there were significant
higher levels of calpastatin in the groups treated with calpastatin
peptide alone or combined with other inhibitors. In addition,
administration with other inhibitors could decrease the levels of
calpastatin (Fig. 4C and D).
In accordance with a previous study (16), we found that both the levels of
µ-calpain and m-calpain remained unchanged in the cachexia and
healthy controls during the experiment (P>0.05), but the
calpastatin level showed a progressive decrease after tumor
transplantation, resulting in a rising imbalance of the
calpain/calpastatin ratio. Moreover, the ratio of calpain to
calpastatin had a positive correlation with calpain activity
(Figs. 4E and 5). The present study indicated that the
levels of µ-calpain, m-calpain, and calpastatin expression in
tumor-bearing mice were affected by different inhibitors and that
the ratio of calpain to calpastatin and the calpain activity was
reduced through the administration of any inhibitor alone or in
combination.
Expression of atrogin-1 and MuRF-1
mRNA
To characterize the effects of calpain inhibitors on
muscle atrophy, mRNA levels of atrogin-1 (MAFbx) and MuRF-1 were
measured. Results from qRT-PCR analyses revealed that atrogin-1 and
MuRF-1 were significantly greater in all cachexia mice than in the
healthy controls (approximately a 20-fold increase). The
mono-treatment of calpain inhibitors significantly downgraded
MuRF-1 levels by 74, 79, and 56% and atrogin-1 levels by 58, 75,
and 61%, respectively, compared to the cachexia control group.
Further reductions were observed when these inhibitors were
administrated together (Fig. 6A and
B).
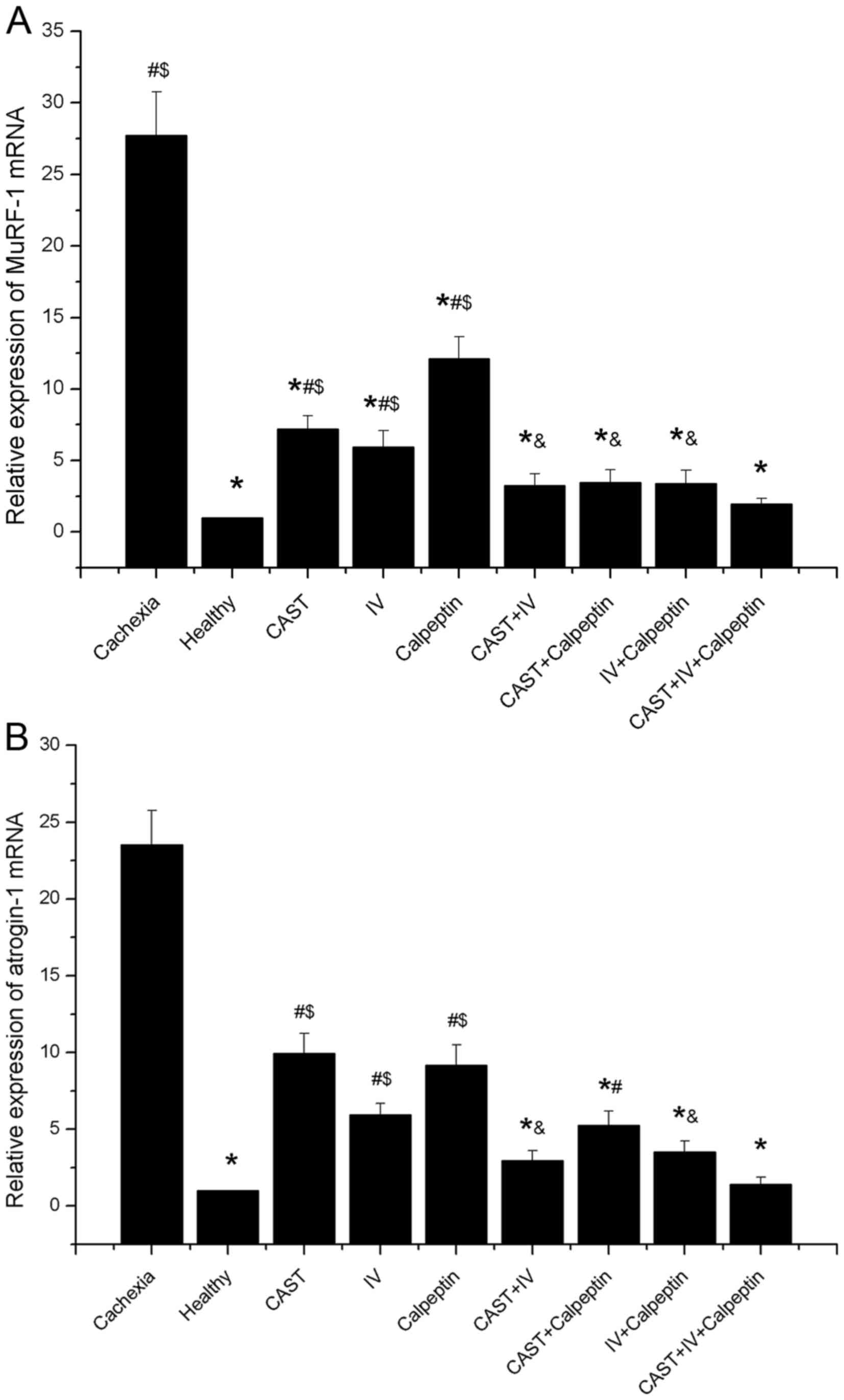 | Figure 6.Effect of different inhibitor
treatments on MuRF-1 and atrogin-1 mRNA expression (n=10 each). (A)
The relative expression of MuRF-1 mRNA. A significant difference
was observed between the cachexia group and the healthy group,
between the cachexia group and any combined treatment group
(*P<0.01), between the healthy group and any mono-treatment
group (#P<0.001), and between the CAST+IV+calpeptin
group and any mono-treatment group ($P<0.05; P=0.003,
P=0.025, P<0.001, respectively). In addition, a significant
difference was also found between the calpeptin group and any
combined treatment group (&P<0.05; P=0.012,
P=0.029, P=0.02, respectively). (B) The relative expression of
atrogin-1 mRNA. A significant difference was found between the
cachexia group and the healthy group, between the cachexia group
and any combined treatment group (*P<0.01), between the healthy
group and any mono-treatment group (#P<0.01;
P<0.001, P=0.004, P<0.001, respectively), between the healthy
group and the CAST+calpeptin group (#P=0.021), and
between the CAST+IV+calpeptin group and any mono-treatment group
($P<0.05; P<0.001, P=0.028, P<0.001,
respectively). In addition, a significant difference was also
observed between the CAST group and the CAST+IV group
(&P=0.007), between the CAST group and the
IV+calpeptin group (&P=0.032). |
Expression of apoptosis-related
protein
To evaluate the effect of calpain inhibitors on
apoptosis, we measured the expression of several widely used
apoptosis-related proteins, including caspase-3, BAX, and BCL-2.
Our results confirmed that apoptosis was present in skeletal muscle
of cachectic mice, as there were significantly higher caspase-3 and
BAX protein levels and lower BCL-2 levels in cachexia control
animals as compared to those observed in the healthy animals.
Mono-treatment with calpain inhibitor IV, calpeptin, or both was
associated with a decrease in the expression of caspase-3 and BAX
and an increase in the expression of BCL-2, which decreased the
BAX-to-BCL-2 ratio. Conversely, administration of calpastatin
peptide increased the protein levels of caspase-3 and BAX, and
reduced the expression of BCL-2, although combination with other
calpain inhibitors, such as calpain inhibitor IV, calpeptin, or
both, could alleviate these changes to some extent (Fig. 7A-E).
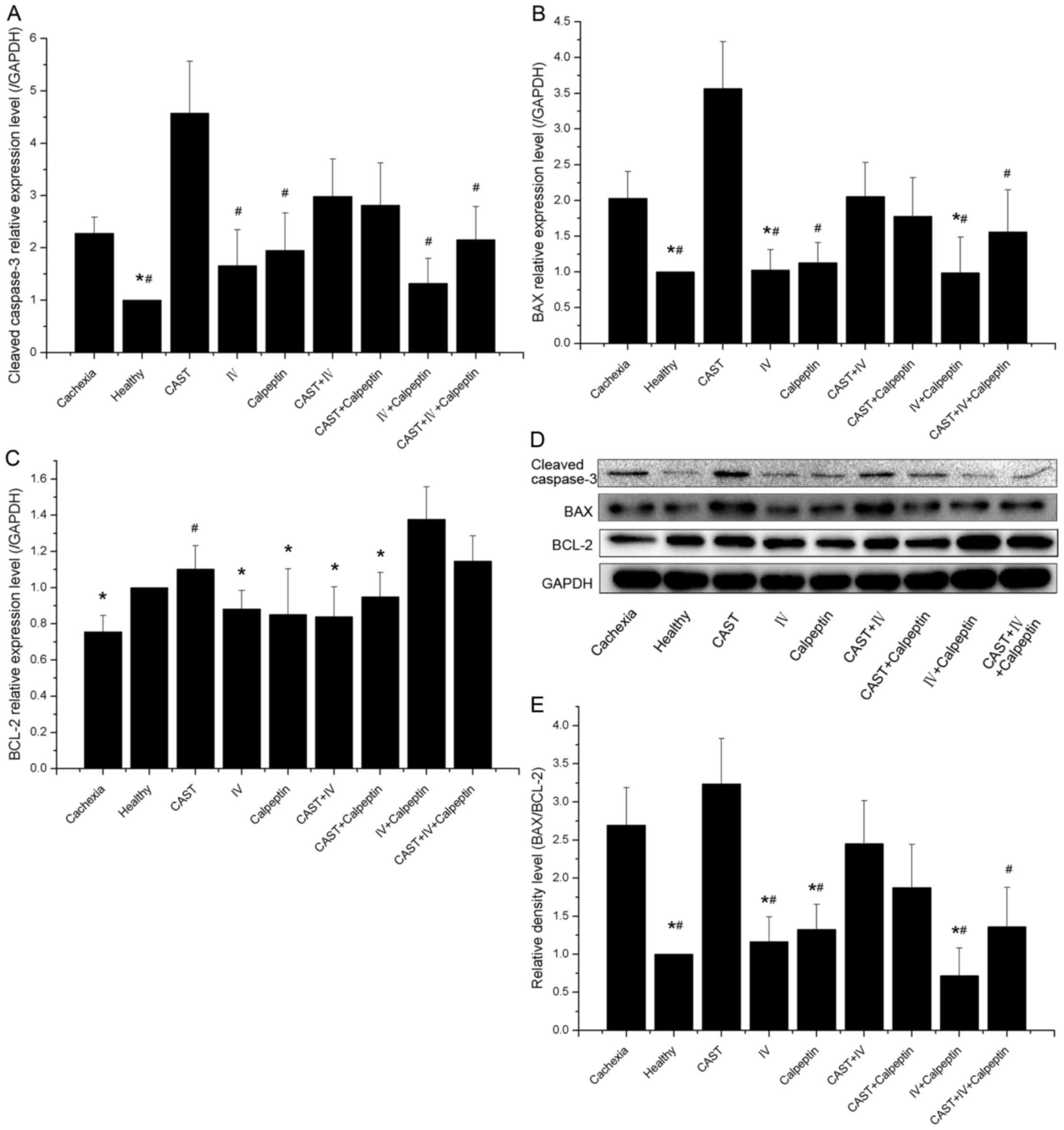 | Figure 7.Effect of different inhibitor
treatments on muscle apoptosis in tumor-bearing and control mice
(n=10 each). (A) The level of cleaved caspase-3 in gastrocnemius
muscle extract. Significant differences were detected between the
cachexia group and the healthy group (*P=0.017), between the CAST
group and any other group (#P<0.001, P<0.001,
P=0.011, P<0.001, P=0.033, respectively) except when compared
with the CAST+IV and the CAST+calpeptin groups (P>0.05). (B) The
level of BAX in gastrocnemius muscle extract. Significant
differences were found between the cachexia group and the healthy
group (*P=0.005), between the cachexia group and the IV group
(*P=0.021), between the cachexia group and the IV+calpeptin group
(*P=0.021), and between the CAST group and any other group
(#P<0.001; P=0.035 when CAST versus
CAST+IV+calpeptin) except when compared with the cachexia, the
CAST+IV and the CAST+calpeptin groups (P>0.05). (C) The level of
BCL-2 in gastrocnemius muscle extract. Significant differences were
observed between the IV+calpeptin group and any other group
(*P<0.001; P=0.024 when IV+calpeptin versus CAST+calpeptin)
except when compared with the healthy, the CAST and the
CAST+IV+calpeptin groups (P>0.05). In addition, a significant
difference was also found between the cachexia group and the CAST
group (#P=0.002). (D) The bands of cleaved caspase-3,
BAX, BCL-2, and the housekeeping protein GAPDH. (E) The
BAX-to-BCL-2 ratio. Significant differences were observed between
the healthy group and the cachexia group (*P<0.001), between the
IV+calpeptin group and the cachexia group (*P<0.001), between
the IV group and the cachexia group (*P=0.008), between the
calpeptin group and the cachexia group (*P=0.047), and between the
CAST group and any other group (#P<0.001, P<0.001,
P=0.007, P<0.001, P=0.011, respectively) except when compared
with the cachexia, the CAST+calpeptin and the CAST+IV groups
(P>0.05). |
Discussion
Cancer cachexia, a debilitating and life-threatening
syndrome, occurs in 22–55% of patients with advanced colorectal
cancer and is associated with high morbidity and mortality rates
(17). As no effective treatment
currently exists, only palliative care is available for patients
(18). Given that skeletal muscle
loss is the primary symptom of cancer cachexia, and that the
calpain system and the ubiquitin-proteasome pathway (UPP) are
critical to proteolysis, we hypothesized that calpain might act
upstream of UPP (11) and that
calpain inhibition could further reduce UPP-dependent protein
breakdown.
After administration with calpain inhibitors, we
observed significant increase in tumor-free body weight and
gastrocnemius muscle mass among the treatment groups compared with
the cachexia control group. In addition, combining multiple agents
was more effective at preserving body weight than mono-treatments.
Most of the treatment groups had a longer survival time than the
cachexia group, indicating that the treated tumor-bearers might
benefit from calpain inhibition. In addition, no statistical
difference in tumor mass was observed among the groups of
tumor-bearing mice, suggesting that these agents have no adverse
effects on tumor growth in the current study. These results support
the concept that calpain inhibitor treatment can alleviate the
muscle loss induced by cachexia.
In this study, the calpain system was activated in
the skeletal muscles of cachectic mice, resulting from an
increasing calpain/calpastatin ratio instead of increased calpains
in absolute amounts, as also seen in previous studies (19). Calpain inhibitor IV and calpeptin
(pharmacological calpain inhibitors) could suppress calpain
activity by reducing their amounts, but calpastatin (an endogenous
calpain inhibitor) downregulated the activity of the calpain system
by raising the level of calpastatin and further decreasing the
calpain/calpastatin ratio. The fact that the calpastatin treatment
group had a significantly higher level of calpain than the other
groups might indicate a positive feedback mechanism. Interestingly,
we found pharmacological calpain inhibitors could ameliorate the
excessive increase of calpains caused by calpastatin during
combination treatment, suggesting that combination therapy might be
an effective strategy for reversing the abnormal activation of the
calpain system.
The unique ubiquitin E3 ligases, muscle atrophy
F-box (MAFbx or atrogin-1), and muscle ring finger-1 (MuRF-1),
essential for the ubiquitin-proteasome pathway, are upregulated in
the process of skeletal muscle atrophy (20). Atrogin-1 or MuRF1 gene knockouts
were confirmed to be resistant to muscle atrophy induced by
different conditions (21,22). Previous studies indicated that
activation of UPP might be the consequence of a growing number of
substrates produced by the activated calpain system rather than the
causative factors of muscle wasting (19). Our study provides further support
for this concept: we found that there was a positive correlation
between atrogin-1/MuRF1 mRNA expression and calpain activity when
they were downregulated synchronously. Furthermore, our data
suggest that enhancing the inhibition action of calpains through
combination therapy could lower the level of atrogin-1/MuRF1 mRNA,
protecting against muscle atrophy. However, further studies will
clarify how cell signaling pathways take part in the linkage
between UPP and the calpain system.
During the past several decades, increasing numbers
of reports have supported the concept that apoptosis is a common
feature in skeletal muscle of tumor-bearing animals and patients
with cancer-related cachexia (9,23).
Apoptosis may account for muscle wasting under cachectic condition
as well as the abnormally activated proteolytic system. In the
present study, the observation that all tumor-bearing mice had
significantly higher levels of cleaved caspase-3 and BAX and lower
levels of BCL-2 compared to healthy controls, is consistent with
previous studies (24). In
addition, the administration of calpain inhibitor IV and calpeptin
alleviated skeletal muscle apoptosis was partly through reducing
the activation of caspase-3 and decreasing the BAX-to-BCL2 ratio,
as other calpain inhibitors have been shown to do during the
process of injury-induced neuronal apoptosis (25,26).
However, the calpastatin treatment group exhibited more apoptotic
proteins (cleaved caspase-3 and BAX) than other groups, which might
be attributed to its higher levels of calpains and calpastatin
(27) and which could be
ameliorated during combination treatment with pharmacological
calpain inhibitors.
In our study, calpain inhibitor IV and calpeptin
alone and in combination could partially reverse tumor-free body
weight loss and muscle wasting, while calpastatin alone was less
effective due to increased calpain levels and apoptosis.
Furthermore, our doses of these inhibitors were derived from
previous reports and were not optimized by titration, which might
lead to an insufficient inhibition of calpain and non-significant
differences between groups. Although no adverse effects on tumor
growth or shortened survival were observed in our study, other side
effects might occur in vivo. Nevertheless, future studies
should focus on the pharmacokinetics and toxicology of these agents
when administrated together, as well as their underlying mechanisms
of action, before they enter into the next phase of clinical
research.
In conclusion, our study showed that treatment of
cachectic tumor-bearing mice with three different calpain
inhibitors, alleviated cachexia-associated symptoms, increased
tumor-free body weight and muscle mass. In addition, improved
concentrations of serum nutritional markers and apoptosis-related
proteins in skeletal muscle, reduced calpain activity and E3
ubiquitin ligase expression were observed. Although the exact
mechanism of calpain inhibitor in cachexia has not been elucidated
fully and needs to be studied further to prove their security, our
results may lead to the development of novel strategies for the
prevention and treatment of cancer cachexia.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (81272465).
Glossary
Abbreviations
Abbreviations:
|
UPP
|
ubiquitin-proteasome proteolysis
|
|
CAST
|
calpastatin
|
|
IV
|
calpain inhibitor IV
|
|
DMSO
|
dimethyl sulfoxide
|
|
PCR
|
polymerase chain reaction
|
|
qRT-PCR
|
quantitative real-time polymerase
chain reaction
|
|
cDNA
|
complementary deoxyribonucleic
acid
|
|
MAFbx
|
muscle atrophy F-box
|
|
MuRF-1
|
muscle ring finger-1
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
BAX
|
BCL2-associated X
|
|
BCL-2
|
B-cell lymphoma/leukemia-2
|
References
|
1
|
Fearon K, Strasser F, Anker SD, Bosaeus I,
Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N,
Mantovani G, et al: Definition and classification of cancer
cachexia: An international consensus. Lancet Oncol. 12:489–495.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baracos VE: Pitfalls in defining and
quantifying cachexia. J Cachexia Sarcopenia Muscle. 2:71–73. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
von Haehling S and Anker SD: Cachexia as
major underestimated unmet medical need: Facts and numbers. Int J
Cardiol. 161:121–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tisdale MJ: Molecular pathways leading to
cancer cachexia. Physiology (Bethesda). 20:340–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Del Fabbro E: Current and future care of
patients with the cancer anorexia-cachexia syndrome. Proc ASCO.
e229–237. 2015.10.14694/EdBook_AM.2015.35.e229.
|
|
6
|
Goll DE, Neti G, Mares SW and Thompson VF:
Myofibrillar protein turnover: The proteasome and the calpains. J
Anim Sci. 86 Suppl:E19–E35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wing SS, Lecker SH and Jagoe RT:
Proteolysis in illness-associated skeletal muscle atrophy: From
pathways to networks. Crit Rev Clin Lab Sci. 48:49–70. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johns N, Stephens NA and Fearon KC: Muscle
wasting in cancer. Int J Biochem Cell Biol. 45:2215–2229. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Busquets S, Deans C, Figueras M,
Moore-Carrasco R, López-Soriano FJ, Fearon KC and Argilés JM:
Apoptosis is present in skeletal muscle of cachectic
gastro-intestinal cancer patients. Clin Nutr. 26:614–618. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Donkor IO: Calpain inhibitors: a survey of
compounds reported in the patent and scientific literature. Expert
Opinion on Therapeutic Patents. 21:601–636. 2011.doi:
10.1517/13543776.2011.568480. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang J and Zhu X: The molecular
mechanisms of calpains on muscle atrophy. Physiol Res. 65:547–560.
2016.PubMed/NCBI
|
|
12
|
Zhang Z, Huang Z, Dai H, Wei L, Sun S and
Gao F: Therapeutic efficacy of E-64-d, a selective calpain
inhibitor, in experimental acute spinal cord injury. Biomed Res
Int. 2015:1342422015.PubMed/NCBI
|
|
13
|
Wang C, Shi D, Song X, Chen Y, Wang L and
Zhang X: Calpain inhibitor attenuates ER stress-induced apoptosis
in injured spinal cord after bone mesenchymal stem cells
transplantation. Neurochem Int. 97:15–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Donkor IO: An updated patent review of
calpain inhibitors (2012–2014). Expert Opin Ther Pat. 25:17–31.
2015.PubMed/NCBI
|
|
16
|
Salamino F, De Tullio R, Mengotti P,
Viotti PL, Melloni E and Pontremoli S: Different susceptibility of
red cell membrane proteins to calpain degradation. Arch Biochem
Biophys. 298:287–292. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thoresen L, Frykholm G, Lydersen S,
Ulveland H, Baracos V, Prado CM, Birdsell L and Falkmer U:
Nutritional status, cachexia and survival in patients with advanced
colorectal carcinoma. Different assessment criteria for nutritional
status provide unequal results. Clin Nutr. 32:65–72. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amano K, Maeda I, Morita T, Okajima Y,
Hama T, Aoyama M, Kizawa Y, Tsuneto S, Shima Y and Miyashita M:
Eating-related distress and need for nutritional support of
families of advanced cancer patients: A nationwide survey of
bereaved family members. J Cachexia Sarcopenia Muscle. 7:527–534.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Costelli P, De Tullio R, Baccino FM and
Melloni E: Activation of Ca(2+)-dependent proteolysis in skeletal
muscle and heart in cancer cachexia. Br J Cancer. 84:946–950. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Foletta VC, White LJ, Larsen AE, Léger B
and Russell AP: The role and regulation of MAFbx/atrogin-1 and
MuRF1 in skeletal muscle atrophy. Pflugers Arch. 461:325–335. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bodine SC, Latres E, Baumhueter S, Lai VK,
Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K,
et al: Identification of ubiquitin ligases required for skeletal
muscle atrophy. Science. 294:1704–1708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furlow JD, Watson ML, Waddell DS, Neff ES,
Baehr LM, Ross AP and Bodine SC: Altered gene expression patterns
in muscle ring finger 1 null mice during denervation- and
dexamethasone-induced muscle atrophy. Physiol Genomics.
45:1168–1185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Royen M, Carbó N, Busquets S, Alvarez
B, Quinn LS, López-Soriano FJ and Argilés JM: DNA fragmentation
occurs in skeletal muscle during tumor growth: A link with cancer
cachexia? Biochem Biophys Res Commun. 270:533–537. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishiko O, Sumi T, Yoshida H, Hyun Y and
Ogita S: Angiogenesis in the adipose tissue of tumor-bearing
rabbits treated by cyclic plasma perfusion. Int J Oncol.
19:785–790. 2001.PubMed/NCBI
|
|
25
|
Ray SK, Samantaray S, Smith JA, Matzelle
DD, Das A and Banik NL: Inhibition of cysteine proteases in acute
and chronic spinal cord injury. Neurotherapeutics. 8:180–186. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ray SK, Matzelle DC, Wilford GG, Hogan EL
and Banik NL: E-64-d prevents both calpain upregulation and
apoptosis in the lesion and penumbra following spinal cord injury
in rats. Brain Res. 867:80–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim KA, Lee YA and Shin MH:
Calpain-dependent calpastatin cleavage regulates caspase-3
activation during apoptosis of Jurkat T cells induced by Entamoeba
histolytica. Int J Parasitol. 37:1209–1219. 2007. View Article : Google Scholar : PubMed/NCBI
|